Abstract
Evidence from clinical trials repeatedly confirms the association of diabetes with heart failure, independent of hypertension, atherosclerosis, coronary artery disease and valvular heart disease. However, the importance of coexistence of diabetes and heart failure is not universally recognized, despite the fact that it may significantly contribute to morbidity and mortality of the diabetic population. It seems that prevention of heart failure, early diagnosis, and appropriate management could improve the outcome. Unfortunately, the etiology of heart failure in diabetic patients is still to be elucidated. It is multifactorial in nature and several cellular, molecular and metabolic factors are implicated. Additionally, there are still no definite guidelines on either the diagnosis and treatment of heart failure in diabetic patients or on the therapy of diabetes in subjects with heart failure. This review focuses on the pathophysiology, diagnosis, and prevention of heart failure in the diabetic population as well as management of both comorbidities.
Keywords: heart failure in diabetes, pathophysiology, screening, therapy
Introduction
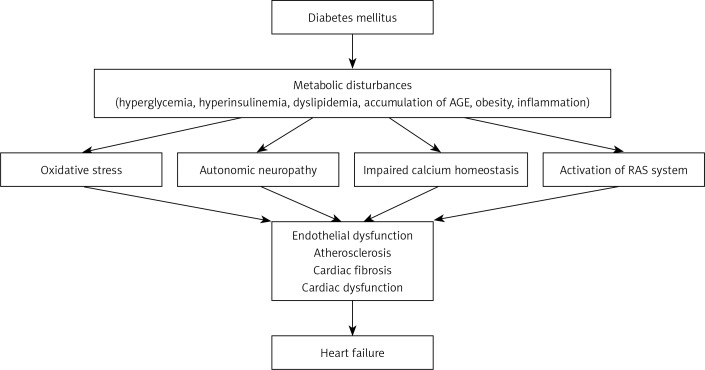
Heart failure (HF) is the most common cardiovascular complication of diabetes mellitus (DM) [1, 2]. This is not surprising since DM and HF share common pathogenetic factors (Figure 1). Heart dysfunction in the diabetic population may develop regardless of typical risk factors such as hypertension and coronary artery disease [3]. The increased incidence of heart failure in diabetic subjects is supported by results of numerous epidemiological studies that indicate the strong association between DM and HF. These observations are further supported by evidence from experimental studies showing structural and functional dysfunction of diabetic myocardium [4]. Certainly, comorbidities accelerate the likelihood of developing cardiac dysfunction in diabetes, but it is still to be elucidated why diabetic patients are at increased risk of HF. The cause of HF in diabetes is certainly multifactorial in nature, but hyperglycemia and insulin resistance seem to be the core factors. Furthermore, the results of some studies suggest that an increased risk of HF may be associated with specific therapies, such as insulin [5], sulfonylurea (SU), gliptins or glitazones [6]. However, the effects of drugs should be assessed with extreme caution, since diabetic patients often receive multiple therapies simultaneously or over time.
Figure 1.
Pathophysiology of HF in diabetic patients
AGE – advanced glycation end-products, RAS – renin angiotensin system
In this review, we discuss the evidence regarding the coexistence of heart failure and diabetes, along with its pathophysiology and management.
We searched using electronic databases (PUBMED/MEDLINE (1966 – December 2012), EMBASE and SCOPUS (1965 – December 2012)). Additionally, retrospective studies, as well as small studies with the number of patients below 100, and animal studies were also included. The main data search terms were: diabetes mellitus, HF, diabetic cardiomyopathy, clinical studies, clinical trials, metabolic disturbances, myocardial fibrosis, small vessel pathology, cardiac autonomic neuropathy, myocardial dysfunction in diabetes, myocardial dysfunction in diabetic animals.
Incidence of heart failure in the diabetic population
The results of the Framingham Heart Study showed that the frequency of HF was twice as high in diabetic men and five times higher in diabetic women compared with control subjects; however, not much is known about the cause of this disparity [7]. This association was independent of age, hypertension, obesity, coronary artery disease (CAD) and hyperlipidemia. Similar results were also seen in other studies after correction for confounding variables [5, 8]. Analysis of data from 9,591 type 2 DM (T2DM) patients registered in the Kaiser Permanente Northwest Division revealed HF in 11.8% of diabetic subjects at baseline, with an additional 7.7% of patients developing HF during a 30-month period of observation [5]. Hamby et al. found that diabetes was present in 22% of patients with idiopathic cardiomyopathy compared to 11% in the control group [9]. Lind et al. analyzed data of 20 985 patients with type 1 DM (T1DM) without concomitant HF registered in the national Swedish registry. The hazard ratio for development of HF was 3.98 in patients with glycated haemoglobin (HbA1c) ≥ 10.5% compared to the reference group of patients with HbA1c < 6.5% even after adjustment for age, sex, duration of diabetes, cardiovascular risk factors, and other comorbidities. Risk of HF increased with age and duration of diabetes [10]. In this group of patients a high level of high-density lipoprotein (HDL) cholesterol was associated with lower risk of HF, but there was no association with low-density lipoprotein (LDL) cholesterol. Bertoni et al. also reported a link between idiopathic cardiomyopathy and diabetes [8]. The prevalence of diabetes in the general population ranges from 4% to 6%. However, diabetes was overrepresented in HF trials such as SOLVD (Studies of Left Ventricular Dysfunction; 26%) [11], ATLAS (Assessment Trial of Lisinopril And Survival; 19%) [12] and V-HeFT II (Vasodilator-HF Trial II; 20%) [13].
In an interesting study Maru et al. analyzed the effects of antidiabetic drugs on the risk of HF in 25 690 patients newly diagnosed with T2DM [14]. The patients were stratified according to the duration and type of treatment. After a mean follow-up of 2.5 years, 1409 patients developed HF, most frequently in SU monotherapy. However, after adjustment for duration of diabetes, the timing and order of therapies received, as well as known risk factors for HF, there was no statistical difference between treatments. Maru et al. revealed that the use of any pharmacological therapy for T2DM was associated with a 4.75-fold increased risk of HF, but this risk did not persist beyond the first year after diagnosis of diabetes and did not differ among the types of examined drug therapies. This suggests that the degree of metabolic disturbances, duration of undiagnosed diabetes, and the need for drug therapy, but not the therapy itself, may all accelerate the development of HF in T2DM patients.
Functional changes in the diabetic heart as an indicator of heart failure
Functional changes occurring in diabetic patients typically involve the impaired diastolic function of the heart, that may precede the systolic dysfunction [15]. However, diastolic dysfunction is not pathognomonic for HF in diabetes [16]. It is estimated that in nearly half of all HF cases, regardless of the presence of diabetes, diastolic dysfunction is present along with normal or near-normal left ventricular ejection fraction. Changes in diastolic function were reported in diabetic patients independent of hypertension, coronary artery disease, or any other known cardiac disease, before they were clinically apparent [17]. The left ventricular (LV) ejection time is often reduced, but the pre-ejection period and the ratio of pre-ejection period to LV ejection time are often increased [18]. Carugo et al. found increased LV wall thickness and LV mass index, as well as an age-related decline in ejection fraction, and an age-related increase in diastolic diameter [19]. The left ventricular mass was 10% greater in woman with diabetes compared to nondiabetics. Left ventricular hypertrophy was observed in 32% of normotensive T2DM patients without known CAD not taking ACE inhibitors [20]. Studies in well-controlled T2DM subjects revealed a prevalence of diastolic dysfunction of up to 30% of patients [21]. The use of new Doppler techniques indicated prevalence of diastolic dysfunction in up to 60% of patients with T1 and T2DM without CAD [22, 23]. Results of several studies suggest that patients with T2DM are more likely to have diastolic dysfunction than T1DM patients. However, diastolic dysfunction was not observed in all studies, especially in younger patients with T1DM.
There is also a significant association of dilated cardiomyopathy with DM [24]. The use of new echocardiographic techniques revealed that systolic dysfunction is also present in a substantial number of diabetic patients. Changes typical for systolic dysfunction may indicate an increased risk for the development of HF, particularly in the presence of coexisting hypertension [25, 26].
In the Strong Heart Study, the severity of diastolic dysfunction correlated with HbA1c level [27]. The most likely cause of diastolic dysfunction is advanced glycation end-product (AGE)-induced formation of reactive oxygen species (ROS), leading to deposition of collagen in myocardium, fibrosis and insulin resistance syndrome leading to left ventricular hypertrophy [27, 28].
Pathophysiology of heart failure in diabetic patients
Several hypotheses have been proposed to explain the mechanisms responsible for decreased myocardial contractility in the diabetic population. These include metabolic disturbances, accumulation of AGE, myocardial fibrosis, small vessel disease, impaired calcium homeostasis, autonomic neuropathy and insulin resistance (Figure 1).
Metabolic disturbances
In diabetes metabolic changes are triggered by hyperglycemia [29]. In normal conditions, the myocytes use free fatty acids (FFA) as a primary source of energy during aerobic exercise. During ischemia or increased work glycolysis and pyruvate oxidation become increasingly important. Positron emission tomography studies in T1DM patients revealed increased myocardial FFA use and reduced glucose oxidation [30]. Altered metabolism in these patients was associated with increased myocardial oxygen consumption and increased concentrations of FFA in serum. In diabetic patients, the major factors limiting glucose utilization are the slow rate of glucose transport into the myocardium probably associated with depletion of glucose transporters (GLUT) 1 and 4 and the inhibitory effect of FA oxidation on pyruvate dehydrogenase complex [31]. In diabetes, an increase in adipose tissue lipolysis and hydrolysis of myocardial triglyceride stores is responsible for elevated circulating levels of FFA [18]. Metabolism of high levels of FFA requires high oxygen consumption and leads to intracellular accumulation of toxic intermediates, which may negatively influence myocardial performance through reduced availability of ATP [32].
Oxidative stress
Although no theory is completely accepted as a single cause of HF in diabetic patients, it is regarded that reactive oxide species (ROS) generated in vivo may play an important role in myocardial damage [33, 34]. In diabetic patients, poor glycemic control leads to chronic hyperglycemia. The oxidation of elevated levels of glucose within the cell stimulates production of ROS and increases oxidative stress [33]. Increased generation of ROS such as superoxide, hydrogen peroxide and hydroxyl radical is the cause of oxidation and modification of structure of cellular proteins, nucleic acids, and membrane lipids. Higher levels of peroxynitrate were found by Al-Nimer et al. in biological fluids of T2DM patients compared to healthy subjects [35]. Damage of the cellular structure and impairment of its function leads to cell necrosis and activation of genes involved in cell damage [36]. Increased ROS-mediated cell death may promote cardiac remodeling, which may contribute to the morphological and functional abnormalities of the heart in diabetic patients. Increased ROS content may lead to cardiac dysfunction via mechanisms other than cellular injury. In diabetic patients increased oxidative stress may be associated not only with overproduction of ROS, but also with a significant decrease in the effectiveness of antioxidant defenses or both. Insufficient anti-oxidative cellular mechanisms may also be involved in cardiac damage. Its seems that the activity of cellular antioxidants such as the enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) may be crucial to this process. Kasznicki et al. demonstrated decreased activity of primary antioxidant enzymes such as catalase, superoxide dismutase and glutathione peroxidase in peripheral blood of T2DM patients with coexisting distal symmetric polyneuropathy (DSPN) [37].
Endothelial dysfunction
Endothelial dysfunction occurs in coronary vessels of diabetic patients and may lead to impaired blood flow. It was observed that in diabetic patients the endothelium-dependent dilatation of the epicardial coronary arteries is impaired [38]. Kasznicki et al. found that chronic high glucose concentration induced the decrease of plasma nitric oxide (NO) level in diabetic patients [37]. It was reported that hyperglycemia resulted in down-regulation of endothelial nitric oxide synthase (eNOS) expression and NO production in cultured human coronary endothelial cells [39]. Endothelial NO, along with prostacyclin I2 (PGI2), is the main vasodilator and the most important factor involved in the function of blood vessels. Endothelial dysfunction is characterized by low bioavailability of endothelium-derived NO. It seems that reduced antioxidative defense in diabetic patients is associated with increased vascular oxidative stress through decreased NO bioavailability. The NO is inactivated by the superoxide radical and the peroxynitrite anion, which can cause endothelial damage. Moreover, free radicals, especially superoxide anion, may react with endothelium-derived nitric oxide (NO) and can inhibit eNOS. This interaction is one of the most important mechanisms involved in the endothelial dysfunction in diabetic patients [40]. The ROS have been reported to contribute to impairment of endothelium-dependent vascular relaxation by the inactivation of NO, and generally to the vascular dysfunction resulting in accelerated atherosclerosis in diabetic patients. It is suggested that increased production of ROS, induced by hyperglycemia, is involved in cardiac tissue remodeling via activation of metalloproteinase 9, which in turn leads to attenuation of sarco-endoplasmic reticulum-calcium ATPase 2 and alters expression of miRNA [41]. Alterations in miRNAs may lead to altered contractility of myocardium.
Accumulation of advanced glycation end-products
Hyperglycemia leads to glycation of numerous macromolecules. Advanced glycation end-products (AGE) accumulate in tissues and may cause morphological changes in the heart. Accumulation of AGE results not only in decreased elasticity of the vessel walls but also in myocardial dysfunction. Berg et al. reported that in diabetic patients, prolongation of isovolumic relaxation time correlates with serum levels of AGE even after adjustment for age, diabetes duration, renal function, blood pressure and autonomic function [42].
Impaired calcium homeostasis
Intracellular calcium (Ca2+) is a major regulator of cardiac contractility and disturbances in its homeostasis may contribute to alteration of cardiac performance. It is believed that diminished activity of ATPases [43], decreased ability of the sarcoplasmic reticulum to take up calcium, and reduced activities of Na+-Ca2+ and Ca2+ ATPase may all contribute to this effect [43, 44]. It was observed that in diabetes reduced activity of the sarcoplasmic reticular calcium pump, and diminished rate of Ca2+ removal from the cytoplasm in diastole, may be responsible for diastolic dysfunction [45].
Myocardial fibrosis
The results of numerous studies have revealed not only structural but also functional changes of the diabetic heart, which may play a role in deterioration of cardiac performance [18, 46]. The most typical finding in diabetic patients is fibrosis, which may be both perivascular and interstitial. Hyperglycemia may cause abnormal gene expression and alteration of signal transduction, which may activate the pathways leading to apoptosis [18]. Hyperglycemia may also directly induce necrosis of myocytes, which results in increased deposition of collagen [47]. Myocardial fibrosis was confirmed in autopsy studies.
Small vessel disease
Alterations of the small vessels’ structure and function may also lead to HF in diabetic patients. Compared to the control subjects, in diabetic patients thickening of the capillary basement membrane and interstitial fibrosis was significantly greater on biopsy [48]. This observation suggests that in this group of patients changes in capillaries may lead to myocardial injury and interstitial fibrosis. The results of an autopsy study revealed endothelial and subendothelial proliferation with fibrosis in small coronary arteries in diabetic patients [48]. However, it seems that pathological changes in capillaries alone may not be responsible for the development of myocardial dysfunction.
Cardiac autonomic neuropathy
Cardiac autonomic neuropathy (CAN) is associated with an increased cardiovascular risk in diabetes and may contribute to impaired diastolic function. The CAN is a frequent complication of both T1DM and T2DM, and is present in nearly all diabetic patients with LV diastolic dysfunction. The CAN may contribute to the development of HF, since significant reductions in coronary blood flow and reserve were observed in diabetic patients with CAN [18]. In diabetics CAN is responsible for an impaired vasodilator response of coronary vessels [49]. In T1DM patients diastolic filling abnormalities were most pronounced in those with CAN and correlated with its severity [50]. Abnormal response to exercise correlates with impairment of cardiac sympathetic innervations [51].
Mustonen et al. reported that an abnormal sympathetic innervation of the heart may be responsible for alterations in LV filling [52]. In T2DM patients both systolic and diastolic dysfunction was related to altered sympathetic function [53]. Several studies also show an association between parasympathetic and cardiac dysfunction with decreased mean heart rate variation [54].
Screening for heart failure in diabetic patients
Screening for HF may help to prevent its progression and reduce mortality in diabetic patients. In everyday practice echocardiogram with Doppler examinations is the most sensitive method to diagnose HF [7, 8]. However, considering the costs of this examination in the diabetic population, one should consider a simpler and less expensive screening test. Bell suggests estimation of microalbuminuria as an adequate prescreening test [55]. The results of the Strong Heart Study showed that the degree of diastolic dysfunction was proportional to the level of microalbuminuria, even after adjusting for age, sex, body mass index (BMI), systolic blood pressure, duration of diabetes, left ventricular mass, and presence of CAD [56]. Additionally, the results of the Heart Outcomes Prevention Evaluation (HOPE) study indicate that microalbuminuria is associated with significant risk for congestive heart failure (CHF) [57]. That is why it seems reasonable to perform echocardiographic screening for HF in diabetic subjects with microalbuminuria. Identification of functional changes should result in the initiation of treatment in order to slow down the progression to congestive HF and reduce mortality.
In the overt HF in the diabetic population symptoms do not differ from those in nondiabetics, and the diagnosis of HF and its staging are typical.
Therapeutic implications of heart failure in the diabetic population
Currently, no specific therapeutic strategies are recommended for treatment of HF in the diabetic population. It seems that management of traditional risk cardiovascular factors and lifestyle modification together with typical pharmacotherapy for diabetes and HF should be instituted.
Glycemic control
It seems that control of glycemia is the most important strategy for preventing the development of HF, but none of the antidiabetic drugs is proved to be more effective in patients with diabetes coexisting with HF [18]. Poor glycemic control is associated with an 11% increase in cardiovascular mortality for every 1% increase in HbA1c [58]. Poorer glycemic control is also responsible for an increased risk of HF. The data from the DCCT/EDIC study, as well as prolonged phases of UKPDS and STENO-2, indicate that intensive glucose control does reduce stroke, microvascular outcomes, death, and nearly all diabetic cardiovascular endpoints [59, 60]. Unfortunately, there are not much data to support the theory that improved glycemic control would reduce the risk of HF.
Lind et al. assessed the relationship between glycemic control and HF in 83 021 patients with T2DM. The patients registered in the Swedish National Register between 1998 and 2003 were followed until hospitalization for HF, death or study termination. After adjusting for known risk factors for HF the hazard ratio for each percentage unit higher HbA1c for HF hospitalization was 1.12. This indicates that poor glycemic control estimated as HbA1c > 7% is associated with an increased risk for hospitalization for HF in T2DM patients [61]. However, the meta-analysis of data from over 20 000 T2DM patients randomized into the Action to Control Cardiovascular Risk in Diabetes (ACCORD), Action in Diabetes and Vascular Disease (ADVANCE), Veterans Affairs Diabetes Trial (VADT) and UK Prospective Diabetes Study (UKPDS) trials failed to reveal any positive effect of intensive therapy in preventing HF.
The analysis of 8890 diabetic patients from the GoDarts study indicated that poor glycemic control was associated with an increased risk of HF. The odds ratio of developing chronic HF with a mean HbA1c > 6.9% was 2.26 (p < 0.01). Surprisingly, intensive glycemic control (HbA1c < 6%) also appeared to increase the risk for chronic HF (OR 2.48, p < 0.01).
The meta-analysis of Castagno et al. compared intensive vs. less intensive strategies of glucose lowering that reported HF events [62]. 37 229 patients were included in the analysis. They noted that the risk of HF-related events did not differ significantly between intensive glycemic control and standard treatment. These findings indicate no direct association between hyperglycemia and HF. Additionally, intensive glycemic control with thiazolidinediones (TZD) significantly increased HF risk. Weight gain and fluid retention were seen during TZD therapy both in monotherapy as well as coadministered with sulfonylurea and metformin. When added to insulin, weight gain was even more dramatic. The weight gain associated with TZD is probably caused by several interacting factors, such as a reduction in renal excretion of sodium and an increase in sodium and free water retention [63]. Increased sympathetic nervous system activity, altered interstitial ion transport, alterations in endothelial permeability, and peroxisome proliferator-activated receptor-γ-mediated expression of vascular permeability growth factor may all be implicated in this side effect [63]. Additionally, the results of the University Group Diabetes Program (UGDP) suggest increased cardiovascular mortality with the sulfonylurea derivative tolbutamide [64].
Dungan et al. conducted a study to assess the effect of glycemic control and glycemic variability on mortality of patients hospitalized with congestive HF. In this study glycemic liability index, indicating increased glycemic variability, was associated with higher mortality, independent of hypoglycemia [65]. This study indicates that further trials are needed to assess the benefit from interventions aimed not only at glucose level but also at glycemic variability.
Metformin seems to be the only drug that has decreased cardiovascular events in T2DM subjects independently of glycemic control, but it is still contraindicated in T2DM patients with coexisting HF [60]. The results of several trials indicate that metformin is not only well tolerated by patients with diabetes and HF but also reduces mortality in this population [66, 67]. Metformin theoretically may increase the risk of lactic acidosis, but there are no clinical data to support this theory. Moreover, the results of numerous trials indicate that there are no significant differences in the incidence of lactic acidosis in patients with diabetes and HF treated with metformin or placebo [68, 69]. Based on those results the Food and Drug Administration (FDA) decided to withdraw HF as a contraindication to metformin. It is now possible to use metformin in patients with diabetes and HF with careful monitoring of therapy. The observation from UKPDS indicating increased mortality in diabetic patients treated concomitantly with metformin and sulphonylurea derivatives was not confirmed by other investigators. It is considered that metformin may be safely used with sulphonylurea derivatives. Moreover, none of the sulphonylurea derivatives registered in Poland is contraindicated in HF. Also insulin is not contraindicated in HF. Theoretically, insulin may increase the severity of HF due to water retention associated with its use. However, it has never been confirmed in clinical trials. Additionally, the results of the UKPDS study indicate that insulin did not increase the frequency of HF in the diabetic population [70].
There is limited information considering the influence of GLP-1 receptor agonists and DPP-IV inhibitors of heart performance in the diabetic population. The data from phase IV trials give contradictory results concerning left ventricular ejection fraction in subjects treated with GLP-1 agonists.
Therapy of heart failure in diabetic patients
None of the drugs routinely used in the therapy of HF is contraindicated in the diabetic population. There are no specific therapeutic strategies for the therapy of HF in the diabetic population. However, considering the central role of the renin-angiotensin system in the development of HF it seems that drugs targeting this system should always be used for this indication (Table I).
Table I.
Evidence-based therapies used in heart failure [79]
| Drugs | Target^/Usual$ daily dose [mg] | Clinical trial |
|---|---|---|
| ACE inhibitor*: | ||
| Captopril | 50 t.i.d^ | SAVE (captopril) |
| Enalapril | 10–20 b.i.d.^ | CONSENSUS, SOLVD (enalapril) |
| Lisinopril | 20–35 o.d.^ | ATLAS (lisinopril) |
| Ramipril | 5 b.i.d.^ | AIRE (ramipril) |
| Trandolapril | 4 o.d.^ | TRACE (trandolapril) |
| ARB*: | ||
| Candesartan | 32 o.d.^ | CHARM (candesartan) |
| Valsartan | 160 b.i.d.^ | Val-HeFT, VALIANT (valsartan) |
| Losartan | 150 o.d.^ | HEAAL (losartan) |
| β-Blockers*: | ||
| Bisoprolol | 10 o.d.^ | CIBIS II (bisoprolol) |
| Carvedilol | 25–50 b.i.d.^ | COPERNICUS (carvedilol) |
| Metoprolol succinate | 200 o.d.^ | MERIT-HF (metoprolol succinate) |
| Nebivolol | 10 o.d.^ | SENIORS (nebivolol) |
| MRA*: | ||
| Spironolactone | 25–50 o.d.^ | RALES (spironolactone) |
| Eplerenone | 50 o.d.^ | EMPHASIS-HF, EPHESUS (eplerenone) |
| H-ISDN | 40 mg/75 mg t.i.d^ | V-HeFT-I, V-HeFT-II, A-HeFT (hydralazine and isosorbide dinitrate) |
| Diuretics# | The effects of diuretics on mortality and morbidity were not studied in HF | |
| Loop diuretics: | ||
| Furosemide | 40–240 usually o.d. or b.i.d.$ | |
| Torasemide | 10–20 usually o.d.$ | |
| Bumetanide | 1–5 usually o.d.$ | |
| Thiazides: | ||
| Hydrochlorothiazide | 12.5–100 o.d.$ | |
| Indapamide | 2.5–5.0 o.d.$ | |
| Ivabradine | 7.5 b.i.d.^ | SHIFT, BEAUTIFUL (ivabradine) |
| Digoxin | 100–500 µg o.d.$ | DIG (digoxin) |
Disease-modifying drugs
Recommended for patients with signs and symptoms of congestion irrespective of ejection fraction
Target dose
Usual dose, ACE inhibitor – angiotensin-converting enzyme inhibitor, ARB – angiotensin receptor blocker, MRA – mineralocorticoid receptor antagonist, H-ISDN – hydralazine and isosorbide dinitrate
The renin-angiotensin system
The role of activation of the renin-angiotensin system (RAS) in the development of heart failure is well recognized [43, 44]. In diabetes activation of RAS occurs despite minimal changes in myocardial loading [71]. The activation of RAS leads to myocardial remodeling, as it is associated with increased oxidative damage, cardiomyocyte hypertrophy and apoptosis [71]. Angiotensin II and aldosterone may induce cardiac fibrosis [69]. These observations suggest that the initial therapy should be started with ACE inhibitors. These drugs decrease left ventricular hypertrophy and myocardial fibrosis, prevent myocardial remodeling, improve endothelial function, and diminish insulin resistance [72]. In diabetes, inhibition of angiotensin II production may delay fibrosis of myocardium and functional and structural changes in small vessels [18]. The ACE inhibitors reduce cardiovascular disease in diabetic patients, especially in those with hypertension [73]. The therapeutic efficacy of ACE inhibitors (captopril, enalapril, lisinopril, trandolapril, ramipril, perindopril) in HF has been proved in both diabetic and non-diabetic populations. The results of the MICRO-HOPE study revealed significant reduction of the incidence of HF in diabetic patients treated with ramipril [74]. In the EUROPA trial diabetic patients treated with perindopril showed an impressive reduction in the rate of hospitalization due to HF [75]. In the ATLAS trial long-term high-dose lisinopril was effective and well tolerated in high-risk patients, including those with diabetes mellitus [76]. The leading role of ACE inhibitors in the therapy of HF in the diabetic population is a result of their cardioprotective action. The ACE inhibitors reduce blood pressure, pre- and afterload and vascular resistance. It is also postulated that ACE inhibitors may positively influence glucose and lipid metabolism [40, 77].
It seems that angiotensin receptor blockers (ARB) may exert similar effects on the diabetic heart as ACE inhibitors. The ARB such as candesartan, losartan, or valsartan may also be used in diabetic patients with HF, especially in subjects in whom ACE inhibitors are contraindicated. The ARB reduce the rate of hospitalization, mortality and morbidity in patients with HF, both with or without diabetes [78–81].
Calcium channel blockers
The intracellular retention of calcium in myocytes of diabetic patients is associated with cardiac dysfunction. Theoretically, calcium channel blockers may reverse intracellular calcium disturbances and prevent myocardial changes induced by diabetes. Unfortunately, data on the use of calcium channel blockers in the treatment of early stages of HF in diabetic patients are scarce. Most clinical trials were performed in the last decades of the twentieth century and these drugs are not routinely used for this indication.
Other drugs
β-Blockers, such as bisoprolol, carvedilol, nebivolol and metoprolol, may be safely used in the therapy of HF in the diabetic population [82]. Their efficacy in HF has been proved in numerous clinical trials. Therapy with β-blockers results in inhibition of the RAS system and reduction of vasopressin and natriuretic peptides release. However, one should also remember that β-blockers may mask the symptoms of hypoglycemia, increase insulin resistance and negatively influence lipid profile.
Data on the efficacy of diuretics in the diabetic population are scarce. It seems there are no important differences in this respect between different classes of diuretics. However, as in the nondiabetic population, mineralocorticoid antagonists such as spironolactone and eplerenone should always be used. Similarly to β-blockers, thiazide diuretics may increase insulin resistance and glycemia, as well as negatively influencing lipid profile. Due to their diabetogenic potential, it seems reasonable to avoid concomitant therapy with thiazide diuretics and β-blockers.
There are insufficient data to estimate the efficacy of digoxin, hydralazine, nitrates, ivabradine and potassium supplementation as well as the role of physical activity in the therapy of HF in the diabetic population [83, 84]. It seems that these drugs may be safely given to diabetic patients when used according to indications [85]. One also has to remember that in patients in whom symptoms of HF may be related to ischemia complete revascularization can further improve HF.
Conclusions
The occurrence of HF in diabetic patients is responsible for increased mortality in this population. However, although the evidence of increased incidence of HF in diabetes is strongly supported by the results of numerous clinical trials, its pathophysiology still remains to be fully elucidated. Metabolic disturbances, interstitial fibrosis, cardiomyocyte loss, small vessel disease, and cardiac autonomic neuropathy are incriminated. It seems crucial to actively screen for patients at risk and institute appropriate therapy as soon as possible. The multifactorial nature of cardiac dysfunction in diabetic patients indicates that various strategies might be effective for preventing or delaying the development of HF and its complications. Presently, tight glycemic control and use of ACE inhibitors seem to be basic therapeutic strategies.
Acknowledgments
The authors would like to thank Polish Society of Metabolic Diseases for the financial suppor.
References
- 1.Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population: sixteen year follow-up study. Diabetes. 1974;23:105–11. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 2.Kolovou G, Marvaki A, Bilianou H. One more look at guidelines for primary and secondary prevention of cardiovascular disease in women. Arch Med Sci. 2011;7:747–55. doi: 10.5114/aoms.2011.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell DSH. Diabetic cardiomyopathy: a unique entity or a complication of coronary artery disease? Diabetes Care. 1995;18:708–14. doi: 10.2337/diacare.18.5.708. [DOI] [PubMed] [Google Scholar]
- 4.Cowie MR, Mosterd A, Wood DA, et al. The epidemiology of HF. Eur Heart J. 1997;18:208–25. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 5.Nichols GA, Hiller TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24:1614–9. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 6.Delea TE, Edelsberg JS, Hagiwara M, Oster G, Phillips LS. Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2003;26:2983–9. doi: 10.2337/diacare.26.11.2983. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 8.Bertoni AG, Tsai A, Kasper EK, Brancati FL. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care. 2003;26:2791–5. doi: 10.2337/diacare.26.10.2791. [DOI] [PubMed] [Google Scholar]
- 9.Hamby RI, Zoneraich S, Sherman L. Diabetic cardiomyopathy. JAMA. 1974;229:1749–54. [PubMed] [Google Scholar]
- 10.Lind M, Bounias I, Olsson M, Gudbjornsdottir S, Svenson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20985 patients with type 1 diabetes: an observational study. Lancet. 2011;378:140–6. doi: 10.1016/S0140-6736(11)60471-6. [DOI] [PubMed] [Google Scholar]
- 11.Shindler DM, Kostis JB, Yusuf S, et al. Diabetes mellitus, a predictor of morbidity and mortality in the studies of the left ventricular dysfunction (SOLVD) trials and registry. Am J Cardiol. 1996;77:1017–20. doi: 10.1016/s0002-9149(97)89163-1. [DOI] [PubMed] [Google Scholar]
- 12.Ryden L, Armstrong PW, Cleland JG, et al. Efficacy and safety of high-dose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J. 2000;21:1967–78. doi: 10.1053/euhj.2000.2311. [DOI] [PubMed] [Google Scholar]
- 13.Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbidedinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–10. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 14.Maru S, Koch GG, Stender M, et al. Antidiabetic drugs and heart failure risk in patients with type 2 diabetes in the U.K. Primary Care Setting Diabetes Care. 2005;28:20–6. doi: 10.2337/diacare.28.1.20. [DOI] [PubMed] [Google Scholar]
- 15.Liu JE, Palmieri V, Roman MJ, et al. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol. 2001;37:1943–9. doi: 10.1016/s0735-1097(01)01230-x. [DOI] [PubMed] [Google Scholar]
- 16.Alagiakrishnan K, Banach M, Jones LG, Datta S, Ahmed A, Aronow WS. Update on diastolic heart failure or heart failure with preserved ejection fraction in the older adults. Ann Med. 2013;45:37–50. doi: 10.3109/07853890.2012.660493. [DOI] [PubMed] [Google Scholar]
- 17.Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98:33–9. doi: 10.1159/000064682. [DOI] [PubMed] [Google Scholar]
- 18.Zhi YF, Johannes BP, Marwick JH. Diabetic cardiomyopathy: evidences, mechanism and therapeutic implications. Endocr Rev. 2004;25:543–67. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 19.Carugo S, Giannattasio C, Calchera I, et al. Progression of functional and structural cardiac alterations in young normotensive uncomplicated patients with type 1 diabetes mellitus. J Hypertens. 2001;19:1675–80. doi: 10.1097/00004872-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Struthers AD, Morris AD. Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deaths. Lancet. 2002;359:1430–2. doi: 10.1016/S0140-6736(02)08358-7. [DOI] [PubMed] [Google Scholar]
- 21.Di Bonito P, Cuomo S, Moio N, et al. Diastolic dysfunction in patients with non-insulin-dependent diabetes mellitus of short duration. Diabet Med. 1996;13:321–4. doi: 10.1002/(SICI)1096-9136(199604)13:4<321::AID-DIA3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Shivalkar B, Dhondt D, Goovaerts I, et al. Flow mediated dilatation and cardiac function in type 1 diabetes mellitus. Am J Cardiol. 2006;97:77–82. doi: 10.1016/j.amjcard.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 23.Di Bonito P, Moio N, Cavuto L, et al. Early detection of diabetic cardiomyopathy: usefulness of tissue Doppler imaging. Diabet Med. 2005;22:1720–5. doi: 10.1111/j.1464-5491.2005.01685.x. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin SS, Pearle DL, Baughman KL, et al. Diabetes mellitus and risk of idiopathic dilated cardiomyopathy. The Washington DC, Dilated Cardiomyopathy Study. Ann Epidemiol. 1994;4:67–74. doi: 10.1016/1047-2797(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 25.Struthers AD, Morris AD. Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deaths. Lancet. 2002;359:1430–2. doi: 10.1016/S0140-6736(02)08358-7. [DOI] [PubMed] [Google Scholar]
- 26.Bella JN, Devereux RB, Roman MJ, et al. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study) Am J Cardiol. 2001;87:1260–5. doi: 10.1016/s0002-9149(01)01516-8. [DOI] [PubMed] [Google Scholar]
- 27.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the Strong Heart Study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 28.Young ME, Mcnulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: part II potential mechanisms. Circulation. 2002;105:1861–70. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 29.Chatham JC, Seymour AM. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res. 2002;55:104–12. doi: 10.1016/s0008-6363(02)00399-1. [DOI] [PubMed] [Google Scholar]
- 30.Herrero P, Peterson LR, McGill JB, et al. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 31.Russell RR, III, Yin R, Caplan MJ, et al. Additive effects of hyperinsulinemia and ischemia on myocardial GLUT1 and GLUT4 translocation in vivo. Circulation. 1998;98:2180–6. doi: 10.1161/01.cir.98.20.2180. [DOI] [PubMed] [Google Scholar]
- 32.Radrigues B, Cam, McNeil JH. Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem. 1998;180:53–7. [PubMed] [Google Scholar]
- 33.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 34.Cai L, Wang Y, Zhou G, et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–97. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Al-Nimer MS, Al-Ani FS, Ali FS. Role of nitrosative and oxidative stress in neuropathy in patients with type 2 diabetes mellitus. J Neurosci Rural Pract. 2012;3:41–4. doi: 10.4103/0976-3147.91932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obrosova IG. How does glucose generate oxidative stress in peripheral nerve? Int Rev Neurobiol. 2002;50:3–35. doi: 10.1016/s0074-7742(02)50071-4. [DOI] [PubMed] [Google Scholar]
- 37.Kasznicki J, Kosmalski M, Sliwinska A, et al. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep. 2012;39:8669–78. doi: 10.1007/s11033-012-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nitenberg A, Valersi P, Sachs R, et al. Impairment of coronary vascular reserve and Ach-induced coronary vasodilatation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42:1017–25. doi: 10.2337/diab.42.7.1017. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan S, Hatley ME, Bolick DT, et al. Hyperglycemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia. 2004;47:1727–34. doi: 10.1007/s00125-004-1525-1. [DOI] [PubMed] [Google Scholar]
- 40.Drzewoski J. Gliclazide, inflammation and atherosclerosis. Antiinflamm Antiallergy Agents Med Chem. 2008;7:224–30. [Google Scholar]
- 41.Chavali V, Tyagi SC, Mishra P. Predictors and prevention of diabetic cardiomyopathy. Diab Metab Syndrome Obesity. 2013;6:151–60. doi: 10.2147/DMSO.S30968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg TJ, Snorgaard O, Faber J, et al. Serum levels of advanced glycation end products are associated with left ventricular diastolic dysfunction in patients with diabetes. Diabetes Care. 1999;22:1186. doi: 10.2337/diacare.22.7.1186. [DOI] [PubMed] [Google Scholar]
- 43.Boudina S, Dale Abel E. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 44.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–67. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 45.Pierce GN, Russel JC. Regulation of intracellular Ca++ in the heart during diabetes. Cardiovasc Res. 1997;34:41–7. doi: 10.1016/s0008-6363(97)00010-2. [DOI] [PubMed] [Google Scholar]
- 46.Hileeto D, Cukiernik M, Mukherjee S, et al. Contributions of endothelin-1 and sodium hydrogen exchanger-1 in the diabetic myocardium. Diabetes Metab Res Rev. 2002;18:386–94. doi: 10.1002/dmrr.322. [DOI] [PubMed] [Google Scholar]
- 47.Cai L, Li W, Wang G, et al. Hyperglycaemia induced apoptosis in mouse myocardium: mitochondrial C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–48. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 48.Kawaguchi M, Techigawara M, Ischiata T, et al. A comparison of ultra structural changes on endomyocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessels. 1997;12:267–74. doi: 10.1007/BF02766802. [DOI] [PubMed] [Google Scholar]
- 49.Di Carli MF, Bianco-Batlles D, Landa ME, et al. Effects of autonomic neuropathy on coronary blood flow in patients with diabetes mellitus. Circulation. 1999;100:813–9. doi: 10.1161/01.cir.100.8.813. [DOI] [PubMed] [Google Scholar]
- 50.Airaksinen K, Kostinen J, Akaheimo M, Huikuri H. Augmentation of atrial contraction to LV filling in IDDM subjects as assessed by Doppler echocardiograph. Diabetes Care. 1989;12:159–61. doi: 10.2337/diacare.12.2.159. [DOI] [PubMed] [Google Scholar]
- 51.Scognamiglio R, Auogaro A, Casara D, et al. Myocardial dysfunction and adrenergic cardiac innervations in patients with insulin dependent diabetes mellitus. J Am Coll Cardiol. 1998;31:404–12. doi: 10.1016/s0735-1097(97)00516-0. [DOI] [PubMed] [Google Scholar]
- 52.Mustonen J, Mantysaari M, Kuikka J, et al. Decreased myocardial 123I-metaiodobenzylguanidine uptake is associated with disturbed LV diastolic filling in diabetes. Am Heart J. 1992;123:804–5. doi: 10.1016/0002-8703(92)90530-9. [DOI] [PubMed] [Google Scholar]
- 53.Annonu AK, Fattah AA, Mokhtar MS, Ghareeb S, Elhendy A. LV systolic and diastolic functional abnormalities in asymptomatic patients with non-insulin-dependent diabetes mellitus. J Am Soc Echocardiogr. 2001;14:885–91. doi: 10.1067/mje.2001.112892. [DOI] [PubMed] [Google Scholar]
- 54.Monteagudo PT, Moises VA, Kohlmann O, Jr, Ribeiro AB, Lima VC, Zanella MT. Influence of autonomic neuropathy upon LV dysfunction in insulin-dependent diabetic patients. Clin Cardiol. 2000;23:371–5. doi: 10.1002/clc.4960230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–50. doi: 10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- 56.Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol. 2003;42:2022–8. doi: 10.1016/s0735-1097(03)00403-0. [DOI] [PubMed] [Google Scholar]
- 57.Arnold JM, Yusuf S, Young J, et al. HOPE Investigators Prevention of heart failure in patients in the Heart Outcome Prevention Evaluation (HOPE) study. Circulation. 2003;107:1284–90. doi: 10.1161/01.cir.0000054165.93055.42. [DOI] [PubMed] [Google Scholar]
- 58.Iribarren C, Karter AJ, Go AS, et al. Glycaemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–73. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 59.United Kingdom Prospective Diabetes Study (UKPDS) Group Investigators. Tight blood pressure control and risk of macrovascular and microvascular complications in type-2 diabetes: UKPDS 38. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 60.Prospective Diabetes Study UK (UKPDS) Group. Effect of intensive blood glucose control with metaformin on complications in overweight patients with type-2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 61.Lind M, Olsson M, Rosengren A, Svensson AM, Bounias I, Gudbjornsdottir S. The relationship between glycaemic control and heart failure in 83021 patients with type 2 diabetes. Diabetologia. 2012;55:2946–53. doi: 10.1007/s00125-012-2681-3. [DOI] [PubMed] [Google Scholar]
- 62.Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: Evidence from a 37,229 patient meta-analysis. Am Heart J. 2011;162:938–48. doi: 10.1016/j.ahj.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 63.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure. A consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108:2941–8. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 64.University Group Diabetes Program. A study of the effects of hypoglycaemic agents on vascular complications in patients with adult-onset diabetes. Diabetes. 1970;19:747–830. [PubMed] [Google Scholar]
- 65.Dungan KM, Binkley P, Nagaraja HN, Schuster D, Osei K. The effect of glycaemic control and glycaemic variability on mortality in patients hospitalized with congestive heart failure. DiabMetabol Res Rev. 2011;27:85–94. doi: 10.1002/dmrr.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah DD, Fonarow GC, Horwich TB. Metformin therapy and outcomes in patients with advanced systolic heart failure and diabetes. J Card Fail. 2010;16:200–6. doi: 10.1016/j.cardfail.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail. 2011;4:53–8. doi: 10.1161/CIRCHEARTFAILURE.110.952556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–51. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 69.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–90. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 70.King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643–8. doi: 10.1046/j.1365-2125.1999.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis treatment. Clin Sci. 2004;107:539–57. doi: 10.1042/CS20040057. [DOI] [PubMed] [Google Scholar]
- 72.Kambara N, Holycross BJ, Wung P, et al. Combined effects of low-dose oral spironolactone and captopril therapy in a rat model of spontaneous hypertension and heart failure. J Cardiovasc Pharmacol. 2003;41:830–7. doi: 10.1097/00005344-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Yusuf S, Slieght P, Pogue J, et al. Effect of an angiotensin converting – enzyme inhibitor, ramipril on cardiovascular events in high risk patients. The Heart Outcome Prevention. Evaluation Study Investigations. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 74.Effects of ramipril on cardiovascular and microvascularoutcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 75.Daly CA, Fox KM, Remme WJ, EUROPA Investigators The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: results from the PERSUADE sub study. Eur Heart J. 2005;26:1369–78. doi: 10.1093/eurheartj/ehi225. [DOI] [PubMed] [Google Scholar]
- 76.Ryden L, Armstrong PW, Cleland JG, et al. Efficacy and safety of high-doselisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J. 2000;21:1967–78. doi: 10.1053/euhj.2000.2311. [DOI] [PubMed] [Google Scholar]
- 77.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–8. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 78.Young JB, Dunlap ME, Pfeffer MA, et al. Candesartan in heart failure assessment of reduction in mortality and morbidity (CHARM) investigators and committees Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110:2618–26. doi: 10.1161/01.CIR.0000146819.43235.A9. [DOI] [PubMed] [Google Scholar]
- 79.Cohn JN, Tognoni G, the Valsartan Heart Failure Trial Investigators A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 80.Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 81.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 82.Vitale C, Iellamo F, Volterrani M, et al. Heart rate control in an unselected consecutive population of outpatients with stable coronary artery disease: analysis of the CARDIf Study Cohort. Angiology. 2010;61:763–7. doi: 10.1177/0003319710369102. [DOI] [PubMed] [Google Scholar]
- 83.Bielecka-Dabrowa A, Mikhailidis DP, Jones L, Rysz J, Aronow WS, Banach M. The meaning of hypokalemia in heart failure. Int J Cardiol. 2012;158:12–7. doi: 10.1016/j.ijcard.2011.06.121. [DOI] [PubMed] [Google Scholar]
- 84.Patel K, Sui X, Zhang Y, et al. Prevention of heart failure in older adults may require higher levels of physical activity than needed for other cardiovascular events. Int J Cardiol. 2013;168:1905–9. doi: 10.1016/j.ijcard.2012.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alagiakrishnan K, Banach M, Jones LG, Ahmed A, Aronow WS. Medication management of chronic heart failure in older adults. Drugs Aging. 2013;30:765–82. doi: 10.1007/s40266-013-0105-9. [DOI] [PubMed] [Google Scholar]