Abstract
Introduction
Tendon tissue engineering (TTE) tries to produce tendinous tissue of high quality to replace dysfunctional tissue. One possible application of TTE might be the replacement of ruptured tissue of the rotator cuff. Autologous tenocytes seem to be most suitable as no differentiation in vitro is necessary. Today it is still uncertain if there is a difference between tendon-derived cells (TDC) of different native tissues. Moreover, the search for suitable scaffolds is another important issue in TTE.
Material and methods
This study compared TDC of the long head of the biceps tendon (LHB), the anterior cruciate ligament (ACL) and the tendon of the musculus semitendinosus (TMS). The TDC were isolated using the cell migration method. Cell morphology was assessed using light microscopy and gene expression was performed using polymerase chain reaction (PCR). Afterwards, cell seeding efficiency and proliferation were tested on a collagen I scaffold using the WST-1 assay. Results were confirmed using H + E staining.
Results
The TDC of the LHB showed higher expression levels of collagen type I and decorin (p < 0.01) compared to TDC of other origin. Results showed efficient cell seeding and proliferation within the scaffold. Proliferation within the scaffold was not as high as when cells were cultivated without a scaffold.
Conclusions
The TDC of the LHB seems to be the most suitable cell source. Further research is necessary to find out if the results can be transferred to an in vivo model. The new collagen I scaffold seems to offer an opportunity to combine good biocompatibility and mechanical strength.
Keywords: tenocytes, tissue engineering, rotator cuff tears, scaffold
Introduction
Musculoskeletal disorders of the shoulder are extremely common and every third person has the experience of an episode of shoulder pain within lifetime [1].
One reason for such a pain episode may be rotator cuff tears. Clinically asymptomatic tears can be observed in 51% of patients over 80 years of age [2]. However, only about half of these tears become symptomatic later [3, 4], causing pain and loss of function. While small tears can occur without any symptoms at all [5, 6], massive transmural tears are frequently symptomatic [7]. Samples of large transmural tears show certain histological characteristics such as a higher amount of fatty tissue and intracellular lipid [8].
These tears, whenever possible, need to be treated by surgical reconstruction. However, the therapeutic management of these tears is difficult as the patient's postoperative satisfaction is unpredictable and ranges from 27% to 74% [9]. A further problem is the high rate of cuff re-ruptures, which can be seen in radiological studies in about 60% of cases after surgery [10]. Therefore, new strategies are necessary to improve the outcome of life quality for patients with massive rotator cuff tears. One of these strategies is allogenic tendon transplants, which are not used routinely in clinical practice today and always bear the risk of infections and rejections [11]. Muscle-tendon-transfer operations are not only limited, but also show unsatisfactory outcomes [12]. The only definite treatment today is the artificial articular replacement with a reverse shoulder prosthesis. Here, loosening of the prosthesis may cause severe problems over time, a situation which is seen in a remarkable number of cases [13, 14].
All these attempts seem to have complications which limit clinical utilisation and therefore new solutions are needed. An innovative new approach to treat massive rotator cuff tears in the future might be tendon tissue engineering (TTE). This approach aims to create tendon tissue of good quality, by seeding cells on scaffolds in vitro, aiming to take over the specific function of the affected tissue after in vivo implantation. Mesenchymal stem cells and tenocytes are frequently used as a cell source in TTE; however, mesenchymal stem cells seem to be restricted as differentiation into multiple cell types is possible [15]. There is also evidence that mesenchymal stem cells respond with osteogenic orientation if cyclic tensile strain is applied [16]. Therefore, tendon-derived cells (TDC) seem to be the most suitable cell type. Until now, however, it has not completely been understood which tendon tissue is to be used to isolate autologous TDC. The following criteria should be fulfilled for optimum results: firstly, a sufficient amount of cells should be available after the cell isolation procedure; secondly, cells should retain a stable phenotype throughout cell passaging; thirdly, the cells should expand remarkably in cell cultures; and finally, the loss of tendinous tissue as a cell source for TDC should only cause little donor site morbidity [17].
The aim of this study was to compare the cells derived from three different native tendinous tissues and ligaments: the long head of the biceps tendon (LHB), the tendon of the semitendinosus muscle (TMS) and the anterior cruciate ligament (ACL). A gene expression analysis was performed on important structural proteins from the extracellular matrix (ECM) of tendons and the results were compared to cells of other musculoskeletal origin such as osteoblasts, chondrocytes and fibroblasts. The expression of the main structural proteins collagen type I and decorin was further investigated using real-time polymerase chain reaction (PCR). The results regarding the LHB were previously published by our study group [18, 19]. The proliferation of TDC from the LHB was analysed on a new collagen matrix scaffold (Shoulder Patch, TETEC, Reutlingen, Germany) using the WST-1 proliferation assay.
Material and methods
Cell isolation
Samples were collected from patients who underwent surgery for primary shoulder arthroplasty (LHB), primary knee arthroplasty (ACL) and anterior cruciate ligament reconstruction using autologous hamstrings (TMS). Demographic data for the three study groups were as follows:
LHB: average age: 67 ±8.9 years, sex ratio female : male = 6 : 1,
ACL: average age: 70 ±6.4 years, sex ratio female : male = 5 : 2,
TMS: average age: 17 ±8.6 years, sex ratio female : male = 5 : 2.
The study was conducted according to the regulations of the Medical Center Ethics Committee of the Ludwig-Maximilians-University of Munich (ethical grant number: 063-09).
Altogether seven biopsies from donors for each tendon type were obtained. After sterile extraction, samples were transported to the laboratory in cell culture medium (DMEM/HAM's F12, Biochrom, Berlin, Germany), where cell isolation was immediately performed. For cell isolation, we used a previously published method by Schulze-Tanzil et al. [20]. Briefly, after washing the samples three times with PBS buffer, the surrounding paratenon was removed carefully and the tendons were minced into small pieces. The tendon specimens were placed into petri-dishes filled with 10 ml of cell culture medium (DMEM/HAM's F12) and were cultivated at 37°C, CO2 5%. Culture medium was changed every second to third day. After approximately 2–4 days, the first colonies of migrating tenocytes around the tendon specimens could be observed. After about 3 weeks, the tendon specimens were transferred into new petri-dishes. Altogether, three migration cycles were performed. Before cell culturing the total number of cells was counted using a haemocytometer.
Cell culture
The TDC were transferred and placed in culture flasks filled with DMEM/HAM's F12 (1 : 1) and supplemented with 10% FCS, 2 mM L-glutamine, 1250 µg/ml ascorbic acid, amino acids, 60 µg/ml penicillin/streptomycin and 25 ng/ml amphotericin B (Biochrom, Berlin, Germany). The seeding density during passaging was about 700 cells/cm2 in all three study groups. As soon as the cultured cells reached 80–90% confluence, they were treated with 0.05% trypsin/0.02% ethylenediaminetetraacetic acid (EDTA) (Biochrom, Berlin, Germany) and subcultured. Osteoblasts and fibroblasts (Provitro, Berlin, Germany) were also subcultured in DMEM cell culture medium with the same supplements used in the TDC group. Chondrocytes were obtained using a method previously described by Meyer-Wagner et al. from our laboratory [21]. Morphological cell assessment was performed using a phase-contrast microscope (Axiostar Plus, Zeiss, Munich, Germany).
Real time-polymerase chain reaction
Cell pallets of the third passage of TDCs were homogenized with lysis buffer (Quiagen, Hilden, Germany) using a QIAshredder (Quiagen) and a centrifuge at 1000 rpm for 3 min. For further RNA purification, the RNeasy Mini Kit (Quiagen) was utilised according to the manufacturer's instructions. DNA leftovers were digested on-column with the RNase-free DNase I Set (Quiagen) for 15 min. cDNA was synthesized by using the Reverse Transcription System Set (Promega, Mannheim, Germany) according to the manufacturer's instructions. Briefly, 1 µg of RNA, 4 µl of MgCl2, 2 µl of 10x reaction buffer, 2 µl of 10 mM deoxynucleotide triphosphate (dNTP) mix, 0.5 µl of RNase inhibitor, 0.6 µl of reverse transcriptase, 0.5 µl of random primer and RNase-free water were mixed up to a final volume of 20 µl. Samples were incubated for 10 min at 25°C, following incubation at 42°C for 60 min. cDNA samples were stored at –20°C until further use.
The RT-PCR was performed for collagen type I, decorin, collagen type III, tenascin-C, fibronectin, scleraxis, tenomodulin, osteopontin and aggrecan. Primer sequences, product lengths and number of amplification cycles are shown in Table I. For the RT-PCR, 1 µl of cDNA was mixed with 0.5 µl of forward and reverse primers, 10x PCR buffer, 0.2 µl of deoxynucleotide triphosphate (dNTP) mix, 1 µl of MgCl2, 0.1 µl of taq-polymerase (Fermentas, St. Leon-Rot, Germany) and filled up to a final volume of 20 µl with RNase-free water. The reaction mixtures were incubated for 3 min at 95°C, followed by 30 s at 95°C, 45 s at 50–64°C and 1 min at 72°C up to 35 cycles, and then 10 min at 72°C. Products were analysed by 2% gel electrophoresis. All experiments were reproduced at least twice.
Table I.
The RT-PCR primer sequences and product length
| Gene | Primer sequence | Length [bp] |
|---|---|---|
| 1. GAPDH Accession No: NM_002046 |
Sense: GAGTCCACTGGCGTCTCCAC Antisense: GGTGCTAAGCAGTTGGTGGT |
188 |
| 2. Collagen type I, α 1 Accession No: NM_000088 |
Sense: GGCCCAGAAGAACTGGTACA Antisense: GGCTGTTCTTGCAGTGGTAG |
200 |
| 3. Collagen type III, α 1 Accession No: NM_033150 |
Sense: CCAGGAGCTAACGGTCTCAG Antisense: CAGGGTTTCCATCTCTTCCA |
103 |
| 4. Decorin Accession No: NM_001920 |
Sense: TGCTGTTGACAATGGCTCTC Antisense: GCCTTTTTGGTGTTGTGTCC |
192 |
| 5. Fibronectin Accession No: NM_212475 |
Sense: ATGATGAGGTGCACGTGTGT Antisense: CTCTTCATGACGCTTGTGGA |
135 |
| 6. Tenascin-C Accession No: NM_002160 |
Sense: TCAAGGCTGCTACGCCTTAT Antisense: GTTCTGGGCTGCCTCTACTG |
230 |
| 7. Scleraxis [20] |
Sense: CCTGAACATCTGGGAAATTTTAC Antisense: CGCCAAGGCACCTCCTT |
111 |
| 8. Tenomodulin Accession No: NM_022144 |
Sense: CCATGCTGGATGAGAGAGGT Antisense: CTCGTCCTCCTTGGTAGCAG |
123 |
| 9. Osteopontin [22] | Sense: TTGCTTTTGCCTCCTAGGCA Antisense: GTGAAAACTTCGGTTGCTGG |
430 |
| 10. Aggrecan [23] | Sense: CACTGTTACCGCCACTTCCC Antisense: ACCAGCGGAAGTCCCCTTCG |
183 |
Quantitative real time-polymerase chain reaction
The gene expression of collagen type I and decorin was also determined by quantitative RT-PCR (qRT-PCR) using a light cycler instrument 2.0 (Roche Diagnostic, Mannheim, Germany). Target sequences were amplified using LightCycler Primer Sets (Search LC, Heidelberg, Germany) with LightCycler-Fast Start DNA Master SYBR Green I Mix (Roche Applied Science, Mannheim, Germany) following the manufacturer's manual. Reactions were performed in duplicate. At least two independent experiments were performed. For relative quantification of the gene expression, samples were normalized to cyclophilin B.
Scaffold
For cell seeding experiments we used a collagen matrix scaffold (Tissue-Tek, Reutlingen, Germany) with a basal strengthening membrane to improve linear stiffness. The scaffold had a height of 3.4–4.0 mm, a grammage of 200–250 g/m2 and a porosity of 110–150 µm (manufacturer's information).
Proliferation assay
To investigate cell proliferation on the collagen matrix scaffold, we modified a method described by Schmitt et al. [24]. Under sterile conditions samples of the collagen scaffold were stamped out using a bone biopsy stamp (Biomed, Jena, Germany) with a diameter of 8.5 mm. The scaffold samples were then transferred to a 96-well plate and incubated in cell culture medium for 24 h. The TDC from the LHB of the fourth cell passage with a seeding density of 1000 cells/µl in a total volume of 20 µl were pipetted onto the stamped scaffold samples. Cells were allowed to adhere to the scaffold for 2 h at 37°C in 5% CO2 atm. Then, culture medium was added to a total volume of 200 µl. 100 µl of the cell culture medium was replaced every second day. After 2 days and 14 days, proliferation analysis was performed using the WST-1 proliferation assay (Roche Diagnostics, Heidelberg, Germany) according to the manufacturer's instructions. Briefly, 20 µl cell culture medium was replaced by 20 µl of WST-1 reagent. After incubation for 2 h the supernatant was mixed at 500 rpm for 3 min using an MTS4 shaker (IKA laboratory technique, Staufen i.B., Germany). 100 µl of the supernatant was measured at 450 nm wavelength with the microplate reader (BWG Biotech, Munich, Germany). The assay was performed in duplicate and optical density (OD) values were converted to total cell number by using the normalized cell number-OD curve for TDC of the same passage. As a control, TDC seeded on a 96-well plate without a scaffold were used. Two independent repeats of each measurement were performed.
Histology
Scaffolds for histology were prepared exactly the same way as for proliferation assay. Samples were then rinsed in phosphate-buffered saline (pH 7.4), incubated in 5% sucrose (48°C, 30 min), embedded in Tissue-Tek cryoprotective media (Sakura, Zoeterwoude, The Netherlands), and frozen in liquid nitrogen. Serial cryosections (10 µm) were cut, mounted on SuperFrost glass slides (Menzel-Gläser, Braunschweig, Germany), fixed in acetone, and dried at room temperature. Samples were stained with hematoxylin and eosin (H + E) using a standard protocol. Finally, samples were embedded and analysed by light microscopy (Axiostar Plus, Zeiss, Munich, Germany).
Statistical analysis
Data are shown as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 3.0 (San Diego CA, USA). The Kruskal-Wallis test with Dunn's post-test was used to analyse significant differences between the three study groups. To analyse the number of cells after cell seeding at two different points of time, the Wilcoxon paired test was used. This test is designed for paired samples. The level of significance was set at p < 0.05.
Results
Cell migration
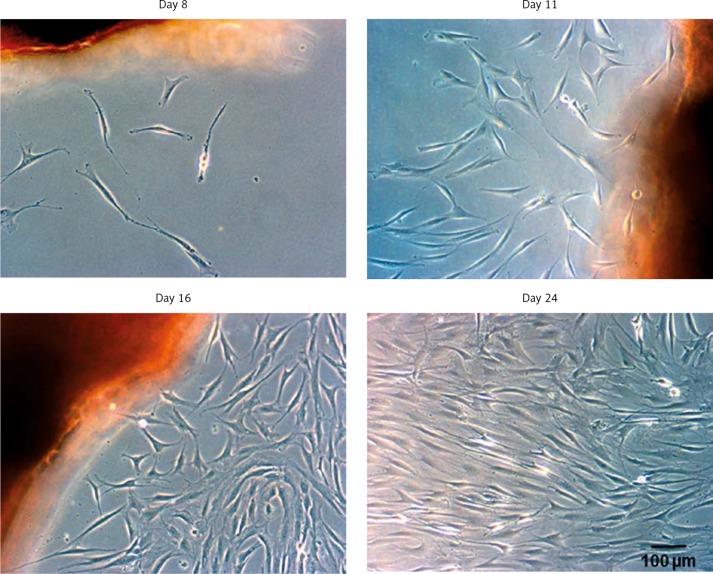
It was possible to isolate approximately the same amount of cells after 6 weeks from all three study groups (about 75 × 103). Similar results were previously published by our study group for TDC of the LHB [18]. The first colonies of migrating cells could be observed around the tendon slices after 1 week. These colonies became denser and reached a confluence of approx. 90% after three weeks. They showed typical tenogenic cell morphology with an elongated cell shape and numerous membrane protrusions. In the following isolation cycles, cell migration accelerated but cell morphology remained constant. Documentation of the cell migration of TDC from the ACL is shown in Figure 1.
Figure 1.
The tendon slices were put into petri-dishes, incubated (37°C, 5% CO2) in cell culture medium (DMEM/ HAM's F12) and analysed by light microscopy. An increasing amount of spindle-like cells could be observed at different time points. The migration process was similar for all study groups. The TDC from the ACL are shown (n = 7)
Cell culture
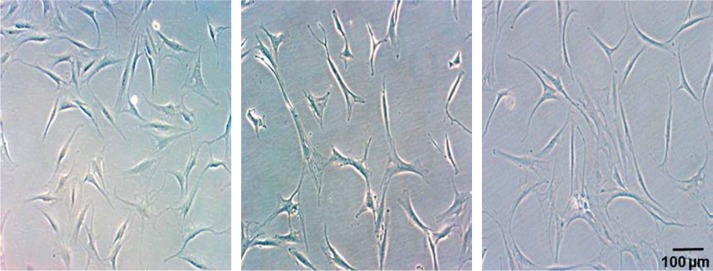
Figure 2 illustrates cell morphology of all three investigated tissues in the third cell passage. Cell number increased significantly from passage 1 to passage 3 in all study groups (results not shown). Cell morphology remained predominantly constant, but an increasing number of flattened cells could be observed in TDC from all three tissues.
Figure 2.
Tendon-derived cell cultures in passage 3. Cells with typical tenocyte morphology as well as polygonal cells are shown. The number of flatter cells increased during in vitro cultivation
Gene expression
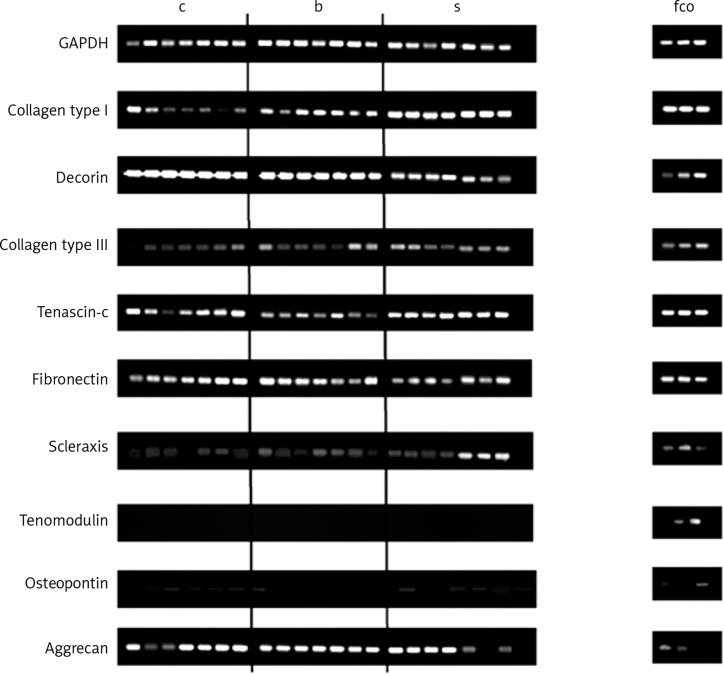
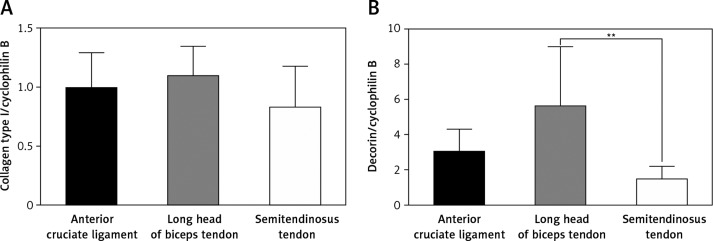
Collagen type III, tenascin-c and fibronectin were expressed continuously in all investigated TDC, but the expression seemed to be weaker compared to the expression of collagen type I and decorin (Figure 3). The RT-PCR results demonstrate that the transcription factor scleraxis, a specific tenocyte marker, was expressed in almost all cell cultures, except in one culture of an ACL donor. Interestingly, the expression of tenomodulin, the most specific tenocyte marker to date, could not be observed in any of the samples. However, it was expressed in chondrocytes and osteoblasts (Figure 3). Osteopontin, a protein which is strongly expressed in bones, could also be seen in some of the cell cultures from tendinous tissues. Aggrecan, a protein of the ECM in cartilage, was quite strongly expressed in TDC (Figure 3). The expression of collagen type I and decorin, the two main compounds of the ECM in tendons, could be detected continuously in cell cultures of the third cell passage of all three tendinous tissues. The highest mRNA expression of both proteins could be detected in cultures of the LHB. Especially the expression of decorin was significantly higher than in cultures of the TMS (p < 0.01) (Figure 4).
Figure 3.
RT-PCR analysis. Cells in the third cell passage were used. cDNA from other musculoskeletal cell types such as f, fibroblasts (passage 6), c, chondrocytes (passage 3) and o, osteoblasts (passage 2) were used for comparison. GAPDH was used for normalization (n = 2)
c – ACL, b – LHB, s – TMS
Figure 4.
Quantitative RT-PCR for collagen I and decorin. Cells in the third cell passage were used. Relative gene expression was estimated against cyclophilin B (n = 7)
**p < 0.01 (Kruskal-Wallis test with Dunn's post-test)
Cell proliferation and histology on the collagen I scaffold
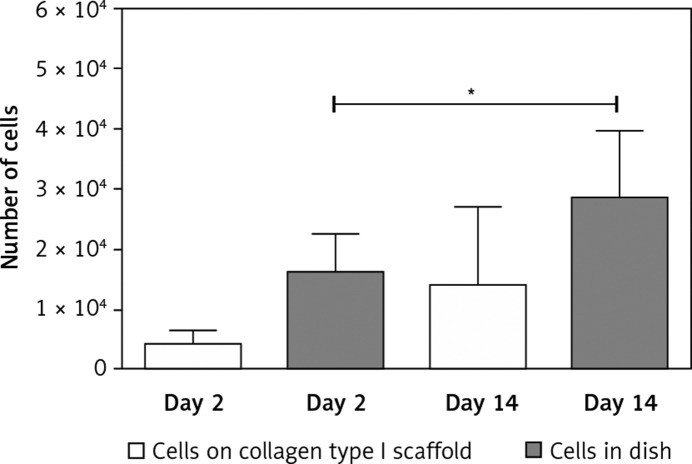
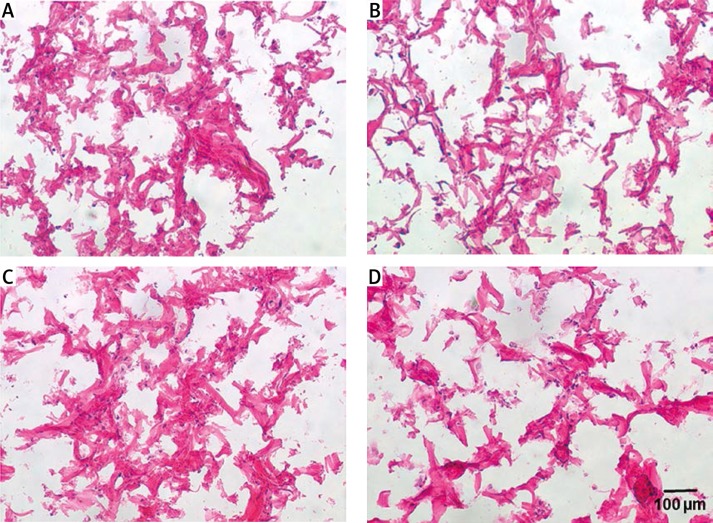
Cells from the LHB were used for cell seeding on the collagen scaffolds. Two days after cell seeding on to the scaffold, a reduced cell number was detected using the WST-1 proliferation assay compared to the cells cultured in flasks. This indicated a certain loss of cells caused by the seeding process (Figure 5). At this time point (2 days), multiple cells could be seen adhering to collagen fibres; however, they were predominantly located in upper layers of the scaffold (Figure 6). After 14 days of cell cultivation on collagen scaffolds, a clear increase in cell number was detected. However, the proliferation index was smaller than that of cells cultured without a scaffold (Figure 5). At day 14 a higher amount of cells could be observed also in deeper layers of the scaffolds, but the majority of cells were still located in upper scaffold layers (Figure 6).
Figure 5.
Proliferation of TDC from LHB on collagen type I scaffolds was analysed using the WST-1 proliferation assay. Results are shown in absolute cell number at 2 days and 14 days after cell seeding. As the control, cells not seeded on collagen scaffolds were used (n = 2)
*p < 0.05 (Wilcoxon paired test)
Figure 6.
Representative H + E-stained histological sections of cell-seeded collagen type I scaffolds at two different time points. A, B – 2 days after cell seeding upper layer and deeper layer. C, D – 14 days after cell seeding upper layer and deeper layer (n = 3)
Discussion
For TTE it is important to identify which native tissue is the most suitable source for the isolation of tendon cells. Some years ago, Cooper et al. made a similar attempt to identify the best cell source for tissue-engineered ligaments. They found a difference in proliferation and gene expression between cells derived from ligaments and cells derived from tendons [25]. In this study we compared TDC from LHB, ACL and TMS, but differences in the gene expression between tendon-derived cells and ligament-derived cells could not be observed.
This study provides evidence that cell isolation is successful for all three investigated tissues. Also, cell morphology during in vitro cultivation does not differ between the study groups. We identified significantly higher expression of decorin in TDC from the LHB. Collagen type I expression was similar in all three study groups, but the highest expression could be found in TDC of the LHB. The expression of collagen type I, an ECM protein responsible for the linear stiffness of tendons, is essential for the formation of high quality tissue after reimplantation [26]. It could be shown that collagen type I and III expression is down-regulated during tendon aging in rats [27]. Since the donors of the LHB and the ACL were older than the TMS donors, we expected higher expression of collagen type I in TDC of the TMS. However, this study could not show differences in the expression of this protein among the study groups, indicating that results observed in animal studies might only partially be valid for humans. Besides, Mazzocca et al. and Güngörmüş et al. could recently show a fast phenotypic drift of TDC during in vitro culture [28, 29]. Further studies are necessary to investigate how the age of tendons as a cell source influences this phenotypic drift. Decorin, a proteoglycan, regulates the assembly and diameter of collagen fibrils [30, 31] and has systemic effects, too [32]. Therefore, it is also essential for the mechanical properties of tendons, especially if TDC are seeded on a scaffold expecting de-novo synthesis of ECM. Scleraxis is commonly used as a tenogenic marker [33, 34] and could be seen in almost every investigated cell culture in this study. This finding is not surprising since it is known that mesenchymal stem cells up-regulate Scleraxis in vitro [35]. Tendons harbour a cell type with the ability to differentiate tendon stem/progenitor cells, too [36, 37]. Interestingly, tenomodulin expression, a mature marker for tenocytes, could not be seen in cell cultures of the third cell passage [38, 39]. The loss of its expression during in vitro cultivation has been described by Yao et al. [40] and Jelinsky et al. [41]. The multipotent cells might be responsible for a certain dedifferentiation in TDC. Our results seem to confirm the findings by Randelli et al., who recently found these pluripotent cells in TDC of the LHB and the supraspinatus tendon [42]. As Randelli used enzymatic digestion as the cell isolation technique, we could show that it is possible to isolate and cultivate this cell type using different tendons as the cell source and using cell migration as the isolation technique. Moreover, our findings seem to demonstrate that this cell population can be found in TDC from young as well as from old donors. Yukata et al. reported that tenocytes from the avascular region in tendons differ in their gene expression profile from tenocytes from other regions [43]. Some of our findings regarding gene expression might be explained by the fact that there is a substantial difference of tenocytes of different tendon origins.
Surprisingly, aggrecan could be detected in all three tenogenic cell cultures. The dedifferentiation potential of tenocytes was observed by Slack et al., who described changes in the matrix composition of tendons during in vitro cultivation [44]. De Mos et al. found that certain genes identifying a chondrogenic differentiation are up-regulated if tenocytes are cultured in vitro [45]. It is also known that chondrocytes are located near the tendon-to-bone junction [46], which might explain these findings.
In summary, all three study groups showed similar morphological characteristics and gene expression profiles. The expression of decorin, which is essential for the de-novo synthesis of the ECM, was significantly higher in samples from the LHB. The highest expression of collagen type I could also be observed in TDC from the LHB. Practically, obtaining TDC of the LHB or the TMS causes only little donor site morbidity [47, 48]. The ACL was used to compare tendon-and ligament-derived cells. As a loss of this ligament might cause remarkable donor site morbidity, it is practically not useful as a cell source. The LHB is often affected in patients with rotator cuff tears causing pain and requires removal by either tenotomy or tenodesis [49]. As TDC from the LHB are isolated from a tendon which imitates the physiological demands of the rotator cuff the best, we continued our cell seeding experiments using TDC from the LHB.
Another important issue in TTE is the development of suitable scaffolds. Numerous materials and cell seeding techniques have been investigated so far, which all have their limitations [50–57]. This study used a collagen matrix scaffold which is hardened at one side with a collagenous membrane to increase linear stiffness. Results of this study verify a successful cell seeding process; however, a certain loss of cells could be detected two days after cell seeding. Nevertheless, proliferation could be seen on collagen scaffold with penetration of cells in the deeper layers of the scaffold. As the release of formazan into the culture medium is complicated due to the 3D structure of the scaffold, the real proliferation of TDC on collagen matrix scaffolds might be higher. However, optical verification of our results seems to confirm the results obtained by Takata et al. and Marinucci et al., who discovered proliferation 2–24 h after seeding tendinous cells on collagen matrices [58, 59].
In conclusion, TDC isolated from LHB show significantly higher expression of decorin compared with TDC isolated from TMS. The expression of collagen type I was the highest in TDC from the LHB, too. The collagen scaffold “Shoulder Patch” supports acceptable initial cell attachment and cell proliferation. Further investigations are necessary to find out how the combination of TDC from the LHB and collagen matrix scaffold performs in vivo. Our group is currently testing the potential of TDC on a collagen matrix scaffold for TTE by using a sheep animal model. Moreover, testing this new scaffold biomechanically will be another task.
Acknowledgments
Matthias F. Pietschmann and Markus U. Wagenhäuser contributed equally.
This study was supported by the “German Shoulder and Elbow Society” with €10 000.
References
- 1.van der Heijden GJ. Shoulder disorders: a state-of-the-art review. Baillieres Best Pract Res Clin Rheumatol. 1999;13:287–309. doi: 10.1053/berh.1999.0021. [DOI] [PubMed] [Google Scholar]
- 2.Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296–9. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10:199–203. doi: 10.1067/mse.2001.113086. [DOI] [PubMed] [Google Scholar]
- 4.Dias D, Matos M, Daltro C, Guimaraes A. Clinical and functional profile of patients with the painful shoulder syndrome (PSS) Ortop Traumatol Rehabil. 2008;10:547–53. [PubMed] [Google Scholar]
- 5.Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995;77:296–8. [PubMed] [Google Scholar]
- 6.Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am. 1995;77:10–5. doi: 10.2106/00004623-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Itoi E, Minagawa H, Sato T, Sato K, Tabata S. Isokinetic strength after tears of the supraspinatus tendon. J Bone Joint Surg Br. 1997;79:77–82. doi: 10.1302/0301-620x.79b1.6860. [DOI] [PubMed] [Google Scholar]
- 8.Steinbacher P, Tauber M, Kogler S, Stoiber W, Resch H, Sanger AM. Effects of rotator cuff ruptures on the cellular and intracellular composition of the human supraspinatus muscle. Tissue Cell. 2010;42:37–41. doi: 10.1016/j.tice.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Joint Surg Am. 2001;83-A:71–7. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Mellado JM, Calmet J, Olona M, et al. MR assessment of the repaired rotator cuff: prevalence, size, location, and clinical relevance of tendon rerupture. Eur Radiol. 2006;16:2186–96. doi: 10.1007/s00330-006-0147-z. [DOI] [PubMed] [Google Scholar]
- 11.Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60:681–4. [PubMed] [Google Scholar]
- 12.Warner JJ. Management of massive irreparable rotator cuff tears: the role of tendon transfer. Instr Course Lect. 2001;50:63–71. [PubMed] [Google Scholar]
- 13.Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–85. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 14.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–95. doi: 10.1302/0301-620x.86b3.14024. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Bao C, Hu J, Yin G, Luo E. Effects of clodronate combined with hydroxyapatite on multi-directional differentiation of mesenchymal stromal cells. Arch Med Sci. 2010;6:670–7. doi: 10.5114/aoms.2010.17079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N, Zhao Z, Zhang L, et al. Up-regulated osteogenic transcription factors during early response of human periodontal ligament stem cells to cyclic tensile strain. Arch Med Sci. 2012;8:422–30. doi: 10.5114/aoms.2012.28810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang D, Balian G, Chhabra AB. Tendon tissue engineering and gene transfer: the future of surgical treatment. J Hand Surg Am. 2006;31:693–704. doi: 10.1016/j.jhsa.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Wagenhauser MU, Pietschmann MF, Sievers B, et al. Collagen type I and decorin expression in tenocytes depend on the cell isolation method. BMC Musculoskelet Disord. 2012;13:140. doi: 10.1186/1471-2474-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagenhauser MU, Pietschmann MF, Docheva D, Gulecyuz MF, Jansson V, Muller PE. Assessment of essential characteristics of two different scaffolds for tendon in situ regeneration. Knee Surg Sports Traumatol Arthrosc. 2014 doi: 10.1007/s00167-013-2820-5. Jan 4 [Epub ahead of print] PMID: 24389992. [DOI] [PubMed] [Google Scholar]
- 20.Schulze-Tanzil G, Mobasheri A, Clegg PD, Sendzik J, John T, Shakibaei M. Cultivation of human tenocytes in high-density culture. Histochem Cell Biol. 2004;122:219–28. doi: 10.1007/s00418-004-0694-9. [DOI] [PubMed] [Google Scholar]
- 21.Mayer-Wagner S, Schiergens TS, Sievers B, et al. Scaffold-free 3D cellulose acetate membrane-based cultures form large cartilaginous constructs. J Tissue Eng Regen Med. 2011;5:151–5. doi: 10.1002/term.300. [DOI] [PubMed] [Google Scholar]
- 22.Crosby AH, Edwards SJ, Murray JC, Dixon MJ. Genomic organization of the human osteopontin gene: exclusion of the locus from a causative role in the pathogenesis of dentinogenesis imperfecta type II. Genomics. 1995;27:155–60. doi: 10.1006/geno.1995.1018. [DOI] [PubMed] [Google Scholar]
- 23.Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt SC, Wiedmann-Al-Ahmad M, Kuschnierz J, et al. Comparative in vitro study of the proliferation and growth of ovine osteoblast-like cells on various alloplastic biomaterials manufactured for augmentation and reconstruction of tissue or bone defects. J Mater Sci Mater Med. 2008;19:1441–50. doi: 10.1007/s10856-007-3238-8. [DOI] [PubMed] [Google Scholar]
- 25.Cooper JA, Jr, Bailey LO, Carter JN, et al. Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials. 2006;27:2747–54. doi: 10.1016/j.biomaterials.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Chokalingam K, Juncosa-Melvin N, Hunter SA, et al. Tensile stimulation of murine stem cell-collagen sponge constructs increases collagen type I gene expression and linear stiffness. Tissue Eng Part A. 2009;15:2561–70. doi: 10.1089/ten.tea.2008.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kostrominova TY, Brooks SV. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age. 2013;35:2203–14. doi: 10.1007/s11357-013-9514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzocca AD, Chowaniec D, McCarthy MB, et al. In vitro changes in human tenocyte cultures obtained from proximal biceps tendon: multiple passages result in changes in routine cell markers. Knee Surg Sports Traumatol Arthrosc. 2012;20:1666–72. doi: 10.1007/s00167-011-1711-x. [DOI] [PubMed] [Google Scholar]
- 29.Gungormus C, Kolankaya D. Gene expression of tendon collagens and tenocyte markers in long-term monolayer and high-density cultures of rat tenocytes. Connect Tissue Res. 2012;53:485–91. doi: 10.3109/03008207.2012.694511. [DOI] [PubMed] [Google Scholar]
- 30.Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997;73:2164–72. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott JE. Proteoglycan: collagen interactions in connective tissues. Ultrastructural, biochemical, functional and evolutionary aspects. Int J Biol Macromol. 1991;13:157–61. doi: 10.1016/0141-8130(91)90041-r. [DOI] [PubMed] [Google Scholar]
- 32.Alan C, Kocoglu H, Altintas R, Alici B, Resit Ersay A. Protective effect of decorin on acute ischaemia-reperfusion injury in the rat kidney. Arch Med Sci. 2011;7:211–6. doi: 10.5114/aoms.2011.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishore V, Bullock W, Sun X, Van Dyke WS, Akkus O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials. 2012;33:2137–44. doi: 10.1016/j.biomaterials.2011.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan Q, Lui PP, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18:840–51. doi: 10.1089/ten.tea.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–47. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 36.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 37.Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549–58. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 38.Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shukunami C, Takimoto A, Miura S, Nishizaki Y, Hiraki Y. Chondromodulin-I and tenomodulin are differentially expressed in the avascular mesenchyme during mouse and chick development. Cell Tissue Res. 2008;332:111–22. doi: 10.1007/s00441-007-0570-8. [DOI] [PubMed] [Google Scholar]
- 40.Yao L, Bestwick CS, Bestwick LA, Maffulli N, Aspden RM. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006;12:1843–9. doi: 10.1089/ten.2006.12.1843. [DOI] [PubMed] [Google Scholar]
- 41.Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28:289–97. doi: 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- 42.Randelli P, Conforti E, Piccoli M, et al. Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am J Sports Med. 2013;41:1653–64. doi: 10.1177/0363546512473572. [DOI] [PubMed] [Google Scholar]
- 43.Yukata K, Matsui Y, Shukunami C, et al. Differential expression of tenomodulin and chondromodulin-1 at the insertion site of the tendon reflects a phenotypic transition of the resident cells. Tissue Cell. 2010;42:116–20. doi: 10.1016/j.tice.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Slack C, Bradley G, Beaumont B, Poole A, Flint M. Changes in the morphology and synthetic activity of cultured rat tail tendon. Cell Tissue Res. 1986;245:359–68. doi: 10.1007/BF00213943. [DOI] [PubMed] [Google Scholar]
- 45.de Mos M, Koevoet W, van Schie HT, et al. In vitro model to study chondrogenic differentiation in tendinopathy. Am J Sports Med. 2009;37:1214–22. doi: 10.1177/0363546508331137. [DOI] [PubMed] [Google Scholar]
- 46.Evans EJ, Benjamin M, Pemberton DJ. Fibrocartilage in the attachment zones of the quadriceps tendon and patellar ligament of man. J Anat. 1990;171:155–62. [PMC free article] [PubMed] [Google Scholar]
- 47.Klonz A, Eggers C, Reilmann H. Proximal and distal biceps tendon rupture: an indication for surgery? Unfallchirurg. 1998;101:735–9. doi: 10.1007/s001130050331. [DOI] [PubMed] [Google Scholar]
- 48.Ejerhed L, Kartus J, Sernert N, Kohler K, Karlsson J. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction? A prospective randomized study with a two-year follow-up. Am J Sports Med. 2003;31:19–25. doi: 10.1177/03635465030310011401. [DOI] [PubMed] [Google Scholar]
- 49.Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. The role of the biceps tendon in massive rotator cuff tears. Instr Course Lect. 2012;61:113–20. [PubMed] [Google Scholar]
- 50.Bostman OM, Pihlajamaki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop Relat Res. 2000;371:216–27. [PubMed] [Google Scholar]
- 51.Cen L, Liu W, Cui L, Zhang W, Cao Y. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008;63:492–6. doi: 10.1203/PDR.0b013e31816c5bc3. [DOI] [PubMed] [Google Scholar]
- 52.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967–79. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 53.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–98. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 54.Lu HH, Cooper JA, Jr, Manuel S, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805–16. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Altman GH, Karageorgiou V, et al. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res A. 2003;67:559–70. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]
- 56.Costa MA, Wu C, Pham BV, Chong AK, Pham HM, Chang J. Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng. 2006;12:1937–43. doi: 10.1089/ten.2006.12.1937. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto E, Kogawa D, Tokura S, Hayashi K. Effects of the frequency and duration of cyclic stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon. J Biomech Eng. 2005;127:1168–75. doi: 10.1115/1.2073587. [DOI] [PubMed] [Google Scholar]
- 58.Takata T, Wang HL, Miyauchi M. Attachment, proliferation and differentiation of periodontal ligament cells on various guided tissue regeneration membranes. J Periodontal Res. 2001;36:322–7. doi: 10.1034/j.1600-0765.2001.360508.x. [DOI] [PubMed] [Google Scholar]
- 59.Marinucci L, Lilli C, Guerra M, et al. Biocompatibility of collagen membranes crosslinked with glutaraldehyde or diphenylphosphoryl azide: an in vitro study. J Biomed Mater Res A. 2003;67:504–9. doi: 10.1002/jbm.a.10082. [DOI] [PubMed] [Google Scholar]