Abstract
Deep brain stimulation (DBS) injects a high frequency current that effectively disables the diseased basal ganglia (BG) circuit in Parkinson’s disease (PD) patients, leading to a reversal of motor symptoms. Though therapeutic, high frequency stimulation consumes significant power forcing frequent surgical battery replacements and causing widespread influence into other brain areas which may lead to adverse side effects. In this paper, we conducted a rigorous study to assess whether low frequency signals can restore behavior in PD patients by restoring neural activity in the BG to the normal state. We used a biophysical-based model of BG nuclei and motor thalamus whose parameters can be set to simulate the normal state and the PD state with and without DBS. We administered pulse train DBS waveforms to the subthalamic nucleus (STN) with frequencies ranging from 1–150Hz. For each DBS frequency, we computed statistics on the simulated neural activity to assess whether it is restored to the normal state. In particular, we searched for DBS waveforms that suppress pathological bursting, oscillations, correlations and synchronization prevalent in the PD state and that enable thalamic cells to relay cortical inputs reliably. We found that none of the tested waveforms restores neural activity to the normal state. However, our simulations led us to construct a novel DBS strategy involving low frequency multi-input phaseshifted DBS to be administered into the STN. This strategy successfully suppressed all pathological symptoms in the BG in addition to enabling thalamic cells to relay cortical inputs reliably.
Index Terms: Deep Brain Stimulation, Parkinson‘s Disease, Spike Trains, Hodgkin-Huxley Models
Introduction
An estimated 6.5 million people world-wide have Parkinson’s Disease (PD), a chronic progressive neurological disorder that occurs when midbrain dopamine neurons of the substantia nigra projecting to the basal ganglia degenerate, causing tremor, rigidity, and bradykinesia [4]. While there is currently no medical treatment to stop disease progression, deep brain stimulation (DBS) injects a current that alters the neural activity of the diseased brain circuit, leading to a reversal of PD symptoms. When appropriately stimulated, patients can regain control of movement and reduce medication use [6].
Although DBS may be the most effective therapy for PD today, the typical stimulation signal is a high-frequency train of square pulses (>100 Hz) which consumes significant power. Consequently, stimulator batteries have to be replaced surgically every 3–5 years for PD patients [4]. stimulation has widespread influence to cortical areas that may cause adverse side-effects such as cognitive impairment and neuropsychiatric effects in PD patients [2][21][31][32].This can cause the DBS system to be ineffective if the side effects are intolerable.
It is important to understand how high-frequency DBS works, as this understanding may lead us to low-powered alternatives. Unfortunately, the basic mechanisms underlying how DBS works are still not fully understood. One hypothesis is based on the similarity of clinical results between DBS and ablative surgeries, where lesions are made in particular basal ganglia (BG) nuclei in the latter [20][22]. In 2004, Rubin and Terman constructed a biophysical model of the BG and proposed that DBS works by replacing the pathological rhythmic output of basal ganglia with high-frequency tonic firing [27]. Due to this high-frequency tonic firing, basal ganglia neurons lose their ability to modulate neuronal activity of neighboring structures, including its output to the thalamus, and thus in a way mimic a lesion in the area. Such suppression of pathological patterns in the BG circuit may minimize PD motor symptoms. This intuition is supported by experimental data in monkeys and humans [12][16].
A recent study used the Rubin-Terman model of the BG and motor thalamus to show that low-frequency stimulation may restore neuronal activity in the globus pallidus internus (Gpi) and motor thalamus to more normal conditions [14]. Specifically, Feng et al used a genetic algorithm to search over two classes of DBS input signals applied to the sub-thalamic nucleus (STN) to find stimulation that enables the thalamic cells to track sensorimotor cortical input and which reduced pathological correlation between globus pallidus internus (Gpi) cells. They showed that certain low frequency pulse train waveforms and stochastic pulse train waveforms (inter-pulse-interval is random) are therapeutic. However, they did not analyze how these DBS signals impact the pathological synchronization in the BG neurons.
Here, we use the Rubin-Terman model to generate data under both normal and PD conditions with and without DBS. We apply square pulse train DBS waveforms into the STN at frequencies ranging from 1Hz–150Hz, and search for waveforms that generate neuronal activity in the BG and thalamus that resemble BG and thalamus activity observed under normal conditions. Our optimality criterion is more stringent than those used in Feng et al described above in that we require the DBS input to eliminate pathological bursting activity, 10–30Hz oscillations, synchronization and correlation within all neurons in globus pallidus externus (GPe), STN, and GPi. Furthermore, we require that thalamus neurons relay the sensorimotor input from cortex.
We defined normality measures for each requirement described above and computed them for each DBS frequency. We found that no periodic square pulse DBS waveform at any frequency met all of our requirements above. In agreement with [11][12][27], we found that high-frequency DBS inputs (90Hz or greater) resulted in reliable relay of cortical information in the thalamic cells. However, the remaining activity in Gpe, STN and Gpi fired tonically at the rate proportional to the DBS input and were thus correlated and synchronized. An important phenomenon that we did observe under our normality measures is that at around 9Hz (near the frequency of oscillations in the STN in the PD state without DBS), the bursting activity in STN diminished even though the neurons remained synchronized and correlated everywhere and even though the thalamic cells failed to relay cortical input. This gave us the idea to administer a different DBS input into each STN neuron in the model. In particular, we applied a 9Hz square pulse waveform into each STN neuron phase shifted by π/4 so that each STN neuron received a delayed version of what the other STN neurons received. Not only did bursting diminish in all neurons, but they all became uncorrelated and desynchronized like in the normal state. Consequently, thalamic cells relayed the cortical input reliably. These findings suggest that an alternative low frequency DBS strategy may be more therapeutic as it is restoring neuronal activity in the diseased circuit.
II. Methods
A. Biophysical Model of Basal Ganglia
Rubin and Terman [27] developed a computational model of the BG sub-circuitry and motor thalamus and included a DBS input signal to the STN. By adjusting values of parameters and applied currents, this model can be used to characterize the PD state without DBS, the PD state with DBS and the normal state. We used their model to generate simulated data under these three conditions and also under different DBS inputs.
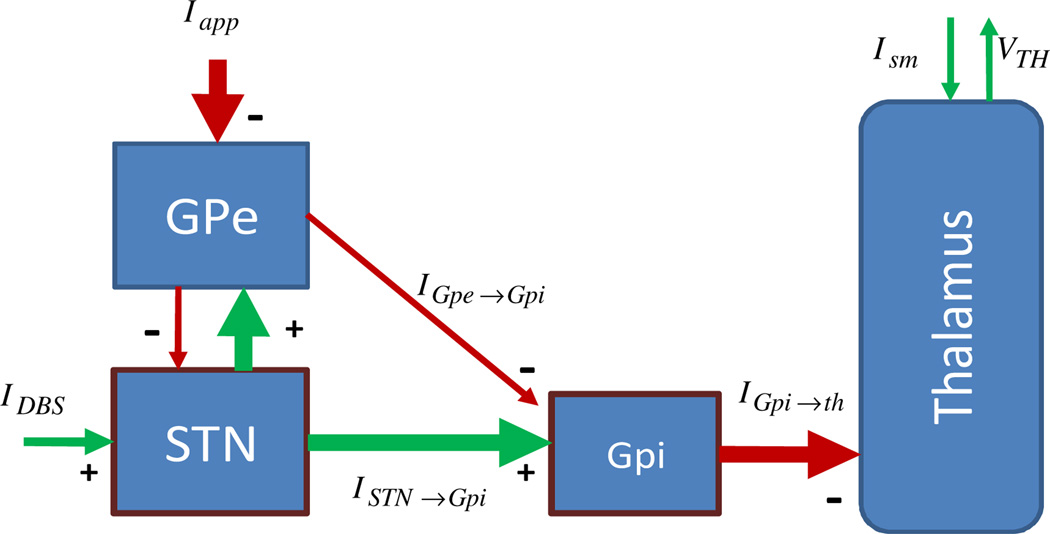
This biophysical model (termed the RT model from here onwards) consists of four neural structures: the GPe, GPi, STN, and motor thalamus, as shown in Figure 1. Each of the BG structures is composed of 8 point neurons and the thalamus has 2 point neurons. The model parameters were estimated from experimental data collected from voltage and current clamp experiments done in vitro [16][27][30]; and the dynamics of each cell in each structure are represented by a single-compartment conductance-based biophysical model. For details see [25][27].
Figure 1.
Rubin Terman Model
B. Normality Measures
In the PD state without DBS, as oppose to normal, firing patterns in all BG structures are correlated and synchronized, there is significant bursting and low frequency oscillations, and thalamic cells do not relay cortical information reliably [5][9][16][22][26][29]. Therefore, we define measures that reflect whether or not neurons in each structure exhibit pathological bursting, low frequency oscillations, correlation, synchronization and thalamic relay reliability. We define all our measures on synaptic gating variable, , for neuron j in structure i, refer [25][27] for details.
1. Bursting and Oscillations
To measure whether the BG circuit is exhibiting bursting and low frequency oscillations, we compute the power spectrum of the synaptic output profiles for each neuron and compare them to those in the normal state for each DBS frequency f. Define as the synaptic output of neuron j in structure i at a DBS frequency f, then the power spectrum of PD with DBS frequency, f is
| (1) |
, ℱMT is the multitaper Fourier transform [7,8]. T is the time window for which we ran the simulations (3000 ms). We discarded the first 500 ms of data to filter out transient responses. Therefore, in our case T=2500 ms. F is , and Δt = 0.2 ms is the time step. Now we define the error between power spectral density of PD with DBS frequency, f, and normal as:
| (2) |
is the spectral density for the normal state. We define the mean error in structure i as:
| (3) |
Here Ni is the number of neurons in the structure i. Under Normal conditions with no DBS we found that power spectrum is almost flat, with no significant peak at any frequency and hence this error takes into account both bursting and oscillations errors. This is because if a neuron is oscillating or bursting then there will be peaks in the and hence the error becomes large, in absence of any peak in remains small.
2. Correlation and Synchronization
In a network of neurons, apart from individual activity of each neuron, it is important to examine the cross activity between different neurons in each structure. This is because in our models the combined activity from an ensemble of neurons in one structure is projected to the neurons from different structures or the same structure. To quantify cross activity, we define the following two measures.
1. Correlation
We computed the cross-power spectrum and define correlation between neuron j and j′ in structure i as:
| (4) |
The mean correlation within structure i is then
| (5) |
Ni is the number of neurons in structure i and f is the DBS frequency.
3. Synchronization
In our models, if spike events in two different neurons continuously occur together in time, then the neurons are synchronized and their contribution to a synaptic input to other neurons add up. To measure synchronization between neurons j and j′ in structure i, we compute
| (6) |
Here . We average over structure i to define synchronization for a structure as:
| (7) |
4. Thalamic Relay Reliability
Thalamic reliability is similar as Rel parameter defined in [27]. Sensorimotor cortical information is modeled as a train of pulses ism. In the ideal case, thalamic cells should spike when there is a pulse in. ism. In the normal state, thalamic cells almost always spike in unison with ism pulses. However in the PD state, thalamus reliability decreases. To quantify this, we define the following measure:
| (8) |
N is the number of neurons in thalamus. We are using 25 ms period because the period of sensory motor input (Ism) to thalamus is 25 ms. We focused on the standard square-pulse train DBS inputs with amplitude (Io), frequency (f) and on duration (D):
| (9) |
In addition to regular DBS signal described above, we used multi-input phase shifted DBS (MIPS-DBS) signal described by following equation:
| (10) |
Here j is the STN neuron to which pulse is applied. Note that f is the frequency in Hertz and D is the duration of each rectangular pulse of Idbs We fixed and D = 0.6 ms and allowed the frequency to vary as f = kf0, k = 1,2,3, …. The amplitude and duration of each are identical to those used in [27]. We conducted all our simulations in XPPaut [13] and Matlab® R 2009a (Mathworks). The numerical method used is an adaptive-step fourth order Runge-Kutta method (QualSt. RK4 in XPP), with time step of 0.1 ms.
III. Results
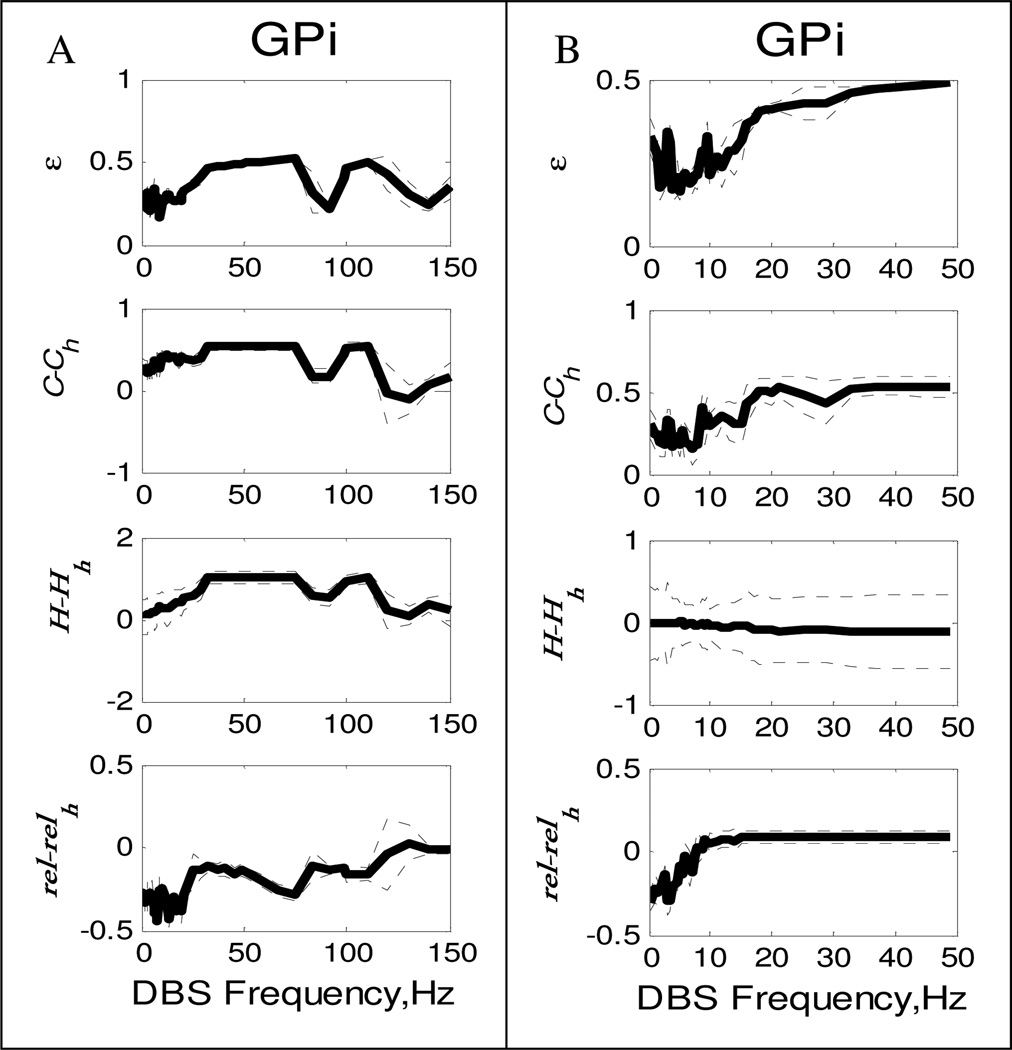
Figure 2 illustrates how each normality measure varies with the DBS frequency for GPi. In each sub plot we have plotted the measure relative to healthy state. Therefore zero in each graph corresponds to healthy state. Also zero DBS frequency denotes PD state. Measures for other structures have similar trends and are thus not shown.
Figure 2.
A)GPi measures and Thalamic Reliability vs regular regular DBS frequency B) GPi measures and Thalamic Reliability vs Multi-Input DBS frequency. All measures are ploted relative to normal state. Dotted line plot ± std across neurons.
We see from Figure 2A, three measures in GPi, generally increase with frequency with the exception of a “dip” in the power spectrum error in the 9Hz range. Although the power spectra in these frequency ranges are close to normal, correlations and synchronization are prevalent at these frequencies. Consequently, thalamic reliability is worse in the 9Hz region. This led us to believe that if we disrupt the bursting in each neuron with the same input, but phase-shifted for each neuron, we could also desynchronize the STN neurons and perhaps Gpe and Gpi neurons would follow. Figure 2B, plots the three measures for GPi in case of shifted DBS. We see that GPi synchronization becomes almost equal to normal condition at 9 Hz, and thalamus reliability goes very high at 9 Hz. The εi is also low as compared to HF DBS.
In our models we use one DBS input per STN neuron to desynchronize each GPi neuron pair, such a high number of DBS input is unpractical. However, we only need to desynchronize clusters of GPi neurons projecting to the same thalamus neuron. Also, degree of synchronization present in PD state will also determine the number of DBS inputs. It may be the case that these two factors may result in number of DBS inputs which would be more practical for implementation purposes.
It is important to note that there have been several studies that look at designing closed-loop DBS strategies to desynchronize neural circuits which can lead to phase-delayed stimulation [1][3][11][17][23] and references therein. Here, we use a network of 26 neurons in 4 different neural structures in the basal ganglia and thalamus and our DBS strategies are all implemented in open-loop. If such an open-loop strategy is effective experimentally, then processing power generated from a feedback controller would be saved.
IV. Future Work
Our preliminary work has encouraged us to test the new DBS strategy on different models of the basal ganglia such as those presented in [10][19][24][28]. That is, our approach is expected to give similar results on different models, with minor changes, provided that such models describe the main features of BG in normal and PD conditions. Later we aim to test this approach on primates.
REFERENCES
- 1.Alexander GE. Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol. 1994 Jul;11(4):420–431. [PubMed] [Google Scholar]
- 2.Aybek S, Vingerhoets FJG. Does deep brain stimulation of the subthalamic nucleus in Parkinson's disease affect cognition. Lancet Neurol. 2000;5:578–588. doi: 10.1038/ncpneuro0379. [DOI] [PubMed] [Google Scholar]
- 3.Barnikol UB, Popovych OV, Hauptmann C, Sturm V, Freund HJ, Tass PA. Tremor entrainment by patterned low-frequency stimulation. Philos Transact A Math Phys Eng Sci. 2008;366:3545–3573. doi: 10.1098/rsta.2008.0104. [DOI] [PubMed] [Google Scholar]
- 4.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 5.Bergman H, Wichman T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of the parkinsonism 1994. J Neurophysiol. 1994;72(2):507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 6.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions Book Series Mathematics in Industry. Springer Berlin Heidelberg. 1990;8 [Google Scholar]
- 7.Bokil H, Pesaran B, Andersen AP, Mitra PP. A method for detection and classification of events in neural activity. IEEE Transactions on Biomedical Engineering. 2006;53:1678–1687. doi: 10.1109/TBME.2006.877802. [DOI] [PubMed] [Google Scholar]
- 8.Bokil H, Purpura K, Schofflen JM, Thompson D, Pesaran B, Mitra PP. Comparing spectra and coherencesfor groups of unequal size. J Neurosci Methods. 2006;159:337–345. doi: 10.1016/j.jneumeth.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. 2003. [DOI] [PubMed] [Google Scholar]
- 10.Cooper SE, Kuncel AM, Wolgamuth BR, Rezai AR, Grill WM. A Model Predicting Optimal Parameters for Deep Brain Stimulation in Essential Tremor. Journal Of Clinical Neurophysiology. 2008 Oct;25(5):265–273. doi: 10.1097/WNP.0b013e318182ed44. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorval AD, Panjwani N, Qi RY, Grill WM. Deep Brain Stimulation that Abolishes Parkinsonian Activity in Basal Ganglia Improves Thalamic Relay Fidelity in a Computational Circuit. 31st Annual International Conference of the IEEE EMBS; September; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson's disease. J Neurophysiol. 2008;100:2807–2818. doi: 10.1152/jn.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ermentrout B. Simulating, Analyzing, and Animating Dynamical Systems. Philadelphia: SIAM Press; 2002. [Google Scholar]
- 14.Feng X, Greenwald B, Rabitz H, Shea-Brownxy E, Kosutz R. Toward Closed-Loop Optimization of Deep Brain Stimulation for Parkinson’s Disease: Concepts and Lessons from a Computational Model. J. Neuroengineering. 2007;4:L14–L21. doi: 10.1088/1741-2560/4/2/L03. [DOI] [PubMed] [Google Scholar]
- 15.Gale JT, Amirnovin R, Williams ZM, Flaherty AW, Eskandar EN. From symphony to cacophony: Pathophysiology of the human basal ganglia in Parkinson disease. Neurosci Biobehav. 2007 doi: 10.1016/j.neubiorev.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Galvan A, Wichmann T. Pathophysiology of Parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauptmann C, Omel'chenko O, Popovych OV, Maistrenko Y, Tass PA. Control of spatially patterned synchrony with multisite delayed feedback. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;76:066209. doi: 10.1103/PhysRevE.76.066209. [DOI] [PubMed] [Google Scholar]
- 18.Hauptmann C, Popovych O, Tass PA. Desynchronizing the abnormally synchronized neural activity in the subthalamic nucleus: a modeling study. Expert Rev Med Devices. 2007;4:633–635. doi: 10.1586/17434440.4.5.633. [DOI] [PubMed] [Google Scholar]
- 19.Modolo J, Mosekilde E, Beuter A. New insights offered by a computational model of deep brain stimulation. Journal of Physiology Paris. 2007;101(1–3):56–63. doi: 10.1016/j.jphysparis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery E, Jr, Baker K. Mechanism of deep brain stimulation and future technical developments. Neurol. Res. 2000;22:259–266. doi: 10.1080/01616412.2000.11740668. [DOI] [PubMed] [Google Scholar]
- 21.Narayana S, Jacks A, Robin DA, Poizner H, Zhang W, Franklin C,Liotti M, Vogel D, Fox PT. A noninvasive imaging approach to understanding speech changes following deep brain stimulation in parkinson’s disease. Am. J. Speech Lang. Pathol. 2009;18:146–161. doi: 10.1044/1058-0360(2008/08-0004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obeso J, Rodriguez M, DeLong M. Basal ganglia pathophysiology: A critical review. In: Obeso J, DeLong M, Ohye C, Marsden C, editors. Advances in Neurology. Vol. 74. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 3–18. [PubMed] [Google Scholar]
- 23.Orosz G, Moehlis J, Murray RM. Controlling biological networks by time-delayed signals. Phil. Trans. R. Soc. 2009;368:439–454. doi: 10.1098/rsta.2009.0242. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka T, Abe T, Tsukagawa T, Song WJ. Conductance- Based Model of the Voltage-Dependent Generation of a Plateau Potential in Subthalamic Neurons. J Neurophysiol. 2004;92:255–264. doi: 10.1152/jn.00508.2003. [DOI] [PubMed] [Google Scholar]
- 25.Pascual A. Details of Rubin and Terman’s model. 2005 Oct 4; [Google Scholar]
- 26.Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and tremulous 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine vervet model of parkinsonism. J. Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. Journal of Computational Neuroscience. 2004;16:211–235. doi: 10.1023/B:JCNS.0000025686.47117.67. [DOI] [PubMed] [Google Scholar]
- 28.Santaniello S, Fiengo G, Glielmo L, Grill WM. Basal Ganglia Modeling in Healthy and Parkinson’s Disease State. II. Network-based Multi-Units Simulation. New York, NY (USA). 26th IEEE American Control Conference; 2007. [Google Scholar]
- 29.Sarma SV, Eden UT, Cheng ML, Williams Z, Hu R, Eskandar EN, Brown EN. Using Point Process Models to Compare Neural Spiking Activity in the Sub-thalamic Nucleus of Parkinson’s Patients and a Healthy Primate. IEEE Transactions on Biomedical Engineering; accepted for publication Dec. 2009; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terman D, Rubin JE, Yew AC, Wilson CJ. Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci. 2002;22(7):2963–2976. doi: 10.1523/JNEUROSCI.22-07-02963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tommasi G, Lanotte M, Albert U, Zibetti M, Castelli L, Maina G, Lopiano L. Transient acute depressive state induced by subthalamic region stimulation. J. Neurol. Sci. 2008;273:135–138. doi: 10.1016/j.jns.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Zahodne LB, Young S, Darrow LK, Nisenzon A, Fernandez HH, Okun MS, dr, et al. Examination of the Lille Apathy Rating Scale in Parkinson disease. Movement Disorders. 2009;24(5):677–83. doi: 10.1002/mds.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]