Abstract
Relay neurons are widely found in our nervous system, including the Thalamus, spinal cord and lateral geniculate body. They receive a modulating input (background activity) and a reference input. The modulating input modulates relay of the reference input. This modulation is critical for correct functioning of relay neurons, but is poorly understood. In this paper, we use a biophysical-based model and systems theoretic tools to calculate how well a single relay neuron relays a reference input signal as a function of the neuron’s electro physiological properties (i.e. model parameters), the modulating signal, and the reference signal parameters. Our analysis is more rigorous than previous related works and is generalizable to all relay cells in the body. Our analytical expression matches relay performance obtained in simulation and suggest that increasing the frequency of a sinusoidal modulating input or decreasing its DC offset increases the relay cell reliability.
I. INTRODUCTION
Relay neurons in the thalamus are believed to relay information from cortical inputs back to the cortex [1], [2], [3], [4]. This relay is modulated by input from other afferent fibres [5], [6]. The function of this type of neuron is to generate an output that relays the reference input conditioned upon modulating input [5]. Relay neurons are also found in areas like olfactory bulb, lateral geniculate body and spinal cord [7], [8], [9]. To enhance our understanding about the relay neurons it is important to characterize the electrophysiological dynamics of a single cell as a function of the cell type and its inputs. Typically, these dynamics are modeled as a set of parametric nonlinear ordinary differential equations, which are not always easy to analyze.
An attempt to study relay neurons is made in [10], [6]. Specifically in [10] they studied effects of the Basal Ganglia (BG) inhibition on the thalamic relay reliability. However, phase-plane analysis is used to argue how reliability changes for only a few types of inputs. Other attempts used simulations to understand how specific inputs impact a cell’s output for a given cell type [11].
Our analysis considers all possible periodic synaptic inputs and is more rigorous. In addition we allow input from cortex to be random, i.e spikes occur at random times at a given rate. Specifically, we use systems theoretic tools to study how well a single neuron’s output relays a reference input signal as a function of the neuron type, the modulating input signal, and the reference signal parameters. The methods used here are generally applicable to understanding cell behavior under various conditions and will enable more rigorous analysis of the electro-physiological changes that occur in diseases.
Finally, we give an analytical expression for relay reliability as a function of the input and the neuron parameters. Our expression suggests that on increasing the frequency of modulating input or decreasing its DC offset the relay reliability of a relay cell increases.
II. METHODS
In this section first we will describe a biophysical model of a relay neuron, and then will use phase plane analysis to define important concepts that will help us to compute reliability.
A. A Relay Neuron Model
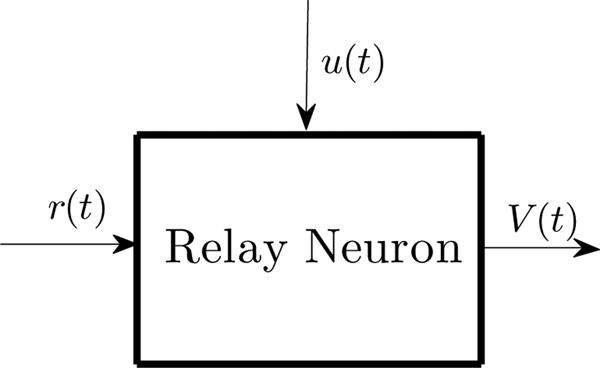
A relay neuron receives two kinds of inputs: a reference input, r(t) and a modulating input u(t), and generates one output, V (t); as shown in Figure 1. This relay neuron model structure has been used many times to model thalamic neurons to study the effect of deep brain stimulation (DBS) in Parkinson’s disease (PD) [2], [10], [11], [12], [13].
Fig. 1. Block diagram of a Relay Neuron.
It receives two inputs 1. reference input r(t) and 2. modulating input u(t), and generates one output V (t).
We would like to understand exactly how the modulating input changes relay reliability of a neuron. To do so, we use a biophysical model to describe the electro-physiological dynamics of a relay neuron. In particular, we choose a simplified model that is driven only by calcium ion and leak currents that is described by the following set of differential equations:
| (1a) |
| (1b) |
| (1c) |
The details of this model can be found in [14] wherein this model is used to model neurons in the inferior olive for the purpose of studying sub-threshold oscillations. We choose this model because it is simple and still contains low threshold calcium currents which are shown to govern input selectivity of relay neurons [12]. In our model we choose Iext = −1μA/cm2,gT = 0.6 and gL = 0.2. All other parameters are same as in [14].
Our model also receives two inputs which are as follows:
Reference Input(r): This input represents the spiking activity from other neurons (e.g. cortical neurons), which the neuron must relay, and belongs to the following class of functions:
| (2) |
Here, I0, ti>, t ∈ ℝ+ and i,n ∈ ℕ. δ (t) is a Dirac delta function [15]. The are generated randomly such that ti+1 = ti + T0 + τ, where T0 ∈ ℝ is a constant that represents the refractory period of reference input, and τ ∈ ℝ+ is exponentially distributed with probability density function:
| (3) |
where λ ∈ ℝ+. Now we define the average interspike interval as T = E (ti+1 − ti) = T0 + 1/λ. Note that as τ′s are characterized completely by λ and T0.
Modulating Input(u): This input represents background activity received by a relay neuron. It modulates the dynamics of the neuron and governs relay performance. It comes into the biophysical model (1) as a synaptic input and belongs to the following class of sinusoidal functions:
| (4) |
Here c1,c2,ω, and t ∈ ℝ+ and c1 ≥ c2. Since u(t) represents the amount of synaptic transmitter released by a neuron, we have the constraint c1 ≥ c2 to ensure that u(t) ∈ ℝ+. Also, c1 and c2 are appropriately small so that the modulating input does not make the relay neuron spike without a reference input. This property of the modulating input will be useful when we linearize (1) for analysis. u(t) is modeled as a deterministic signal because it represents ensemble synaptic activity from a group of neurons.
Finally, by defining a state vector X = [(V − Vsyn),h]T, we can write an equivalent but compact state space representation of (1) as:
| (5) |
B. Response to u(t) = c1 + c2sin(ωt) and r(t) = 0
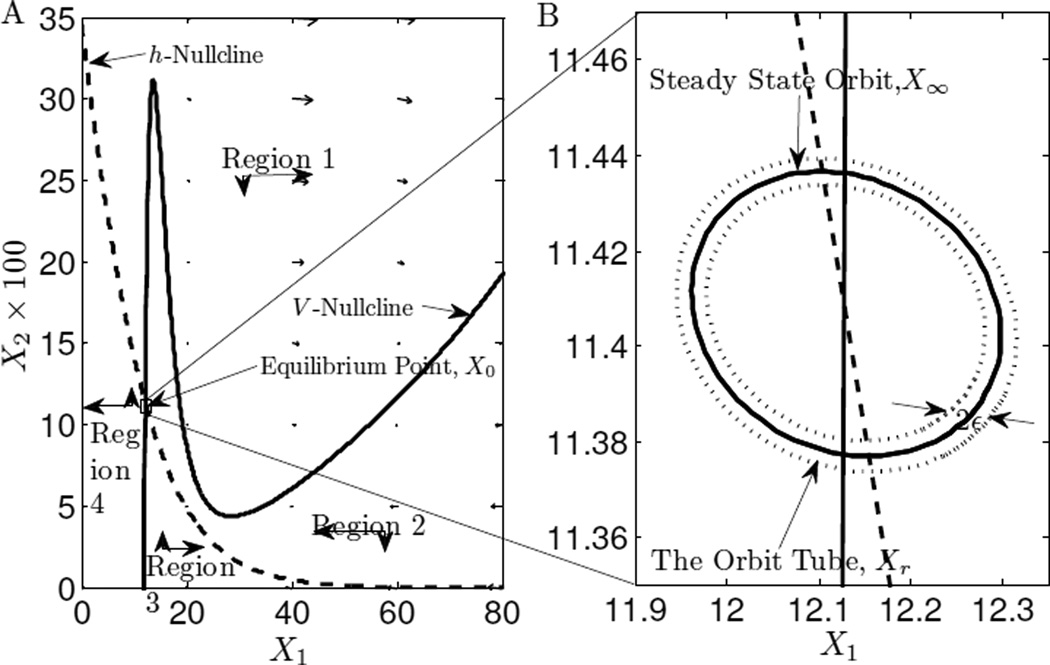
A neuron characterized by (5) approaches a closed trajectory as t → ∞ if r(t) = 0 and u(t) = c1 + c2sin (ωt). We define this closed trajectory as the steady state orbit of a relay neuron. If the system does not converge to a closed trajectory or a point, then it has no steady state orbit.
Now, we define 𝕏∞ as the collection of all points in the steady state orbit. 𝕏∞ is not achievable in finite time, therefore, we relax the definition to the collection of all points inside a tube of ε thickness around the steady state orbit as the set 𝕏r, i.e.
| (6) |
If state X ∈ 𝕏r, then we say that it is in the orbit tube. Note that for a given ε > 0 ∃ t0 ∈ ℝ+ such that ∀t > t0, X (t) ∈ Xr. We set ε = 10−3 in our study. Figure 2 B plots steady state orbit and the orbit tube for a neuron (5).
Fig. 2. A. Phase-Plane diagram B. Steady State Orbit and The Orbit Tube of a Relay Neuron.
A. The v-nullcline denotes the collection of points where Ẋ1 = 0 and the h-nullcline is where Ẋ2 = 0. These two lines divide the phase-plane into four different regions marked by balck arrows. In each region, the black arrows indicate the direction in which the state vector evolves. B. Shows the steady state orbit and the orbit tube of a relay neuron
C. Response to pulses in r(t) and u(t) = c1
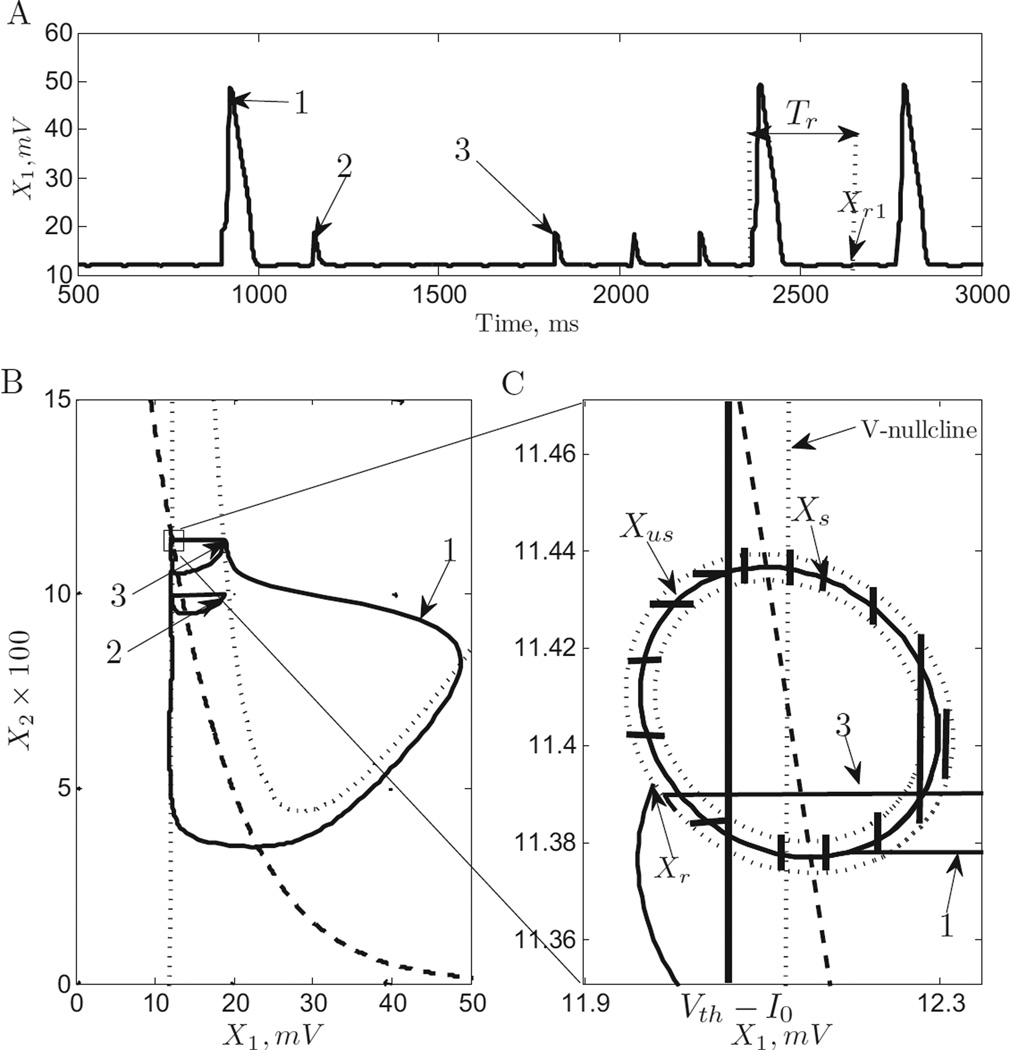
When a reference pulse arrives, there are 2 possible responses of system in (5). The neuron either successfully spikes or unsuccessfully spikes. In Figure 3 A, we have plotted these two types of pulse responses.
Fig. 3. Successful Spike, Unsuccessful Spike and Time to Resting State.
A. The time profile of a successful spike (1) and unsuccessful spikes (2,3). Tr is the time between spike onset and point where system state enter orbit tube at point Xr. B. Successful spike and unsuccessful spike in the phaseplane. C. 𝕏s and 𝕏us. Note that unsuccessful spike(2) occurs as a result of the reference pulse occurring when X ∉ 𝕏r.
It is straightforward to see how these two responses occur. The reference pulse causes the state vector to “ jump” to . This is easy to show by direct integration of (5), on the time interval limΔt→0[ti − Δt, ti]. If the dynamics of second component of state, X2, are slow and X (ti) falls into region 1 (see Figure 2 A) the neuron will generate a successful spike, otherwise it will return back to the equilibrium point generating unsuccessful spike (note the directions of arrows in Figure 2 A). We define, the threshold current, Ith, such that X0 + [Ith,0]T, falls in region 1 and the system generates a successful spike. We define quantity X01 + Ith as threshold voltage Vth. Note that X0 is the equilibrium point of the system.
The system that we are interested in studying, i.e. (5), receives a time varying input u(t) with c2 ≠ 0, making the threshold voltage Vth time dependent, but for simplicity we assumes that Vth is a constant in time. We later show that this assumption does not significantly impact our expression for relay reliability of the neuron, and our expression matches well with reliability calculated through simulation of (1). Finally, we define time taken by system state to return to 𝕏r after shooting a successful spike as time to orbit tube, Tr.
D. Full response to both r(t) and u(t)
When r(t) and u(t) are both applied to (5), whether a pulse in r(t) induces a successful spike or unsuccessful spike depends on the system state when the pulse occurs. Therefore, we define set 𝕏s ⊆ 𝕏r as the collection of all points that result in successful spikes after a pulse in r(t), and 𝕏us ⊆ 𝕏r as the collection of points that results in unsuccessful spikes after a pulse in r(t). These sets are illustrated in Figure 3 C. Note that if the reference pulse does not occur for a Tr time interval i.e, the system state will move into 𝕏r.
From Figure 3 C, one can see that 𝕏us is the region in 𝕏r to the left of vertical line at Vth − I0 and 𝕏s is the region in 𝕏r to the right of vertical line at Vth − I0. This is because for X1 < Vth − I0, a delta pulse of height I0 will not make the system state jump to region 1 and hence will result in an unsuccessful spike. Similarly, if X1 ≥ Vth − I0 a delta pulse will result in a successful spike.
E. Relay Reliability
We define relay reliability as:
| (7) |
In this manuscript we will study reliability under the condition that Tr < T0. This condition will ensure that state X (t) ∈ 𝕏r whenever a pulse in r(t) occurs. Therefore we could rewrite definition of reliability as:
| (8) |
R can also be approximated as the ratio of the time spent by the steady state solution of (5) (r(t) = 0), X (t) in 𝕏s to the time it takes to complete one orbit cycle in 𝕏r. R converge to this approximation in case T >> 2π/ω, with T being average inter-pulse interval in r(t) and ω being the frequency of u(t). The time taken to complete one orbit cycle in 𝕏r is 2π/ω. To calculate time spent in 𝕏s, we must find the solution to (5) when the state is in orbit tube 𝕏r. This solution is given by steady state solution of (5) with r(t) = 0.
To find it, we linearize (5) about the nominal solution X0 (t) = X0 given the nominal input u0 (t) = c1. Now, if the input is perturbed such that u(t) = u0(t) + δu(t) and initial condition is perturbed such that X (0) = X0(0) + δx (0), the state trajectory will also be perturbed to X (t) = X0 (t)+δx (t). Upon substituting these values and performing a first order Taylor series expansion of (5) about the nominal solution and nominal input we get:
| (9) |
which can be equivalently written as:
| (10) |
Note that here δu(t) = c2sin(ωt).
The solution of (10), with δx(0) = 0, in the Laplace domain [16] is:
| (11) |
Substituting δu and calculating (sI − A)−1B, we get:
| (12) |
For our model we found and . From (12), one can compute the steady state solution of (10) by taking inverse Laplace transform of (12) and taking the limit t → ∞. This gives steady state solution as:
| (13) |
Here, ℑ (a) denotes the imaginary part of complex number a. Note that δu(t) = c2sin(ωt) for u(t) ∈ 𝕌. (10) approximates (5) in steady state when c2 is small, which is always the case by definition of 𝕌. Also note that, we will get the same steady state even if δx(0) ≠ 0. Using (13), we can write the steady state solution, of (5) as:
| (14) |
Now the time spent in 𝕏us in one cycle is the difference between the two values of time, t, when X1 (t) = X01 + δx1 (t) = Vth − I0. Substituting the value δx1 (t) from (13) and observing that Vth = X01 + Ith, we get:
| (15a) |
| (15b) |
Here Ψ = ∠H1(jω). The roots of this equation are:
| (16a) |
| (16b) |
Finally the time spent in 𝕏us is μ2 − μ1. Therefore time spent in 𝕏s is:
| (17) |
Now we calculate, R, as ratio of and 2π/ω. That is,
| (18) |
III. Results & Discussion
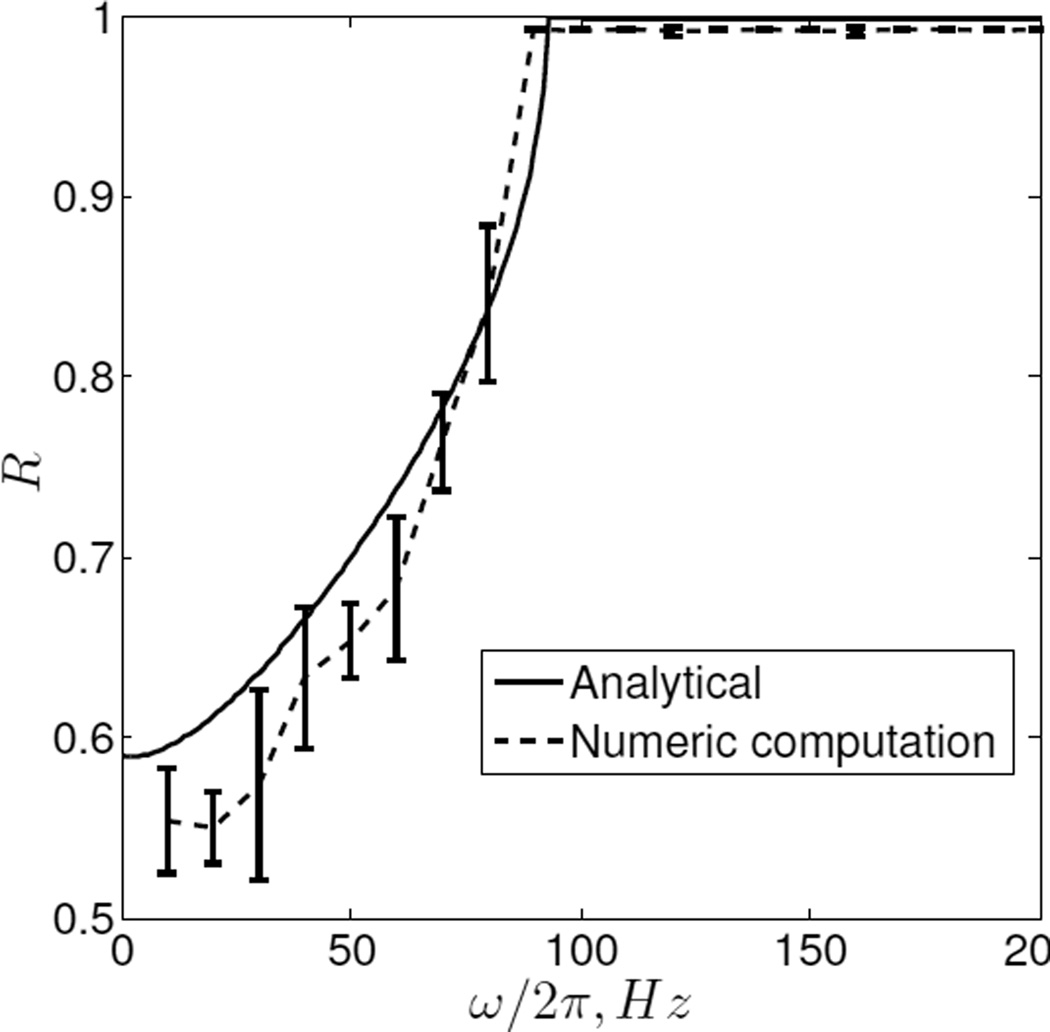
Equation (18) gives dependence of reliability of a relay neuron on different input and model parameters. Most important among which is dependence on the frequency of modulating input. In Figure 4 we plot the R as a function of frequency , for fixed c1 = c2 = 0:0025,I0 = 6:71,Tr = 520ms,T0 = 1000,T = 1500. From the analytical curve we see that reliability is almost constant for low ω and then increases steeply until it again becomes constant. Figure 4 also plots the simulation results from (5). While simulating, we set T0 > Tr, to ensure that every pulse in r(t) occurs when the neuron state is in 𝕏r. This allows us to compute R as given by (7), through simulation. We see that the analytic expression for R fits well to the simulated curve, indicating that all our approximations and assumptions are reasonable.
Fig. 4. R vs ω/2π.
Solid line is from the (18). Dotted line is plotted by running simulations on (1), for different ω.
Our analysis also gives insights on how modulating input frequency effects relay reliability. One can see decrease in gain H1 (jω) is responsible for increase in reliability. This is because a relay neuron acts as a low pass filter and filters out high frequency components of the modulating input. Therefore a high frequency modulating input effect becomes similar to zero modulating input effect, as far as relay reliability is concerned. Finally, (4) may find wide use in studying neuronal circuits where relay neurons are involved, e.g. BG-Thalamus circuit in PD, reflex circuitry in spinal cord. Furthermore, as thalamic relay reliability is hypothesized to be related to PD [5], [10], (4) may also be useful in answering questions such as why lesion and DBS has similar therapeutic effects in PD, why low frequency DBS do not work etc.
Acknowledgments
This work was supported by Burroughs Wellcome fund
Contributor Information
Rahul Agarwal, Email: rahul.jhu@gmail.com, Department of Biomedical Engineering, Johns Hopkins University, 3400 N Charels Street, Baltimore, MD 21218, USA.
Sridevi V. Sarma, Email: ssarma2@jhu.edu, Faculty of Biomedical Engineering, Johns Hopkins University, 3400 N Charels Street, Baltimore, MD 21218, USA.
References
- 1.Jones Edward G. The Thalamus. Cambridge Uni Press; 2007. [Google Scholar]
- 2.Huguenard JR, Prince DA. A novel t-type current underlies prolonged calcium-dependent burst firing in gabaergic neurons of rat thalamic reticular nucleus. J Neurophysiol. 1992 doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. JN Physiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- 4.Daesoo Kim, Inseon Song, Sehoon Keum, Taehoon Lee, Myung-Jin Jeong, et al. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking 1g t-type ca2+ channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 5.Guillery RW, Murray Sherman S. Thalamic relay functions and their role in corticocortical communication: Generalizations from the visual system. Neuron. 2002;33:163–176. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 6.Le Masson Gwendal, Renaud-Le Masson Sylvie D, Bal Thierry. Feedback inhibition controls spike transfer in hybrid thalamic circuits. Nature. 2002;417:854–858. doi: 10.1038/nature00825. [DOI] [PubMed] [Google Scholar]
- 7.Samuel Lagier, Alan Carleton, Pierre-Marie Lledo. Interplay between local gabaergic interneurons and relay neurons generates γ oscillations in the rat olfactory bulb. The Journal of Neuroscience. 2004;24:4382–4392. doi: 10.1523/JNEUROSCI.5570-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch JC, Fourment A, Marc ME. jByvnp title = Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. 2004 doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- 9.Seki Kazuhiko, Perlmutter Steve I, Fetz Eberhard E. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nature Neuroscience. 2003;6:1309–1316. doi: 10.1038/nn1154. [DOI] [PubMed] [Google Scholar]
- 10.Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci. 2004;16:211–235. doi: 10.1023/B:JCNS.0000025686.47117.67. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies in deep brain stmulation protocols in data-driven computational models. J Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. [DOI] [PubMed] [Google Scholar]
- 12.Hayriye Cagnan, Meijer Hil GE, Gils Stephan A van, Krupa Martin, Heida Tjitske, et al. Frequency-selectivity of a thalamocortical relay neuron during parkinsons disease and deep brain stimulation: a computational study. European Journal of Neuroscience. 2009;30:13061317. doi: 10.1111/j.1460-9568.2009.06922.x. [DOI] [PubMed] [Google Scholar]
- 13.Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low threshold calcium currents in thalamic relay cells. J Neurosci. 1998;18:3574–3588. doi: 10.1523/JNEUROSCI.18-10-03574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manor Y, Rinzel J, Segav I, Yarom Y. Low amplitude oscillations in inferior olive: A model based on electrical coupling of neurons with heterogeneous channel densities. J Neurophysiol. 1997 doi: 10.1152/jn.1997.77.5.2736. [DOI] [PubMed] [Google Scholar]
- 15.Paul Dirac. Principles of quantum mechanics. 4th ed. Oxford at the Clarendon Press; 1958. [Google Scholar]
- 16.Arendt Wolfgang, Batty Charles JK, Hieber Matthias, Neubrander Frank. Vector-Valued Laplace Transforms and Cauchy Problems. Birkhuser Basel. 2002 [Google Scholar]