Abstract
Background
Critical illness is associated with cognitive impairment, but mental health and functional disabilities in general intensive care unit (ICU) survivors are inadequately characterized and there are a paucity of data regarding the relationship between age and delirium and these outcomes.
Methods
In this prospective, multisite cohort study, we enrolled medical/surgical ICU patients with respiratory failure or shock, collected detailed demographics and in-hospital variables, and assessed survivors at 3 and 12 months with measures of depression, posttraumatic stress disorder (PTSD) and functional disability. We used linear and proportional odds logistic regression to examine the independent associations between age and delirium duration versus mental health and functional disabilities.
Findings
We enrolled 821 patients with a median (interquartile range) age of 61 (51, 71), assessing 448 patients and 382 patients 3 and 12 months after discharge. At 3- and 12-month follow-up, 37% (149/407) and 33% (116/347) of subjects reported at least mild depression, driven primarily by somatic rather than cognitive symptoms. Depressive symptoms were common even among those with no proxy reported history of depression, reported at 3- and 12-month follow-up by 30% (76/255) and 29% (62/217) of these individuals. At either follow-up assessment, only 7% (27/415, 24/361) of subjects had symptoms consistent with PTSD. Disabilities in basic activities of daily living (ADLs) and instrumental activities of daily living (IADLs) were present in 32% (139/428) and 26% (108/422) of individuals at 3 months and in 27% (102/374) and 23%(87/372) at 12 months. Mental health and functional difficulties were prevalent in young and old patients. Although older age was frequently associated with mental health and functional disabilities, no consistent association was observed between delirium and these outcomes.
Interpretation
In contrast with early single-center reports, data from this large, multicenter investigation reveal depression is much more common than PTSD after critical illness and is driven by somatic symptoms indicative of physical disabilities rather than by cognitive symptoms. Poor mental health and functional disability were common, and persistent in up to a quarter of patients.
Introduction
Well over 5 million individuals are admitted to medical/surgical critical care units in North America annually and approximately 80% survive – more than are diagnosed annually with cancer.1, 2 For them, critical illness may be a gateway to Post-Intensive Care Syndrome (PICS)3 – cognitive impairment, depression, post-traumatic stress disorder (PTSD), functional disabilities and quality of life decrements.4-9
The aforementioned outcomes have been studied in specialized critically ill populations (e.g., sepsis and Acute Respiratory Distress Syndrome - ARDS), typically with individuals of a narrow age range (preventing an understanding of whether these outcomes differ in young versus old patients), yet they have rarely been evaluated in general medical and general surgical intensive care unit cohorts.10,11 Depression has been observed in a third of survivors of ARDS, but the precise nature of their symptoms or those of medical and surgical intensive care unit (ICU) survivors is unknown.4 Select studies have reported PTSD symptoms in up to half of survivors of critical illness, yet PTSD prevalence using Diagnostic and Statistical Manual (DSM) criteria in general medical and surgical populations is also unknown, particularly in the context of critical illness as a traumatic stressor.5 Functional disability in critical care survivors beyond ARDS is poorly understood, though it has been evaluated in some other specific contexts (e.g. septic patients).12 Lastly, few investigations have explored ICU delirium—a potentially modifiable risk factor—in the emergence of mental health and functional difficulties.4, 5,13
We undertook this investigation for 3 reasons. First, in a general ICU population, we wanted to characterize mental health outcomes and functional disabilities and explore the hypothesis that depressive symptoms after discharge are more somatic than cognitive. Secondly, we wanted to study the effects of age across outcomes, testing the hypothesis that younger patients experience symptoms of depression, PTSD, and functional disability similarly to those of older patients at 3 and 12 months. Finally, we sought to determine if delirium is a risk factor for poor mental health and functional outcomes at 3 and 12-month follow-up, testing the hypothesis that delirium is associated with depression, PTSD, and functional disabilities.
Methods
Patients and Setting
This institutional review board (IRB) approved prospective observational cohort (NCT00392795) study screened patients in the ICUs at Vanderbilt University Medical Center and Saint Thomas Hospital in Nashville, Tennessee. We included adults admitted to medical/surgical ICUs with respiratory failure, cardiogenic shock, or septic shock. We excluded individuals mechanically ventilated at any time two months before the current ICU admission, spent >5 days in an ICU during the month before the current ICU admission, or spent >72 hours with organ dysfunction in the current ICU admission, and individuals with significant preexisting cognitive impairment identified using the Short Informant Questionnaire on Cognitive Decline in the Elderly [IQCODE]14 and the Clinical Dementia Rating [CDR] Scale.15 Specifically, patients who were suspected to have preexisting cognitive impairment on the basis of a score of 3.3 or more on the Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE; on a scale from 1.0 to 5.0, with 5.0 indicating severe cognitive impairment) were assessed by certified evaluators with the use of the Clinical Dementia Rating (CDR) scale (with scores ranging from 0 to 3.0, and higher scores indicating more severe dementia). Patients with a CDR score of more than 2.0 (moderate dementia) were excluded.11 We additionally excluded individuals at high risk for preexisting cognitive deficits owing with neurodegenerative disease, recent cardiac surgery (within the past 3 months), or suspected anoxic injury; blindness, deafness, or non- English speaking; individuals for whom follow-up would be difficult due to substance abuse, psychosis, homelessness, or residence ≥200 miles from Nashville; those unlikely to survive 24 hours; and those for whom informed consent could not be obtained. Informed consent was obtained from patients or their healthcare proxy (hereafter referred to simply as “proxy”); if consent was initially obtained from a proxy, the patient provided informed consent when mentally competent. The index presentation of data from this BRAIN-ICU study included cognitive outcomes but did not contain any mental health, functional or quality of life outcomes.11 All of the data presented in this manuscript other than demographics and in-hospital descriptive data are original – i.e. they have not been previously published.
We collected baseline patient characteristics: age, preexisting cognitive impairment,14 history of proxy reported depression and other mental health illnesses (using questions that inquired about whether a patient had ever been diagnosed with specific psychiatric conditions by a healthcare professional), education, activities of daily living and instrumental activities of daily living (IADLs) at enrollment using the Katz Activities of Daily Living (ADL)16 and Pfeffer Functional Activities Questionnaire17 (FAQ) questionnaires (completed by a proxy), illness severity using the Acute Physiology and Chronic Health Evaluation (APACHE) II score,18 comorbidities using the Charlson Comorbidity Index19 and admission diagnoses. Up to 30 days while in the hospital, we evaluated patients twice daily in the ICU and once daily in the wards, assessing level of consciousness using the Richmond Agitation-Sedation Scale (RASS)20 and delirium using the Confusion Assessment Method for the ICU (CAM-ICU).21 Patients were delirious if they were responsive to verbal stimuli (RASS -3 or more alert) and CAM-ICU positive.21 Patients were comatose if they were unresponsive to verbal stimuli (RASS -4 or -5). Data on ongoing organ dysfunctions via the Sequential Organ Failure Assessment score (SOFA),22 severe sepsis, and vital signs were collected daily through ICU discharge. Throughout the hospitalization, we collected information about sedative and analgesic exposure from the medication administration record.
At 3 and 12 months (+/-1 month) after hospital discharge, a doctoral level neuropsychologist or masters level professional assessed patients using a battery including: 1) the Beck Depression Inventory-II (BDI-II),23 2) the Post-Traumatic Stress Checklist – Specific Version (PCL-S),24 3) the Katz Activities of Daily Living (ADL) Scale,16 4) the Pfeffer Functional Activities Questionnaire (FAQ), assessing IADLs17 and 5) the Short Form-36 (SF-36) Health Survey – Mental Component Score (MCS) and Physical Component Score (PCS), assessing quality of life25 (see Table 1). On the BDI-II, we characterized depressive symptoms as either cognitive or somatic, following the approach of test developers, Beck and Steer.26 On the PCL-S, we used an item mapping approaching to determine if individuals met the DSM-IV criteria for PTSD27 (described in the footnotes of Table 1).
Table 1. Description of Follow Up Data.
| Test | Description | Area Measured | Scoring |
|---|---|---|---|
| BDI-II1 | Assesses the presence and severity of depressive symptoms | Depression | 21 questions with a range of 1-3 each (total range, 0-63), with scores >13 suggesting depression. Items 1-14 address cognitive/affective symptoms while items 15-21 pertain to somatic symptoms |
| PCL-S2 | Assesses the presence and severity of PTSD symptoms | PTSD | DSM-IV- based, 17 questions range of 1-5 each (total range, 17-85), with high scores indicating high likelihood of PTSD |
| Katz ADL | Assesses basic abilities such as bathing, feeding and transferring | Basic functional abilities | 6 questions (total range, 0-18), with high scores indicating dependence |
| FAQ | Assesses higher order functioning (e.g., managing finances, medications, etc.) | Instrumental Activities of daily living | 10 questions with a range of 0-3 each (total range, 0-30), with scores ≥9 indicating impaired functioning |
| SF-36 | Assesses quality of life and overall functioning across physical and mental health domains | Generic quality of life | 36 questions across 8 domains, each with a range of 0-100, with low scores indicating poor quality of life |
Definition of abbreviations: ADL = activities of daily living; BDI-II = Beck Depression Inventory – II; FAQ = Functional Activities Questionnaire; PCL-S = Posttraumatic Stress Disorder Checklist, Event Specific Version; SF-36 = Short Form-36.
BDI-II: The BDI-II is a comprised of 2 primary factors, cognitive/affective and somatic. Cognitive/affective questions (1-14) pertain to symptoms such as sadness, tearfulness, and feelings of failure; somatic questions (15-21) pertain to symptoms such as loss of energy, changes in appetite, and fatigue.
The PCL-S comprises 17 questions that map onto the Diagnostic and Statistical Manual (DSM)-IV criteria for PTSD (the DSM-IV was the version of the DSM current at the time this study was begun. Questions 1-5 on the PCL map to Criterion B (intrusion symptoms), questions 6-12 map to Criterion C (avoidance), and questions 13-17 map to Criterion D (negative alterations in cognition and mood). All patients met Criterion A (exposed to stressor) since all were survivors of critical illness. We used the mapped DSM-IV criteria as the main determinant of prevalence of PTSD: to be diagnosed with PTSD, patients were required to have at least 1 positive symptom (defined as > 3 on a 5 point scale indicating being moderately affected) from the PCL questions mapping to Criterion B, at least 3 positive symptoms from items correlating to Criterion C, and at least 2 positive symptoms from items matching Criterion D, where “positive” is defined as ≥3 on a 5-point scale (indicating being at least moderately affected). We also used a PCL threshold of > 50 as a secondary measure of PTSD symptom prevalence.
Statistical Analysis/Covariates
Continuous data are presented as medians and interquartile ranges; categorical variables are presented as frequencies and proportions. In addition to descriptive data, we sought to determine whether age and delirium duration were independent risk factors for our key outcomes. Delirium duration was calculated as the number of days a patient was CAM-ICU positive during the 30-day study period. Simple imputation was used for missing delirium and coma evaluations (3% of all assessments) – for example, on the few days with missing data, we took a random draw from the patient's mean mental status conditioned on his or her status on the days immediately prior to and after the missing day in the computations. We used multiple imputation to assign values to missing risk factors and outcomes in regression modeling and to reduce potential bias resulting from loss to follow-up, a conservative approach. In the imputation, we included all covariates used in the models as well as other variables which might predict missingness (including additional information on hospital illness, such as worst severity of illness and time on the ventilator, or cognitive and HRQL test scores from non-missing follow-up data). We again used the aregImpute function provided in R's Hmisc library, which is an approximation to full Bayesian multiple imputation procedures using the bootstrap. We specified ten imputations in aregImpute and used the results in conjunction with the fit.mult.impute function, also in Hmisc, which replaces individual model coefficient vectors with the average of all coefficient estimates and creates an imputation-corrected variance-covariance matrix. We specified five imputations in fit.mult.impute. The method we used in multiple imputation is thought to be valid under MAR.28 We did not, however, impute data for patients who had no mental health and functional data (e.g., due to death or withdrawal). We used R version 3.0.1 for all analyses.
Outcome variables were: BDI-II, PCL-S, Katz ADL, FAQ and SF-36 physical component scores (PCS) and mental component scores (MCS). Since missing data are rarely random and patients who had partial missing outcome data were different from those with complete data, we used multiple imputation during modeling to reduce bias.28 Model covariates were chosen a priori as potential confounders, i.e., they had known or suspected relationships with both the independent and outcome variables. Covariates included age, education level, Short IQCODE,14 depression and other mental health illnesses history, baseline ADL,16 baseline Charlson Comorbidities Index,19 cerebrovascular disease burden via the Framingham Stroke Risk Profile,29 mean modified SOFA score (using RASS rather than GCS scores) over ICU duration,22 duration of severe sepsis and coma, episodes of hypoxemia, and mean daily doses of benzodiazepines, propofol, dexmedetomidine, opiates and haloperidol.
We used multivariable regression models to study the independent association of age and delirium duration with functional and mental health outcomes, using separate models for 3- and 12-month time points. SF-36 PCS and MCS were normally distributed, and we used linear regression for these outcomes. Because all other outcomes were not normally distributed and assumptions for linear regression would not be satisfied, we used proportional odds logistic regression for all other models. In all models, drug doses were transformed with the use of their cube root to reduce the influence of extreme outliers, and continuous variables were modeled with the use of restricted cubic splines to allow for nonlinear associations (with the exception of dexmedetomidine and haloperidol doses, used so infrequently that the number of unique doses was too small for splines). We examined model diagnostics, using residual plots versus predicted plots and quantile-quantile plots for linear regression models and graphically examining the proportional odds assumption for proportional odds logistic regression models. All model assumptions were met adequately. Variance inflation due to multiple imputation was not problematic.
Prior to initiation of the BRAIN-ICU study, we developed formal estimates with regard to enrollment and generated sample size and power analysis calculations based on 12-month follow-up. We anticipated enrolling 800 subjects in the study and assessing 320 at 12 month follow-up; we calculated a priori that the assessment of 285 patients at 12 month follow-up would provide us at least 80% power to answer key questions of interest related to the association between the primary risk factor of interest (delirium) and mental health, functional, and quality of life outcomes.
The sponsor of the study had no role in study design, data collection, analysis, or interpretation, or paper writing. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 1, 2007 and June 30,2010, we enrolled 826 patients. Five withdrew consent and permission to use collected data; thus, we had 821 patients with in-hospital data and assessments. Between enrollment and the 3-month follow-up, 252 patients (31%) died; 448 of the 569 surviving patients (79%) participated in follow-up testing 3 months after discharge. Another 59 patients (7% of the original cohort) died before the 12-month follow-up, and 382 of the 510 remaining survivors (75%) were tested 12 months after discharge. For a full description of details to enrollment, reasons for loss to follow-up, death, and withdrawals, refer to our CONSORT diagram (Appendix, Supplemental Figure - SF1).11
The enrolled 821 patients had a median age of 61 years and high severity of illness (median APACHE II score of 25). Only 6% (51/821) of patients had preexisting cognitive impairment (score of 3.6 or greater on the Short IQCODE or medical history). Demographic data and descriptive data for all patients that had assessments at either follow-up time points are presented in Table 2. Of the outcomes assessed at 3 and 12 month follow-up (BDI-II, PCL-S, Katz ADL, FAQ, SF-36 PCS and MCS) complete data were obtained in at least 91% of patients (408/448 at 3 month follow-up and 347/382 at 12 month follow-up). (Appendix, Supplemental Table - ST1). Small but potentially meaningful differences were observed with regard to specific baseline and enrollment characteristics between those with and without complete outcome data. Individuals with partial HRQL outcomes had poorer proxy reported ADL and IADL functioning at enrollment and had higher Framingham stroke risk scores (ST2). Additionally, they were older and were more likely to experience delirium. The median age of patients with complete vs. partial outcomes data was 64 (IQR of 51, 72) vs. 49 (48, 68) and delirium occurred in 74% (282/383) of individuals with complete outcomes data vs. 83% (70/84), respectively (ST2). For a description of additional characteristics of those with complete versus partial outcomes data, refer to ST3-ST4).
Table 2. Demographic and In-Hospital Data* ¶.
| Variable | In hospital cohort (N=821) |
Follow-up cohort (N=467) |
|---|---|---|
| Age at enrollment (years) | 61 (51, 71) | 59 (49, 69) |
| Caucasian, N (%)+ | 740 (90%) | 413 (88%) |
| Males, N (%) | 420 (51%) | 234 (50%) |
| Education (years) | 12 (12, 14) | 12 (12, 14) |
| IQCODE at enrollment± | 3.0 (3.0, 3.1) | 3.0 (3.0, 3.1) |
| History of depression, N (%) | 261 (33%) | 159 (35%) |
| History of mental health illness (not depression), N (%) | 66 (8%) | 44 (10%) |
| History of ADL disability | 229 (29%) | 120 (26%) |
| History of IADL disability | 93 (12%) | 44 (10%) |
| Charlson comorbidity score† | 2 (1,4) | 2 (1,4) |
| Framingham stroke risk | 9 (6, 14) | 9 (5, 14) |
| APACHE II Score at enrollment§ | 25 (19, 31) | 24 (19, 30) |
| SOFA Score at enrollment‖ | 9 (7, 12) | 9 (7, 12) |
| Admission Diagnosis, N (%) | ||
| Sepsis | 244 (30%) | 136 (29%) |
| Acute respiratory failure†† | 135 (16%) | 71 (15) |
| Cardiogenic shock, myocardial ischemia, or arrhythmia | 141 (17%) | 79 (17%) |
| Upper-airway obstruction††† | 87 (11%) | 49 (10%) |
| Gastric/colonic surgery | 63 (8%) | 29 (6%) |
| Neurologic disease or seizure | 11 (1%) | 7 (1%) |
| Other surgical procedure‡‡ | 82 (10%) | 65 (14%) |
| Other diagnosis | 58 (7%) | 31 (7%) |
| ICU Type, N (%) | ||
| Medical ICU | 559 (68%) | 298 (64%) |
| Surgical ICU | 262 (32%) | 169 (36%) |
| Mechanical ventilation during study period, N (%) | 746 (91%) | 421 (90%) |
| Days of mechanical ventilation among exposed to MV | 3 (1, 8) | 2 (1, 6) |
| Delirium during study period, N (%) | 606 (74%) | 352 (75%) |
| Delirium days among those with delirium | 4 (2, 7) | 3 (2, 7) |
| Coma during study period, N (%) | 517 (63%) | 265 (57%) |
| Coma days among those with coma | 3 (2, 6) | 3 (1, 5) |
| Severe sepsis during study period, N (%) | 572 (70%) | 297 (64%) |
| ICU length of stay (days) | 5 (3, 11) | 5 (3, 10) |
| Hospital length of stay (days) | 10 (6, 17) | 10 (6, 18) |
Abbreviations: IQCODE, Informant Questionnaire On Cognitive Decline in the Elderly; APACHE, Acute Physiology and Chronic Health Evaluation; ICU, Intensive Care Unit; SOFA, Sequential Organ Failure Score; MV, mechanical ventilation
Percentages may not sum to 100, owing to rounding. Of the 821 patients with in-hospital data, 448 patients were assessed at 3 months and 382 patients were assessed at 12 months. Nineteen patients were not assessed at 3 months, but were available at 12 months, making the total number of patients who had follow-up at either one or both time points 467.
Median (interquartile range) unless otherwise indicated
Race was determined according to the medical record or was reported by the patient's surrogate.
Scores on the Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) range from 1 to 5, with a score of 3 indicating no change in cognition over the past 10 years, a score lower than 3 indicating improvement, and a score higher than 3 indicating decline in cognition, as compared with 10 years before. A score of 3.3 or higher indicates an increased probability of cognitive impairment, and a score of 3.6 or higher indicates preexisting cognitive impairment
Scores on the Charlson comorbidity index range from 0 to 33, with higher scores indicating a greater burden of illness; a score of 1 or 2 is associated with mortality of approximately 25% at 10 years.
Scores on the Acute Physiology and Chronic Health Evaluation (APACHE) II range from 0 to 71, with higher scores indicating worse outcomes.
Scores on the Sequential Organ Failure Assessment (SOFA) range from 0 to 24 (from 0 to 4 for each of six organ systems), with higher scores indicating more severe organ dysfunction. We used a modified SOFA score in our regression models, which excluded the Glasgow Coma Scale components, since coma was included separately in our models.
Acute respiratory failure included the acute respiratory distress syndrome, acute exacerbations of chronic obstructive pulmonary disease or asthma, pulmonary edema, and fibrosis.
Upper airway obstruction refers to patients with airway swelling or obstruction due to otolaryngeal, tracheal or maxillofacial pathology or surgery, sleep apnea, angioedema, inability to protect airway from secretions, etc.
Other surgical procedures included hepatobiliary surgery, liver transplantation, and orthopedic, obstetrical or gynecologic, vascular, otolaryngologic, and urologic surgery.
Mental Health Outcomes (Depression and PTSD)
Depression
A total of 37%(149/407) and 33% (116/347) of respondents reported at least mild depressive symptoms (BDI-II >13) at 3 and 12 month follow-up respectively (Table 3). Mild and moderate symptoms were similarly prevalent at 3 months and 12 months and severe depressive symptoms were relatively uncommon (Table 3). Depressive symptoms existed among both younger and older patients (Figure, for an additional presentation of outcome data across ages ranges in histogram form, refer to SF2, in the Appendix). At 3-month follow-up, BDI-II scores >13 were present in 52% (71/137) of patients with a reported depression history and 30% (76/255) without such a history. At 12-month follow-up, BDI-II scores > 13 were present in 43% (49/115) and 29% (62/217) of those with and without a reported history of depression. Among the 117 patients reporting depressive symptoms at 3 months with 12 month data, 73 (62%) had depressive symptoms at 12 months. Only 15% (30/200) of those without depressive symptoms at initial follow-up displayed such symptoms at 12 months; in only 2% (3/200) of individuals with no depression at 3 months were depressive symptoms severe at 12 months. In contrast, 80% (20/25) of those with severe depression at 3 months had symptoms of depression at 12 month follow-up, though depression at 12 months was severe in only 36% (9/25) of patients with severe depression at 3 months.
Table 3. Long-Term Outcomes at 3 and 12 months.
| Outcome | 3-Month Follow-Up Cohort (N = 448) |
12-Month Follow-Up Cohort (N = 382) |
|---|---|---|
| Psychological (N/%) | ||
| Depression | ||
| BDI-II (N:407, 347)× | 10.0 (5.0, 17) | 10.0 (4.6, 16.5) |
| Somatic-affective | 8 (5, 13) | 8 (4, 13) |
| Cognitive | 2 (0, 4) | 1 (0, 5) |
| No Dep. (0-13) | 257 (63%) | 231 (67%) |
| Mild Dep. (14-19) | 66 (16%) | 43 (12%) |
| Mod. Dep. (20-28) | 47 (12%) | 48 (14%) |
| Severe Dep.(29+) | 36 (9%) | 25 (7%) |
| BDI-II (History Dep/No Dep)* | 14.0 (9, 24) vs. 9.0 (4, 14.8) | 12 (5, 22) vs. 9 (4, 15) |
| BDI-II (History Dep/No Dep) | 52% vs. 30% | 42% vs. 29% |
| PTSD | ||
| PCL-S (N:415, 361)× | 23 (19, 29) | 22 (19, 28) |
| Probable PTSD+ | 27 (7%) | 24 (7%) |
| Functional | ||
| ADLs | ||
| Katz ADL (N:428, 371)× | 0 (0, 1) N = 428 | 0 (0, 1) N = 374 |
| ≥ Partial disability± | 139 (32%) | 102 (27%) |
| ADLs (History Dis/No Dis)∞ | 77% vs. 27% | 55% vs. 22% |
| IADLs | ||
| FAQ (N:422, 372)× | 3 (0, 9) | 2 (0, 8) |
| Functional Disability§ | ||
| FAQ (History Dis./No Dis)£ | 9.5 (7.0, 16.8) vs. 2.2 (0.0, 8.0) | 10 (6.0, 14.0) vs. 2.0 (0.0, 7.0) |
| Functional Disability (History of Dis/No Dis)β | 56% vs. 23% | 62% vs. 20% |
| Quality of Life | ||
| SF-36 (N:416, 361)× | ||
| Mental Component | 56 (40, 62) | 55 (45,60) |
| Physical Component | 29 (22, 38) | 32 (25, 43) |
refers to the total N for the patients who completed these assessments at 3 and 12 months, reflecting the fact that a small percentage of patients did not complete every test in the battery at each follow-up time point.
BDI-II (History Dep/No Dep) refers to % with at least mild depression symptoms by baseline depression status versus no depression symptoms at baseline.
- Probable PTSD was defined as at least 1 positive symptom (defined as > 3 on a 5 point scale indicating being “moderately affected”) from the PCL-S questions mapping on to Criterion B, at least 3 positive symptoms mapping on to Criterion C, and at least 2 positive symptoms from items mapping onto Criterion D,
- At least partial disability in activities of daily living is defined as a score of >0 on 1 of 6 ADL items.
- ADLs (History Dis/No Dis) refers to % of individuals with at least partial disability among those with a baseline history of at least partial disability (dependence) vs. those with no disability (dependence).
- Functional disability was defined as score of >9 on the FAQ.
- FAQ (History Dis./No Dis) refers to FAQ scores by baseline functional status (disability vs. no disability)
- Functional Disability (History of Dis/No Dis) refers to % functional disability by baseline functional status (disability vs. no disability).
Figure. A Comparison of Mental Health and Functional Related Scores by Age at 3 and 12 Months.
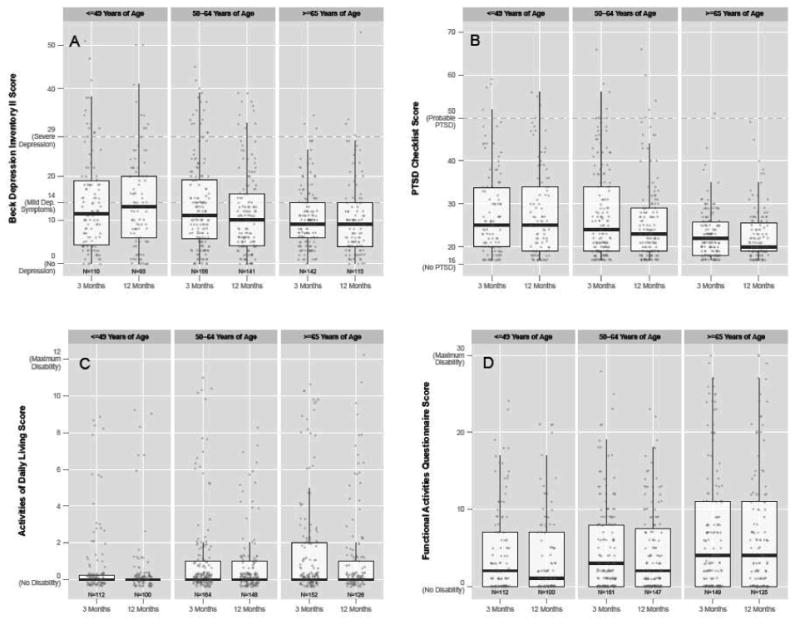
This figure displays scores on our 4 primary outcome measures separated by age group (≤49, 50-64, and ≥65 years): A) Beck Depression Inventory II (BDI-II), which assesses depressive symptoms; B) PTSD Checklist (PCL), which assesses symptoms of PTSD; C) Katz Activities of Daily Living (ADL), which assesses disability in activities of daily living; and D) Pfeffer Functional Activities Questionnaire (FAQ), which assesses disability in instrumental activities of daily living (IADLs). †Jittering was used to prevent overplotting of equivalent points at integer values.
Somatic versus Cognitive Depression Criteria
Even though somatic items account for only 21 of 63 points on the BDI-II, test scores on the BDI-II were much higher on the somatic than the cognitive/affective scale at 3 [8.0 (5.0, 13.0) vs. 2.0 (0.0 vs. 4.0)] and 12 months; [8.0 (4.0, 13.0) vs. 1.0 (0.0, 5.0)](Table 3). Among the 149 patients with at least mild depression at 3 months, 66% (99/149 patients) would have met criteria for depression based entirely on the 7 items comprising the somatic subscale, while only 8% (12/149 patients) would have met criteria based entirely on the cognitive-affective subscale. Among the 116 patients with at least mild depression at 12 months, 72% (83/116 patients) would have met depression criteria based only on the somatic items and 4% (5/116 patients) would have met criteria using only cognitive-affective items.
PTSD
At both 3- and 12-month follow-up, 7% (27/415 and 24/361) of individuals had probable critical illness-related PTSD, using PCL-S scoring criteria based on the DSM-IV (see footnote Table 1).25 Clinically significant elevations on the PCL-S were rare (Figure), with 3% (13/415) of respondents scoring >50 at 3 month follow-up and 3% (10/361) at 12 month follow-up (Figure).
Co-Morbid Depression and PTSD
A total of 6% of patients had symptoms of both depression and PTSD at both 3 (23/404) and 12 months (21/346) (BDI-II >13 and PCL-S DSM-IV defined criteria). Among patients with severe depression (BDI-II >29), 25% (9/36) had PTSD symptoms at 3 months and 42% (10/24) had PTSD symptoms at 12 months. Of the 251 patients without depression at 3 months, only two had symptoms of PTSD; at 12 months, only 1 patient out of 231 had PTSD without depression.
Functional Status (ADL and IADL) and Health-Related Quality of Life (HRQL)
At least partial ADL disability (>0 on the Katz ADL), was seen in 32% (139/428) and 27% (102/374) of individuals at 3 and 12 months (Table 3). Among those fully independent at enrollment, at least partial ADL disability was observed in 27% (98/367) and 22% (69/319) of patients at 3- and 12-month follow-up. IADL disability (>8 on the FAQ) was seen in 26% (108/422) and 23% (87/372) of individuals at 3 and 12 months. When comparing patients with and without baseline IADL disability, respectively, we found that 56% (19/34) vs. 23% (87/384) had IADL disability at 3 months follow-up. Similarly at 12 months, 62% (21/34) vs. 20% (66/333) had IADL disability among those with and without baseline IADL disability.
SF-36 PCS scores were approximately 2 standard deviations below the mean of the general US population of 50±10, whereas MCS scores were consistent with those reported by general US population (Table 3).
Age as a Risk Factor for Mental Health and Functional Outcomes
After adjusting for covariates, younger age was associated with a probability of higher scores (worse PTSD) on the PCL-S at 3 and 12-month follow-up (Table 4). Older age was independently associated with a probability of higher BDI-II scores at 12-month follow-up, higher ADL scores at 3-month follow-up, higher FAQ scores at 3 and 12 month follow-up, and lower SF-36 MCS scores at 12-month follow-up (Table 4). No association was observed between age and a variety of other outcomes/time points.
Table 4. Effect of Duration of Delirium on Mental Health and Functional Outcomes.
| Delirium: 25th percentile (0 delirium days) vs. 75th percentile (5 delirium days) | ||||
|---|---|---|---|---|
|
| ||||
| Outcome | 3 Mo F/Up | P Value | 12 Mo F/Up | P Value |
| BDI-II‡ | 1.63 (0.94 to 2.85) | 0.38 | 2.31 (1.25 to 4.27) | 0.05 |
| PCL-S‡ | 1.39 (0.78 to 2.47) | 0.59 | 1.92 (1.03 to 3.55) | 0.18 |
| Katz ADL‡ | 1.50 (0.63 to 3.57) | 0.18 | 0.63 (0.27 to 1.46) | 0.51 |
| FAQ‡ | 1.57 (0.89 to 2.77) | 0.35 | 1.84 (0.96 to 3.54) | 0.21 |
| SF-36 PCS‡ ‡ | 0.79 (-2.59 to 4.18) | 0.77 | -3.72 (-8.60 to 1.16) | 0.09 |
| SF-36 MCS‡ ‡ | -4.53 (-8.81 to -0.24) | 0.10 | -5.79 (-10.26 to -1.31) | 0.18 |
Definition of abbreviations: ADL = activities of daily living; BDI-II = Beck Depression Inventory – II; FAQ = Functional Activities Questionnaire; PCL-S = Posttraumatic Stress Disorder Checklist; SF-36 = Short Form-36
Results shown are from linear regression models in which outcome variables were scores on the BDI, PCL, Katz ADL, FAQ, and SF-36 PCS and MCS. The independent variables were duration of delirium and age and covariates were the following potential confounders, which were selected a priori: age, education level, Short IQCODE, 10 history of depression and other mental health illnesses, baseline ADL, baseline Charlson Comorbidities Index, cerebrovascular disease burden via the Framingham Stroke Risk Profile, mean modified SOFA score over ICU duration, duration of severe sepsis and coma, episodes of hypoxemia, and mean daily doses of benzodiazepines, propofol, dexmedetomidine, opiates and haloperidol.
Proportional Odds Logistic Regression (POLR): Proportional odds logistic regression (POLR) was used for the BDI-II, PCL, Katz ADL and FAQ models. Therefore, the point estimates indicate odds ratios (95% Confidence Intervals); here, they indicate the odds ratio between the 75th and 25th percentiles of the exposure in question, holding all other covariates constant. For example, a patient with 5 days of delirium (our 75th percentile) had, on average, 2.3 times the odds of a higher (worse) score on the BDI-II at 12-month follow-up than a patient with no days of delirium (our 25th percentile), assuming all other covariates were equal. Similarly, a 71-year-old patient (our 75th percentile) had, on average, 0.3 times the odds of a higher BDI-II score at 12 months than a 51-year-old patient (our 25th percentile).
Linear (note: not logistic) regression was used for the SF-36 PCS and MCS models; therefore, the point estimates (95% Confidence Intervals) indicate the average difference in SF-36 component scores for patients at the 75th percentile of the exposure compared to patients at the 25th percentile. For example, a patient with 5 days of delirium (our 75th percentile) had, on average, a 12-month SF-36 MCS score 5.8 points lower than a patient with no delirium (our 25th percentile).
Although p-values may indicate significance (and is correct), due to the use of nonlinear terms for continuous exposures, the comparison of the 25th and 75th percentiles may yield a point estimate with a confidence interval that crosses zero, or vice versa.
Delirium as a Risk Factor for Mental Health and Functional Outcomes
At 3 months, no significant associations were observed between delirium duration and depression, PTSD symptoms, ADL, IADLs, or quality of life after adjusting for covariates (Table 4). At 12-month follow-up, longer delirium duration was associated with higher BDI-II scores (i.e., greater depression) and lower SF-36 MCS scores (i.e., worse mental health status) after adjusting for covariates (Table 4). We found no consistent associations between delirium and any other 12 month outcomes, and at neither 3 nor 12 months did we find any consistent associations between sedative and analgesic medications and outcomes.
Discussion
This large prospective cohort investigation has yielded two main sets of observations related to mental health and functional outcomes in ICU survivors. First, we found that depression was 4 times more frequent than PTSD, and its symptoms were driven dramatically more often by somatic rather than cognitive components. Second, we found that patients in this large cohort (including a substantial number with no previous history of psychiatric treatment per proxy report) demonstrated the presence of mental health and functional deficits across the entire spectrum of age (although depression, functional disability and mental health quality of life were worse with older age), and we did not observe a consistent relationship between delirium and our long-term outcomes of interest.
Our investigation highlights the finding that depression is substantially more prevalent than PTSD after critical illness, although these conditions are admittedly frequently comorbid as reflected in the fact that in our cohort virtually everyone who had PTSD symptoms also suffered from depression. Our study also suggests that individuals with more severe expressions of depression in the early post-critical illness recovery period (3 months) tend to experience persistent depressive symptoms at 1 year, though symptom severity may decrease slightly. Given our data and the configuration of somatic symptoms (pervasive) versus cognitive-affective symptoms (uncommon) in this population of ICU survivors (an overall difference of at least 5 points on the BDI-II is clinically significant30 and the difference on somatic and cognitive-affective scales exceeded this), it appears that a significant portion of our “depressed” patients were manifesting symptoms of physical debility. Studies have shown that medically ill individuals with chronic physical problems are misdiagnosed as having depression when their complaints may be illness-driven.31 Our data support the hypothesis that survivors of critical illness, as in cardiac patients,32 largely experience depression through somatic complaints (this hypothesis has been previously unexplored in cohorts of survivors of critical illness, perhaps in part because investigators9,33,34 have frequently used the Hospital Anxiety and Depression Scale – HADS – which does not contain questions about somatic symptoms of depression). Literature to date4 has focused on risk factors and mechanisms of post-ICU depression mainly in the realm of inflammation or neurotransmitter imbalances with virtually no studies attempting to explicate roles of psychopathology versus physical pathology. These findings are of utmost importance in that somatic depression tends to be resistant to antidepressant medications, which are often frequently used both in the ICU and thereafter.35 The management of somatically depressed ICU survivors represents an untapped opportunity for non-pharmacological intervention through physical and occupational rehabilitation,36 although this is a hypothesis in need of further testing.
In the same way that critical illness may be a gateway to the development of dementia-like cognitive impairment11, these data support that critical illness is also a gateway to the develop of persistent physical disability that may be a driver of a closely-related somatic-type of depression in the young and the old. Physical disability, reflected in ADL deficits, has been studied widely in individuals after intensive care, but IADL functioning, particularly in general medical and surgical ICU populations has not.12,37 Using conservative criteria, which potentially underestimate disability rates, we found that a quarter of patients had Pfeffer FAQ scores suggestive of IADL disability and, indeed, the IADL scores in this cohort were significantly worse than individuals with early dementia.38
These findings challenge conventional thinking about the prevalence of PTSD. Early studies reported that PTSD occurred in 50%of ICU survivors and more recent reports suggest rates close to 20%,5 though a few studies done with general critically ill patients have described rates between 5% and 13%.39,40 We found a 7% rate of PTSD symptoms – lower than was has typically been reported, though this is not to diminish the importance of this malady in ICU survivors as it is still twice as high as the overall prevalence rates of 3% in the general population.27 Reasons for the discrepancy could include the larger sample size of our study and inclusion of general ICU patients, as opposed to just one category of ICU illness such as ARDS. Importantly, we identified individuals as having probable PTSD only if they met pertinent DSM-IV criteria (the definitive criteria employed at the time of this investigation). We also asked individuals about symptoms in reference to the trauma specific to intensive care and critical illness—an approach different than that used in most ICU studies, although there are a few investigations that have focused explicitly on the critical illness as the traumatic stressor of interest.39, 40 This approach, while effective in identifying individuals with PTSD symptoms due to critical illness related trauma, likely failed to capture the effects of critical illness on individuals with pre-existing PTSD due to other causes. Evidence suggests that for such individuals, the stress of intensive care and critical illness could exacerbate underlying symptoms. Substantial time and energy been invested in addressing PTSD in survivors of critical illness, yet our findings suggest that among mental health concerns, it is less pervasive than depression. These data suggest at a minimum that for ICU survivors, it is likely in the interest of public health to focus with added emphasis on the prevention and management of depression rather than so pointedly on PTSD, though these conditions often overlap considerably – particularly in the context of high symptom severity - and cannot be optimally studied, treated, or understood in isolation. Indeed, it may be individuals with both severe depression and PTSD who represent the group most pointedly in need of clinical intervention.
Evidence suggests that advanced age confers unique risks for the ability of older adults to recover from critical illness.41 Whereas older age was a risk factor for depression and functional disability, younger patients were not immune to these outcomes; we found that ADLs and IADLs, usually impaired predominantly in geriatric populations, were affected in both young and middle age patients. Thus, as previously reported among ARDS survivors,10 functional disability among survivors of critical illness is not only an “old age” problem. These findings have potential implications for those who develop ADL/IADL disability during “working years,” since it may adversely affect an individual's ability to hold a job or alter their vocational trajectory. Future research is needed to identify interventions that can effectively rehabilitate functional disability, enabling critical illness survivors to return to work and engage in many other activities that are prevented by these disabilities.
Our study had strengths, including reliance on a large, mixed ICU population, and the use of a comprehensive battery comprised on instruments with robust psychometric properties which have been used extensively with medical populations though in some cases their validity has not specifically been studied in survivors of critical illness. The BDI-II, for instance, has not been validated with general medical and general surgical ICU populations but has been validated in other medical cohorts (e.g. cardiac, HIV, chronic fatigue, and general medical patients)42 and we believe that findings from these validation studies – which highlight the possibility that the BDI-II may slightly overestimate depression prevalence due to its inclusion of somatic items – likely apply to critically ill populations as well.
Our study also had other limitations. We identified pre-existing depression and mental health by asking proxys whether the individual patient in question had ever been diagnosed with particular psychiatric conditions of interest and this approach may have resulted in an underestimation of the true prevalence of premorbid depression and related difficulties. It was not feasible to directly observe patient behaviors, thus we relied on self-report measures. In this cohort that has previously been reported as having high levels of newly-acquired cognitive impairment11, the duration of delirium was not associated with either disability outcome (i.e., IADL or ADL status), a finding in contrast to disability outcomes in populations with similar levels of cognitive impairment such as mild cognitive impairment and Alzheimer's disease.43 While in need of further study in survivors of critical illness, the difference in these outcomes could be explained by differing underlying pathologies between these syndromes, the potential for functional disability to be expressed differently in cognitively impaired ICU survivors than individuals with progressive dementia, the neuropsychiatric domains that are impaired, or simply that the data supporting an association between cognition and functional status are conflicting and in some cases modest.44 At least 2 studies in medically ill cohorts – one in ICU patients45 and one in patients following surgery46 – have demonstrated an association between delirium and ADL disability, in contrast to the current study. Reasons for this discrepancy are unclear, though in light of the findings from these two studies – one of them which was quite large46 – it is possible that the absence of an association between delirium and ADL and IADL functioning in the BRAIN-ICU study reflects a Type II error. Yet another limitation in our investigation is that patients had unplanned critical illnesses that did not allow pre-ICU testing thus, like other studies10 we relied both on patients and qualifying proxys.
Conclusion
Depression and PTSD are important mental health problems after significant critical illness, yet our findings reveal that depression is at least 4 times more common than PTSD and largely somatic in nature. This suggests that physical disability47 contributes predominantly to this depression, which has implications for scientific discovery related to the roles of physical rehabilitation versus anti-depressant medications in the prevention and management of post-ICU depression. Our data further indicate that monitoring these outcomes is relevant for young and old survivors, and that non-pharmacological interventions during the ICU stay36 previously found to improve physical outcomes in ICU survivors should be tested in terms of their ability to alter mental health and functional outcomes.
Research In Context
Systematic Review
No formal systematic review was conducted prior to the initiation of the BRAIN-ICU cohort study. However, we knew from our engagement with relevant literature over the previous decade that little if any evidence existed exploring the association between delirium and long-term mental health and functional outcomes, even as a dearth of information existed - particularly in the context of large cohort studies – related to the prevalence of depression, PTSD, and ADL and IADL functioning in survivors of critical illness. At the time of the BRAIN-ICU's inception, evidence from numerous investigations suggested that significant depression occurred in approximately a third of survivors of critical illness and that PTSD symptoms impacted between 15% and 50% of individuals following intensive care. None of these investigations focused on determining the relative prevalence of somatic versus cognitive depression symptoms and few evaluated PTSD using instruments oriented to DSM-based diagnostic criteria.
Interpretation
Our investigation represents one of the largest cohort studies to evaluate wide-ranging mental health and functional outcomes in survivors of critical illness and to explore delirium as a risk factor these outcomes over time. Our results show that depression is more common than PTSD after critical illness and more common than in the general population (where the prevalence of depression is estimated to be ∼ 10%) and that it is driven predominantly by somatic rather than cognitive symptoms. These results underscore the importance of addressing mental health difficulties and functional disabilities should be monitored across the age range and that physical and non-pharmacologic interventions may to impact depression symptoms in particular merit further study.
Table 5. Effect of Age on Mental Health and Functional Outcomes.
| Age: 25th percentile (51 years) vs. 75th percentile (71 years) | ||||
|---|---|---|---|---|
|
| ||||
| Outcome | 3 Mo F/Up | P Value | 12 Mo F/Up | P Value |
| BDI-II‡ | 0.52 (0.29 to 0.97) | 0.10 | 0.34 (0.17 to 0.69) | 0.005 |
| PCL-S‡ | 0.39 (0.21 to 0.74) | 0.02 | 0.43 (0.22 to 0.84) | 0.02 |
| Katz ADL‡ | 1.41 (0.63 to 3.16) | 0.01 | 3.94 (0.78 to 4.38) | 0.10 |
| FAQ‡ | 0.89 (0.49 to 1.62) | <0.0001 | 0.93 (0.47 to 1.81) | <0.0003 |
| SF-36 PCS‡ ‡ | 0.50 (-3.15 to 4.16) | 0.79 | -0.13 (-4.37 to 4.12) | 0.18 |
| SF-36 MCS‡ ‡ | 1.58 (-3.02 to 6.19) | 0.56 | 5.15 (0.42 to 9.89) | 0.04 |
Definition of abbreviations: ADL = activities of daily living; BDI-II = Beck Depression Inventory – II; FAQ = Functional Activities Questionnaire; PCL-S = Posttraumatic Stress Disorder Checklist; SF-36 = Short Form-36
Results shown are from linear regression models in which outcome variables were scores on the BDI, PCL, Katz ADL, FAQ, and SF-36 PCS and MCS. The independent variables were duration of delirium and age and covariates were the following potential confounders, which were selected a priori: age, education level, Short IQCODE, 10 history of depression and other mental health illnesses, baseline ADL, baseline Charlson Comorbidities Index, cerebrovascular disease burden via the Framingham Stroke Risk Profile, mean modified SOFA score over ICU duration, duration of severe sepsis and coma, episodes of hypoxemia, and mean daily doses of benzodiazepines, propofol, dexmedetomidine, opiates and haloperidol.
Proportional Odds Logistic Regression (POLR): Proportional odds logistic regression (POLR) was used for the BDI-II, PCL, Katz ADL and FAQ models. Therefore, the point estimates indicate odds ratios (95% Confidence Intervals); here, they indicate the odds ratio between the 75th and 25th percentiles of the exposure in question, holding all other covariates constant. For example, a patient with 5 days of delirium (our 75th percentile) had, on average, 2.3 times the odds of a higher (worse) score on the BDI-II at 12-month follow-up than a patient with no days of delirium (our 25th percentile), assuming all other covariates were equal. Similarly, a 71-year-old patient (our 75th percentile) had, on average, 0.3 times the odds of a higher BDI-II score at 12 months than a 51-year-old patient (our 25th percentile).
Linear (note: not logistic) regression was used for the SF-36 PCS and MCS models; therefore, the point estimates (95% Confidence Intervals) indicate the average difference in SF-36 component scores for patients at the 75th percentile of the exposure compared to patients at the 25th percentile. For example, a patient with 5 days of delirium (our 75th percentile) had, on average, a 12-month SF-36 MCS score 5.8 points lower than a patient with no delirium (our 25th percentile).
Although p-values may indicate significance (and is correct), due to the use of nonlinear terms for continuous exposures, the comparison of the 25th and 75th percentiles may yield a point estimate with a confidence interval that crosses zero, or vice versa.
Acknowledgments
Disclosure of Funding: This project was supported by the National Institutes of Health AG027472 and the Geriatric Research, Education and Clinical Center (GRECC), Department of Veterans Affairs Medical Center, Tennessee Valley Healthcare System, Nashville, TN.
Dr. Jackson is supported by the National Institute of Health AG 1K23AG031322. Dr. Pandharipande is supported by the VA Clinical Science Research and Development Service and by the National Institutes of Health HL111111, AG027472, and AG035117. Drs. Girard and Vasilevskis are supported by the National Institutes of Health AG034257 and AG040157, respectively. Dr. Brummel is supported by National Institutes of Health AG045095 and by the Vanderbilt Clinical and Translational Scholars program. Dr. Hughes received salary support from the Foundation for Anesthesia Education and Research Mentored Research Training Grant now from the National Institutes of Health HL111111. Dr. Ely is supported by the VA Clinical Science Research and Development Service and the National Institutes of Health AG027472, AG035117 and HL111111. Dr. Bernard is supported by National Institutes of Health HL087738-06, AG027472, AG035117 and HL111111. Dr. Dittus is supported by the National Institutes of Health AG027472, AG035117 and HL111111. Drs. Ely, Girard, Vasilevskis and Dittus are all supported by the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC), Nashville, Tennessee, USA.
Footnotes
Contributors: JCJ, PPP, TDG, AKS, GRB, RSD, and EWE designed the study. AKS and JLT analyzed the data. JCJ, PPP, TDG, AKS, JLT NEB, CGH, BP, EEV, AM, ROH, and EWE interpreted the data analysis. JCJ wrote the first draft of the manuscript; all the other authors reviewed the manuscript for intellectual content and approved the final draft.
Conflicts of Interest: Dr. Pandharipande has received research funding and honoraria for nonpromotional research purposes from Hospira Inc. Dr. Girard and Ms. Pun have received honoraria for nonpromotional research purposes from Hospira Inc. Dr. Ely has received honoraria from Hospira, Abbot, and Orion Pharma for nonpromotional research purposes. Dr. Hughes has received honoraria from Orion Pharma for nonpromotional research purposes. The other authors have no financial disclosures.
References
- 1.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38(1):65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2013. 2013 [Google Scholar]
- 3.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–9. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 4.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512–9. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. GenHospPsychiatry. 2008;30(5):421–34. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182(2):183–91. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus D, Musthafa AA, Clermonte G, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1389–94. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 8.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in severe sepsis: a prospective cohort study of older Americans. Am J Geriatr Psychiatry. 2013;21:887–97. doi: 10.1016/j.jagp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Depressive symptoms and impaired physical function after actue lung injury: a 2 year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 11.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svenningsen H. Associations between sedation, delirium and post-traumatic stress disorder and their impact on quality of life and memories following discharge from an intensive care unit. Dan Med J. 2013;60(4):B4630. [PubMed] [Google Scholar]
- 14.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–53. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 15.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24(4):637–9. [PubMed] [Google Scholar]
- 16.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–9. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) JAMA. 2001;286(21):2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–8. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Steer RA, Brown G. BDI-II depression inventory manual. New York: Harcourt Brace; 1996. [Google Scholar]
- 24.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist. Behaviour Research and Therapy. 1996;34:669–73. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE. SF-36 Physical and Mental Health Summary Scales: a user's manual. Boston: Health Assessment Lab, New England Medical Center; 1994. [Google Scholar]
- 26.Beck AT, Steer RA. Beck Depression Inventory Manual. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- 27.Diagnostic and statistical manual of mental disorders. 4th. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 28.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley Classics Library: John Wiley & Sons; 2004. [Google Scholar]
- 29.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 30.Hiroe T, Kojima M, Yamamoto I, Nojima, et al. Gradations of clinical severity and sensitivity to change assessed with the Beck Depression Inventory-II in Japanese patients with depression. Psychiatry Res. 2005;135(3):229–35. doi: 10.1016/j.psychres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Peavaler RCA, Rodin G. Depression in medical patients. BMJ. 2002;325(149) doi: 10.1136/bmj.325.7356.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.dJ P, J O, van Den Brink RHS, et al. Symptom Dimensions of Depression following Myocardial Infarction and Their Relationship with Somatic Health Status and Cardiovascular Prognosis. Am J Psychiatry. 2006;163(1):138–44. doi: 10.1176/appi.ajp.163.1.138. [DOI] [PubMed] [Google Scholar]
- 33.Sukantarat KT, Williamson RC, Brett SJ. Psychological assessment of ICU survivors: a comparison between the Hospital Anxiety and Depression scale and the Depression, Anxiety and Stress scale. Anaesthesia. 2007;62(3):239–43. doi: 10.1111/j.1365-2044.2006.04948.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones C, Skirrow P, Griffiths RD, et al. Rehabiliation after critical illness: a randomized, controlled trial. Crit Care Med. 2003;31(10):2456–2461. doi: 10.1097/01.CCM.0000089938.56725.33. [DOI] [PubMed] [Google Scholar]
- 35.Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47(5):399–407. doi: 10.1176/appi.psy.47.5.399. [DOI] [PubMed] [Google Scholar]
- 36.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183(8):1037–42. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown PJ, Devanand DP, Liu X, Caccappolo E. Alzheimer's Disease Neuroimaging I. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68(6):617–26. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones C, Backman C, Capuzzo M, et al. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised controlled trial. Crit Care. 2010;14(5):R168. doi: 10.1186/cc9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones C, Backman C, Capuzzo M, et al. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med. 2007;33:978–985. doi: 10.1007/s00134-007-0600-8. [DOI] [PubMed] [Google Scholar]
- 41.Somme D, Maillet JM, Gisselbrecht M, Novara A, Ract C, Fagon JY. Critically ill old and the oldest-old patients in intensive care: short- and long-term outcomes. Intensive Care Med. 2003;29(12):2137–43. doi: 10.1007/s00134-003-1929-2. [DOI] [PubMed] [Google Scholar]
- 42.Yuan-Pang W, Gorenstein I. Assessment of depression in medical patients: A systematic review of the utility of the Beck Depression Inventory-II. Clincs (Sao Paulo) 2013;68(9):1274–1287. doi: 10.6061/clinics/2013(09)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hesenberg K, Bentzen H, Ranhoff AH, et al. Disability in instrumental activities of daily living in elderly patients with mild cognitive impairment and Alzheimer's disease. Dement Geriatr Cogn Disord. 2013;36(3-4):146–53. doi: 10.1159/000351010. R57. [DOI] [PubMed] [Google Scholar]
- 44.Royall DR, Lauterbach EC, Kaufer D, et al. The cognitive correlates of functional status: a review from the committee on research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19(3):249–65. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 45.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–77. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abelha FJ, Luis C, Veiga D, et al. Outcome and quality of life in patients with postoperative delirium during and ICU stay following major surgery. Critical Care. 2013;17 doi: 10.1186/cc13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–41. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]


