Abstract
Preeclampsia affecting 3-5% of all pregnancies is a major cause of maternal and perinatal morbidity and mortality worldwide. This disorder is characterized by a constellation of signs and symptoms, most notably new onset hypertension and proteinuria during the last trimester of pregnancy. In this review, the molecular mechanisms of preeclampsia with an emphasis on the role of circulating anti-angiogenic proteins in the pathogenesis of preeclampsia and its complications will be discussed.
Keywords: preeclampsia, angiogenesis, cardiovascular disease, proteinuria
Introduction
Preeclampsia, that affects 3-5% of all pregnancies is defined as new onset hypertension and proteinuria occurring after 20 weeks gestation 1. Other features of the preeclampsia syndrome include seizures (eclampsia), thrombocytopenia, elevated transaminases, and microangiopathic hemolytic anemia (HELLP syndrome) 2. Neonatal complications of preeclampsia include preterm delivery, fetal growth restriction, hypoxia-related neurologic injury, perinatal death, and long-term cardiovascular morbidity associated with low birthweight 1. Delivery of the placenta is the only known treatment at this time, suggesting that this is a placental disease 3.
While edema is often noted in preeclampsia, it is not very specific for the disease. Moreover, there is evidence that even with patients who develop eclampsia before or after 32 weeks gestation, there are significant number of patients who do not develop edema 4. Maternal and perinatal outcomes are better in patients with mild disease developing after 36 weeks’ gestation, but in patients with who develop the disease prior to 33 weeks have a higher maternal and perinatal morbidity and mortality2, 1. Mortality increases with maternal age for both preeclampsia and eclampsia, and black women were 3.1 times more likely to die from preeclampsia or eclampsia than white women 5.
Factors that increase the risk for preeclampsia development in a woman include prior history of preeclampsia, chronic hypertension, chronic kidney disease, pregestational diabetes, multiple gestations, obesity, and age >40 years old 6. Other factors include a partner who fathered preeclamptic pregnancy with another woman, woman born as small for gestational age, and adverse outcomes in a previous pregnancy 7. Women with a history of preeclampsia also develop cardiovascular disease later in their life. In this review, we will summarize the molecular mechanisms of preeclampsia and its related complications.
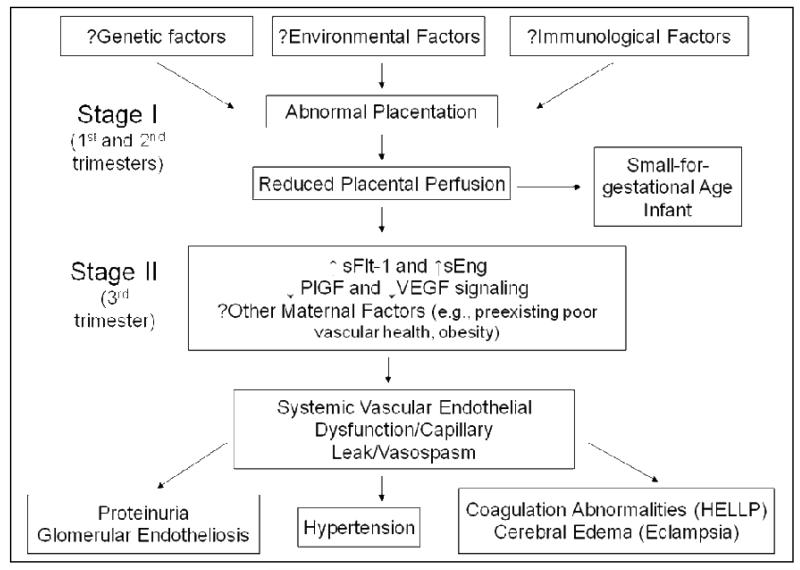
Pathogenesis of Preeclampsia (see Figure 1 for summary)
Figure 1.
Summary of the Pathogenesis of Preeclampsia
The placenta is the central to the pathogenesis of preeclampsia. Preeclampsia occurs only in the presence of the placenta, even when there is no fetus (as in hydatidiform mole) and usually remits when the placenta is delivered. Pathology specimens have shown placental infarcts, likely due to ischemia and occlusion of the spiral arteries. The physiological vascular remodeling of spiral arteries by trophoblasts does not occur in preeclampsia8. At the cellular level, cytotrophoblasts undergo pseudovasculogenesis by switching their adhesion molecules to mimic those of vascular cells 9. In preeclampsia, this process does not occur appropriately, and therefore invasion into the spiral arteries is incomplete. Various pathways including deficient heme oxygenase expression, genetic factors, oxidative stress, immune factors such as angiotensin receptor autoantibodies or altered natural killer cell signaling and, more recently deficient catechol-O-methyl transferase or deficient corin enzymes have been all proposed to have key roles in inducing placental disease 10-15. However it is not known whether any one of more of these pathways play a casual role in mediating the abnormal placentation noted in humans with preeclampsia. It is currently believed that abnormal placentation occurs early in pregnancy and that this leads to placental ischemia (Stage I). The ischemic placenta is thought to secrete soluble factors during the third trimester that in turn induces systemic endothelial dysfunction and the maternal syndrome of preeclampsia (Stage II)16.
The maternal syndrome and clinical features of preeclampsia are unified by the presence of systemic endothelial dysfunction and microangiopathy, in which the target organ may be the brain (seizures or eclampsia), liver (HELLP syndrome), or kidney (glomerular endotheliosis and proteinuria)17. Recent studies by our group and others have led to an extremely plausible hypothesis that the clinical manifestations of preeclampsia result, in part, from an imbalance between circulating pro-angiogenic and anti-angiogenic factors in the maternal circulation 18-25. The two placental derived circulating anti-angiogenic factors that have received greatest attention are soluble vascular endothelial growth factor 1 (sVEGFR1) (also referred to as soluble fms-like tyrosine kinase 1 or sFlt1) and soluble endoglin (sEng) whose levels are elevated in women with preeclampsia, while the pro-angiogenic proteins, whose circulating concentrations (free levels) are reduced in women with the disease are vascular endothelial and placental growth factors (VEGF, PlGF) 6. sFlt1 is an endogenous soluble anti-angiogenic protein that acts by binding pro-angiogenic proteins – VEGF and PlGF. Soluble endoglin is another anti-angiogenic protein that is thought to act by disrupting transforming growth factor beta signaling in the vasculature 6. Overexpression of sFlt1 and sEng in pregnant rats appear to induce severe preeclampsia-like syndrome including severe hypertension, nephrotic range proteinuria, glomerular endotheliosis thrombocytopenia, and fetal growth restriction25. The renal manifestations of preeclampsia can be explained almost entirely by excess sFlt1 with accompanying loss of VEGF actions in the glomeruli, as genetic deficiencies of VEGF in mice also lead to glomerular endotheliosis, the classic histological lesion of preeclampsia26. Loss of endothelial fenestrae due to lack of glomerular VEGF signaling leads to significant reduction in glomerular filtration rate and renal failure in preeclampsia 27. Overexpression of sFlt1 and sEng in rodents also has been shown to induce focal vasospasm, hypertension, increased vascular permeability, and cerebral edema that resembles the reversible posterior leucoencephalopathy of human eclampsia 28. Interesting several anti-angiogenic compounds (anti-VEGF antibodies) used as part of cancer chemotherapy to treat tumor related angiogenesis is also been associated with preeclampsia/eclampsia like changes such as hypertension, proteinuria and reversible posterior leucoencephalopathy 29-32. While there a number of signaling pathways downstream of anti-angiogenic factors, increased endothelin-1 (ET1) signaling may be a critical pathway that mediates sFlt1 induced endothelial dysfunction 33.
Placental syncytiotrophoblasts and in particular syncytial knots were identified as a major source of sFlt1 and soluble endoglin production 34, 35. Syncytial knots are induced by placental hypoxia and are noted predominantly in preeclamptic placentas. Syncytial knots have been shown to release aggregates into the maternal circulation suggesting an additional source of increased sFlt1 in the maternal blood besides secretion by the placenta 35. It has been suggested that shed syncytial aggregates get trapped in the capillary beds of lung tissue, where they further undergo disaggregation or apoptosis/necrosis to release the smaller microparticles into the systemic circulation. The relative contribution of these processes to the formation of trophoblast microparticles within the maternal circulation remains to be determined. .
Epidemiologic studies have revealed that blood levels of angiogenic proteins (sFlt1, PlGF and sEng) are altered in women with preeclampsia both during and prior to clinical signs and symptoms of the disease, consistent with a pathogenic role for these angiogenic factors in preeclampsia 21, 22, 24, 36-38. In addition, several studies have demonstrated that the alterations in circulating angiogenic factors may explain a number of risk factors for preeclampsia such as multiple gestation, trisomy 13, nulliparity and molar pregnancies 39-43. More recent data suggests that alterations in sFlt1, PlGF, and sEng in women with preeclampsia correlate with maternal vascular dysfunction as measured by flow mediate vasodilation and uterine artery pulsatility index24. In addition, alterations in these angiogenic factors have been found in complications of preeclampsia. In preeclampsia-associated placental abruption, sFlt1, PlGF, and sEng levels have all been shown to be altered 44, 45. In eclampsia, sFlt1, PlGF, and sEng are also altered to a similar degree as in patients with severe preeclampsia, reiterating synergistic role of these factors in both of these conditions 46. In a prospective study of pregnant women, patients who develop preeclampsia had higher levels of sFlt1, higher sEng, and lower PlGF but these findings were not seen in patients with gestational hypertension. Those patients that developed gestational hypertension, but not preeclampsia, were found to have higher brachial flow-mediated dilatation, suggesting a more hyperdynamic circulation as compared to patients with preeclampsia 24. These findings suggest that the pathophysiologies of these two diseases are fundamentally different, but that there may be some overlap with risk factors for patients who are likely to develop these diseases. Finally, other predisposing factors such as obesity and chronic hypertension may sensitize the maternal vascular endothelium to the anti-angiogenic effects of sFlt1 and sEng and thus lower threshold to develop preeclampsia. Clinical studies support this hypothesis as obese women tend to have lower circulating sFlt1 and sEng 21, 22.
Clinical Implications for Diagnosis and Treatment of Preeclampsia
A number of recent studies have suggested that circulating angiogenic factors in plasma or urine can be used to differentiate preeclampsia from other diseases that mimic preeclampsia such as chronic hypertension, gestational hypertension, lupus nephritis and gestational thrombocytopenia 47-56. To demonstrate clinical utility, we prospectively studied the role of angiogenic biomarkers in the prediction of preeclampsia related adverse outcomes among women evaluated at our institution for suspected preeclampsia. We found that the plasma sFlt1/PlGF ratio on presentation predicts adverse maternal and perinatal outcomes (occurring within two weeks) in the preterm setting. This simple, quantitative, rapid test outperformed the standard battery of clinical diagnostic measures including blood pressure, proteinuria, uric acid, and other laboratory assays 57. Importantly, sFlt1 and/or PlGF levels at presentation were strongly associated with the remaining duration of pregnancy 53, 57-60. We also recently evaluated the role of sEng measurements and found that it has comparable performance to sFlt1/PlGF ratio61. Interestingly, a number of patients with preeclampsia, particularly those presenting late in pregnancy present with no angiogenic factor abnormalities62. Whether these patients are misclassified as preeclampsia or if they truly represent a benign variant of preeclampsia remain unknown. In summary, current evidence supports the hypothesis that circulating angiogenic factors are useful in risk stratification of women with preeclampsia to predict development of complications.
Animal studies have suggested that sFlt1 ligands such as VEGF or PlGF itself can safely ameliorate preeclampsia 63-65. In addition, compounds that upregulate pro-angiogenic factors such as statins have been used to ameliorate preeclampsia in animal models 66. A clinical proof-of-concept trial to test the effects of pravastatin (that does not cross the placenta) in preeclampsia is ongoing 67. Extracorporeal apheresis to lower circulating sFlt1 has also been attempted as a treatment modality in women with preeclampsia. In exciting studies, Thadhani et al using dextran sulfate apheresis have been able to extend three preeclamptic pregnancies by 2-4 weeks, all of which resulted in healthy deliveries with no neonatal or maternal morbidity 68. During the course of the treatment, the extracorporeal adsorption device only lowered soluble sFlt1 levels by 30% on average, validating the idea that just partially lowering circulating sFlt1 levels is sufficient to successfully prolong preeclamptic pregnancies. Experimental studies suggest that the hormone relaxin may also be used to ameliorate preeclampsia by improving vascular compliance and upregulating VEGF locally69. A phase I study to test the safety of human relaxin in women with preeclampsia has recently been completed70.
Long-term Complications of Preeclampsia
As endothelial dysfunction is a hallmark of preeclampsia, it is not surprising that the long-term sequelae in women with a history of preeclampsia are centered around cardiovascular complications such as hypertension, ischemic heart disease and stroke. Women with a history of a preeclampsia are several times more likely to die from cardiovascular disease (CVD) 71, 72. This risk is greatest among women who have a history of preterm preeclampsia or preeclampsia that was complicated with a growth-restricted fetus73, 74. It is not know whether preeclampsia directly causes CVD or merely unmask subclinical CVD risk. Recent study of CVD risk factors present before and after pregnancy suggests that nearly half of the elevated risk for future hypertension after preeclampsia can be explained by pre-pregnancy risk factors75. Therefore, pregnancy may be viewed as a stress test that can reveal subclinical CVD phenotypes long before overt disease. Small studies have shown that levels of sFlt-1 remained higher in women with a history of preeclampsia in the post-partum period 76, 77 Whether a persistent anti-angiogenic milieu in the post-partum period may contribute to lasting endothelial dysfunction and an elevated risk of CVD in women remains unknown. Recent studies also suggest that preeclampsia is a major risk factor for peripartum cardiomyopathy and that imbalances in angiogenic factors may be critical for both disorders 78. Babies born to preeclampsia are also at risk for pulmonary hypertension in the long term 79.
Epidemiologic studies have also shown an increased risk for end-stage renal disease (ESRD) in women with a history of preeclampsia 80, 81. Although the relative risk for ESRD in women with preeclampsia is robust with hazard ratios ranging from 4-12, the absolute risk is still quite low. A recent meta-analysis suggested there is at least four times increased risk of microalbuminuria in women following preeclampsia, possibly due to persistent kidney damage after preeclampsia 82. Other studies have also suggested that familial factors may not be responsible for the development of chronic kidney disease following preeclampsia 83. A small pilot study suggested that angiotensin II sensitivity was noted in the post-partum period in women with a history of preeclampsia 76. However, it is not known whether the increased angiotensin II sensitivity contributes to the development of hypertension and/or chronic renal disease.
Conclusion
In summary, current evidence suggests that placental derived anti-angiogenic factors such as sFlt-1 and sEng play a role in the development of the maternal syndrome of preeclampsia. The identification and characterization of circulating anti-angiogenic factors in preeclampsia syndrome has allowed a better understanding of not only the pathogenesis, but has raised hope that this research area will lead to early identification and specific therapies in the immediate future. More work is needed to further define the regulation of placenta vascular development and expression of these angiogenic factors in normal and in preeclamptic pregnancies. Following pregnancy, women with preeclampsia have a higher risk for developing hypertension, renal disease and CVD in future years. Although the precise mechanisms for these long term vascular complications among women with preeclampsia are not known, these patients should be assessed yearly for the development of hypertension and/or proteinuria and managed appropriately to reduce the risk for CVD and ESRD.
Clinical Summary.
Increased levels of circulating anti-angiogenic proteins contribute to the pathogenesis of the maternal syndrome
Targeting anti-angiogenic proteins may safely ameliorate preeclampsia and prolong pregnancy, however randomized trials are still needed
Preeclampsia is associated with hypertension and cardiovascular disease in later life
Footnotes
Disclosures
Dr. Karumanchi is a co-inventor of multiple patents related to angiogenic proteins for the diagnosis and therapy of preeclampsia. These patents have been licensed to multiple companies. Dr. Karumanchi reports having served as a consultant to Roche and Beckman Coulter and has financial interest in Aggamin LLC. Dr. Naljayan reports no conflicts.
References
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 2.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American journal of obstetrics and gynecology. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 3.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annual review of pathology. 2010;5:173–92. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 4.Mattar F, Sibai BM. Eclampsia. VIII. Risk factors for maternal morbidity. American journal of obstetrics and gynecology. 2000;182:307–12. doi: 10.1016/s0002-9378(00)70216-x. [DOI] [PubMed] [Google Scholar]
- 5.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstetrics and gynecology. 2001;97:533–8. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 6.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–69. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton JR, Sibai BM. Prediction and prevention of recurrent preeclampsia. Obstetrics and gynecology. 2008;112:359–72. doi: 10.1097/AOG.0b013e3181801d56. [DOI] [PubMed] [Google Scholar]
- 8.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by smallfor-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–64. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–97. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y, Wang W, Dong N, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–50. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiby SE, Walker JJ, O’Shaughnessy KM, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–65. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanasaki K, Palmsten K, Sugimoto H, et al. Deficiency in catechol-Omethyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–21. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 14.Zhou A, Carrell RW, Murphy MP, et al. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–11. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou CC, Zhang Y, Irani RA, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–62. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 17.Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. Preeclampsia: a renal perspective. Kidney Int. 2005;67:2101–13. doi: 10.1111/j.1523-1755.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–91. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 19.Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–7. doi: 10.1016/j.ajog.2004.03.043. discussion 7-50. [DOI] [PubMed] [Google Scholar]
- 20.Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. The New England journal of medicine. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 22.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. The New England journal of medicine. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 23.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–87. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 25.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 26.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lafayette RA, Druzin M, Sibley R, et al. Nature of glomerular dysfunction in pre-eclampsia. Kidney international. 1998;54:1240–9. doi: 10.1046/j.1523-1755.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- 28.Maharaj AS, Walshe TE, Saint-Geniez M, et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. The New England journal of medicine. 2008;358:1129–36. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. The New England journal of medicine. 2006;354:980–2. doi: 10.1056/NEJMc052954. discussion -2. [DOI] [PubMed] [Google Scholar]
- 31.Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. The New England journal of medicine. 2006;354:980–2. discussion -2. [PubMed] [Google Scholar]
- 32.Patel TV, Morgan JA, Demetri GD, et al. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. Journal of the National Cancer Institute. 2008;100:282–4. doi: 10.1093/jnci/djm311. [DOI] [PubMed] [Google Scholar]
- 33.Li F, Hagaman JR, Kim HS, et al. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol. 2012;23:652–60. doi: 10.1681/ASN.2011040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sela S, Itin A, Natanson-Yaron S, et al. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res. 2008;102:1566–74. doi: 10.1161/CIRCRESAHA.108.171504. [DOI] [PubMed] [Google Scholar]
- 35.Rajakumar A, Cerdeira AS, Rana S, et al. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension. 2012;59:256–64. doi: 10.1161/HYPERTENSIONAHA.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsatsaris V, Goffin F, Munaut C, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–63. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 37.Thadhani R, Mutter WP, Wolf M, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–5. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silasi M, Rana S, Powe C, et al. Placental expression of angiogenic factors in Trisomy 13. Am J Obstet Gynecol. 2011;204:546–e1-4. doi: 10.1016/j.ajog.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 40.Mijal RS, Holzman CB, Rana S, Karumanchi SA, Wang J, Sikorskii A. Midpregnancy levels of angiogenic markers in relation to maternal characteristics. Am J Obstet Gynecol. 2011;204:244–e1. doi: 10.1016/j.ajog.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanter D, Lindheimer MD, Wang E, et al. Angiogenic dysfunction in molar pregnancy. Am J Obstet Gynecol. 2010;202:184–e1. doi: 10.1016/j.ajog.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faupel-Badger JM, Wang Y, Karumanchi SA, et al. Associations of pregnancy characteristics with maternal and cord steroid hormones, angiogenic factors, and insulin-like growth factor axis. Cancer Causes Control. 2011;22:1587–95. doi: 10.1007/s10552-011-9835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bdolah Y, Lam C, Rajakumar A, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198:428–e1-6. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- 44.Signore C, Mills JL, Qian C, et al. Circulating angiogenic factors and placental abruption. Obstet Gynecol. 2006;108:338–44. doi: 10.1097/01.AOG.0000216014.72503.09. [DOI] [PubMed] [Google Scholar]
- 45.Signore C, Mills JL, Qian C, et al. Circulating soluble endoglin and placental abruption. Prenat Diagn. 2008;28:852–8. doi: 10.1002/pd.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaisbuch E, Whitty J, Hassan S, et al. Circulating Angiogenic and Anti-Angiogenic Factors in Women with Eclampsia. Am J Obstet Gynecol. 2010 doi: 10.1016/j.ajog.2010.08.049. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buhimschi CS, Norwitz ER, Funai E, et al. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol. 2005;192:734–41. doi: 10.1016/j.ajog.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 48.Qazi U, Lam C, Karumanchi SA, Petri M. Soluble Fms-like tyrosine kinase associated with preeclampsia in pregnancy in systemic lupus erythematosus. J Rheumatol. 2008;35:631–4. [PubMed] [Google Scholar]
- 49.Rolfo A, Attini R, Nuzzo AM, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney international. 2013;83:177–81. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 50.Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 2007;197:28–e1. doi: 10.1016/j.ajog.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Sunderji S, Gaziano E, Wothe D, et al. Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: a prospective clinical study. Am J Obstet Gynecol. 2010;202:40–e1-7. doi: 10.1016/j.ajog.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161–e1-e11. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58–e1-8. doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 54.Young B, Levine RJ, Salahuddin S, et al. The use of angiogenic biomarkers to differentiate non-HELLP related thrombocytopenia from HELLP syndrome. J Matern Fetal Neonatal Med. 2010;23:366–70. doi: 10.1080/14767050903184207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levine RJ, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 56.Perni U, Sison C, Sharma V, et al. Angiogenic Factors in Superimposed Preeclampsia: A Longitudinal Study of Women With Chronic Hypertension During Pregnancy. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.111.181735. [DOI] [PubMed] [Google Scholar]
- 57.Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–9. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187–207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651–7. doi: 10.3109/14767058.2012.713055. [DOI] [PubMed] [Google Scholar]
- 60.Sibiude J, Guibourdenche J, Dionne MD, et al. Placental growth factor for the prediction of adverse outcomes in patients with suspected preeclampsia or intrauterine growth restriction. PLoS One. 2012;7:e50208. doi: 10.1371/journal.pone.0050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rana S, Cerdeira AS, Wenger J, et al. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS One. 2012;7:e48259. doi: 10.1371/journal.pone.0048259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powers RW, Roberts JM, Plymire DA, et al. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension. 2012;60:239–46. doi: 10.1161/HYPERTENSIONAHA.112.191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–5. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Zhang Y, Ying Ma J, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–92. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki H, Ohkuchi A, Matsubara S, et al. Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension. 2009;54:1129–35. doi: 10.1161/HYPERTENSIONAHA.109.134668. [DOI] [PubMed] [Google Scholar]
- 66.Kumasawa K, Ikawa M, Kidoya H, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A. 2011;108:1451–5. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed A. New insights into the etiology of preeclampsia: identification of key elusive factors for the vascular complications. Thromb Res. 2011;127(Suppl 3):S72–5. doi: 10.1016/S0049-3848(11)70020-2. [DOI] [PubMed] [Google Scholar]
- 68.Thadhani R, Kisner T, Hagmann H, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124:940–50. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]
- 69.McGuane JT, Danielson LA, Debrah JE, Rubin JP, Novak J, Conrad KP. Angiogenic growth factors are new and essential players in the sustained relaxin vasodilatory pathway in rodents and humans. Hypertension. 2011;57:1151–60. doi: 10.1161/HYPERTENSIONAHA.110.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unemori E, Sibai B, Teichman SL. Scientific rationale and design of a phase I safety study of relaxin in women with severe preeclampsia. Ann N Y Acad Sci. 2009;1160:381–4. doi: 10.1111/j.1749-6632.2009.03838.x. [DOI] [PubMed] [Google Scholar]
- 71.Funai EF, Friedlander Y, Paltiel O, et al. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–15. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- 72.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ (Clinical research ed. 2001;323:1213–7. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 74.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56:166–71. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in Pregnancy and Later Cardiovascular Risk. Common Antecedents? Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 76.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 2010;55:1239–45. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolf M, Hubel CA, Lam C, et al. Preeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89:6239–43. doi: 10.1210/jc.2004-0548. [DOI] [PubMed] [Google Scholar]
- 78.Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jayet PY, Rimoldi SF, Stuber T, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–94. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 80.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. The New England journal of medicine. 2008;359:800–9. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 81.Wang IK, Muo CH, Chang YC, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013 doi: 10.1503/cmaj.120230. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. 2010;55:1026–39. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 83.Vikse BE, Irgens LM, Karumanchi SA, Thadhani R, Reisaeter AV, Skjaerven R. Familial factors in the association between preeclampsia and later ESRD. Clin J Am Soc Nephrol. 2012;7:1819–26. doi: 10.2215/CJN.01820212. [DOI] [PMC free article] [PubMed] [Google Scholar]