Abstract
Domestication is an ongoing process continuously changing the lives of animals and humans and the environment. For the majority of European cattle (Bos taurus) genetic and archaeozoological evidence support initial domestication ca. 11'000 BP in the Near East from few founder aurochs (Bos primigenius) belonging to the mitochondrial DNA T macro-haplogroup. Gene flow between wild European aurochs of P haplogroup and domestic cattle of T haplogroup, coexisting over thousands of years, appears to have been sporadic. We report archaeozoological and ancient DNA evidence for the incorporation of wild stock into a domestic cattle herd from a Neolithic lake-dwelling in Switzerland. A complete metacarpus of a small and compact adult bovid is morphologically and genetically a female. With withers height of ca. 112 cm, it is comparable in size with small domestic cattle from contemporaneous sites in the area. The bone is directly dated to 3360–3090 cal BC and associated to the Horgen culture, a period of the secondary products revolution. The cow possessed a novel mtDNA P haplotype variant of the European aurochs. We argue this is either a single event or, based on osteological characteristics of the Horgen cattle, a rare instance of intentional breeding with female aurochs.
It is becoming increasingly clear, that the processes of domestication are more complex than previously thought1,2,3. Genetic signatures of these past human-animal relationships may be lost or blurred in modern livestock, but can be directly detected by ancient DNA analyses in a chronological and spatial setting2,4,5,6. The aurochs, roaming large parts of Eurasia and North Africa7, was the largest animal to be domesticated with withers height between ca. 132 cm (small female) and 189 cm (large males)8,9,10. In the archaeological record, large and robust bones signify the presence of wild cattle, however, due to pronounced sexual dimorphism in bovids, morphological identification is ambiguous because small female aurochs overlap with robust male domestic cattle8. Moreover, the sizes of aurochs vary depending on geographical and chronological setting. Animals tend to be smaller in the southern regions and younger prehistoric periods of Europe11. Ancient DNA surveys of the maternally inherited mitochondrial DNA (mtDNA) of European aurochs revealed haplogroups P and E present in Northern/Central Europe12,13, and aurochs of haplogroup T coexisting with P types in Italy14,15. Traces of female aurochs inheritance were detected in very few modern cattle through haplogroups P, R, Q16. Gene flow between wild and domestic cattle was a rare event2. The occasional archaeogenetic evidence of crossbreeding, namely the identification of morphological aurochs carrying the T haplotype, has been rejected on the plausible identification problems associated with fragmented remains12. Yet, the incorporation of wild animals into domestic stocks has been argued, for example, for pigs2,17,18 and horses19.
Results
Presented here are metric analyses and ancient DNA typing of mtDNA d-loop and allosomic zinc finger gene (zfx/zfy) of a complete left metacarpus from a Bovidae. The bone (Inv. Number 2048.1) is part of a large assemblage of slaughter or kitchen waste8, excavated at Twann, Lake Biel, Switzerland (Twann Bahnhof), one of the famous Neolithic lake shore settlements in the Circum-Alpine area (Fig. 1, Supplementary note). It is associated with the uppermost layer (OH) of the Horgen culture, dated by dendrochronology to 3093–3072 BC20. The chronological position is confirmed by direct AMS-14C-dating of the bone to 3360–3090 cal BC (ETH-42968: 94.5%)21,22 (OxCal version v3.10).
Figure 1. The site.
Location of Twann in Switzerland. Map was created with ArcGIS version 10 using SRTM data.
Archaeozoological status identification as small domestic cow
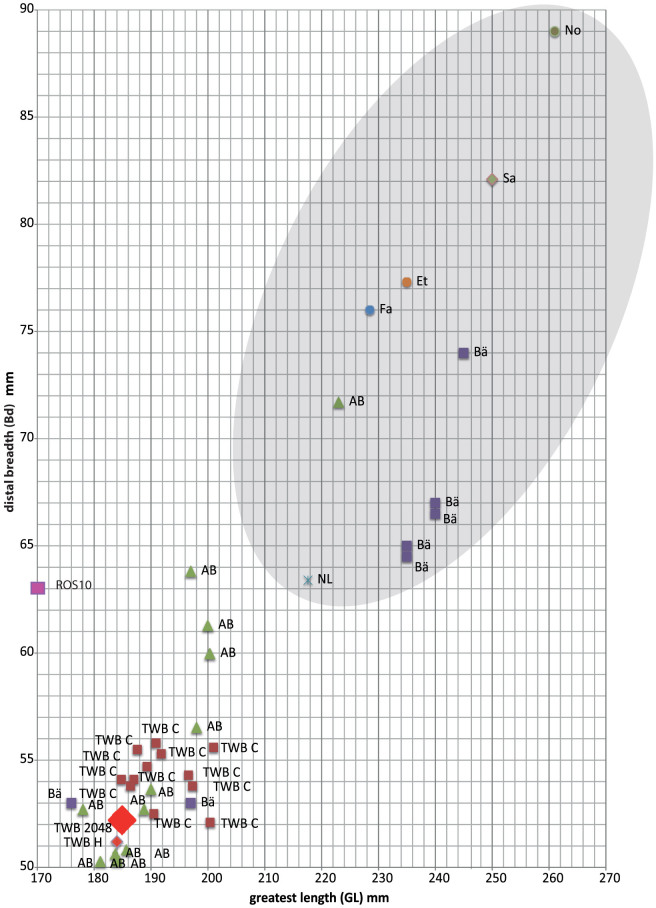
The bone belonged to an adult (older than 2 years) – the distal end is fully fused, the epiphyseal line is not visible – and does not show any pathologies (Fig. 2). The measurements taken according to von den Driesch23 are: GL 184.9; Ll 181.1; Bp 52.6; SD 28.2; DD 17.4; Bd 52.2. These measurements are in accordance with an archaeozoological status assignment to fully domestic female cattle. The withers height calculated for the cow is 111.8 cm24, which is the size of small modern cattle breeds such as the alpine Raetian Grey. A comparison of greatest length (GL) and distal breath (Bd) from complete cattle and aurochs metacarpi of contemporaneous sites in the area with metacarpus “2048.1” places the bone into the group of small domestic cattle (Fig. 3). Morphologically, the bone clearly lies outside the size of any known aurochs specimen. It should be noted, however, that findings of complete long bones are extremely rare in the archaeological record.
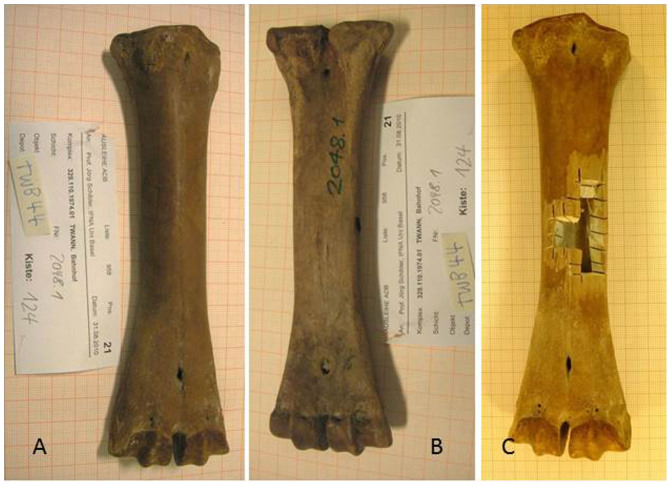
Figure 2. The metacarpus sinister 2048.1 from Twann Bahnhof.
(A): front, (B): back, (C): after sampling for genetic analysis.
Figure 3. Measurements of contemporary metacarpi from domestic and wild cattle from selected Swiss Neolithic sites and others.
Greatest length (GL) and distal breath (Bd) of complete metacarpi from: AB = Arbon-Bleiche 3 (Horgen culture: 3384–3370 BC); TWB C = Twann (Cortaillod culture: 3838–3532 BC); TWB 2048; TWB H = Twann (Horgen culture 3360–3090 cal BC)38; BA = Burgäschisee-Süd (Cortaillod culture: 3820–3680 BC); Ros 10 = Rosenhof 10 (4770 ± 40 cal BC), not complete13; NL = Mesolithic aurochs River Tjonger valley25; Early/Late Neolithic aurochs43 Et = Etival; Fa = Farges; Sa = Sauge, No = Novéant. Gray ellipse: morphological aurochs.
Genetic mtDNA haplotype and sex identification
A fragment of the mtDNA d-loop, diagnostic to distinguish between P and T maternal lineages in bovids, and nuclear zfx/zfy genes were PCR amplified and sequenced. The concatenated 361 bp mtDNA d-loop sequence displayed the characteristic substitutions of European aurochs P haplogroup and three further polymorphisms (pos. 16'074, 16'084, 16'085) in relation to the main P haplotype (DQ915577, Fig. 4, Supplementary Fig. S1). Sexing with zfy/zfx genes confirmed that the bone belonged to a female (Supplementary Fig. S1).
Figure 4. Network comprising bovine P haplotypes.
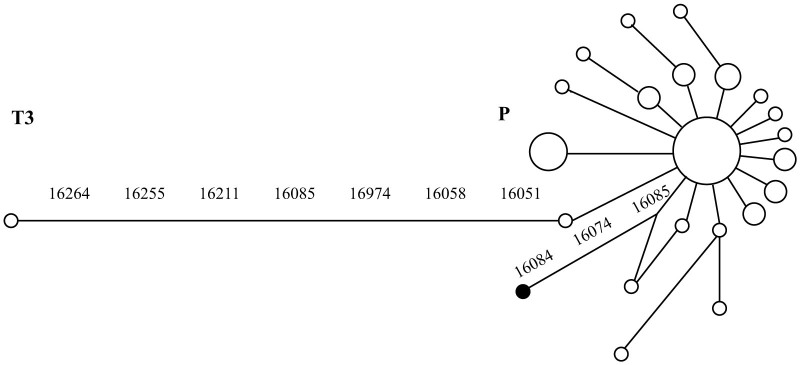
Median joining network of published mtDNA d-loop sequences (pos. 16'042–16'152 and 16'185–16'262 from V00654) from aurochs (Supplementary table S3). The position of the Twann metacarpus 2048.1 is indicated by a filled circle.
Discussion
Several scenarios may explain this finding. It may be argued that, similar to pigs, wild or domestic status in cattle cannot be reliably identified morphologically and that female aurochs size variability was much larger than expected. If this is true, miniature aurochs individuals or populations must have undetectably coexisted with large ones, a scenario we consider highly unlikely. Currently the smallest securely identified aurochs had 132 cm withers height (cited in25:), 20 cm larger than 2048.1 (Fig. 3).
Single events of gene flow between wild and domestic cattle sharing the same habitat at the same time is another possible explanation26,27. Yet genetic and morphometric evidence for crossbreeding in the archaeological record is controversial. Rare morphologically intermediate size types are often explained as crossbreeds, but the issue is disputed (e.g.28,29,30). The P lineage was previously identified in different skeletal elements from few bovids with ambiguous domestic status (Supplementary Table S2). Of these, the only one directly comparable to 2048.1 is the metacarpus Ros 10 belonging most probably to a small female aurochs (4770 ± 40 cal BC) (Fig. 3)13 which is larger than 2048.1. Therefore, 2048.1 is definitely not of intermediate size. This also raises the question of how dramatic the height decrease of the offspring, in this case of domestic bull and a wild cow, in the first generation is. Chance events are even more unlikely considering both archaeozoological and -botanical data from the area suggesting that due to increasing human impact on the environment, local aurochs populations experienced greater competition for resources with domestic cattle and most probably withdrew to other areas31,32.
Another, more likely explanation is repeated and intentional crossbreeding between the offspring of a female aurochs with domestic bulls over an extended time period, consequently keeping the females close to the settlement, starting in an unknown location sometimes before 3300 BC. Chance crossbreeding may have been the initial event but it was followed by capture and human interaction. Amongst others, this scenario was previously proposed for the bovine R lineage in modern Italian cattle33. Intentional breeding can lead to the appearance of domestic traits in a few generations, as has been shown for deer34, and changes from wild to domestic appearance in cattle under management is suggested to be visible within 150–180 years35. It implies that early farmers took the opportunity to restock their cattle herds with wild aurochs. Such events appear to have been rare, as today no unambiguous domestic cattle with a P haplotype from the Mesolithic onwards has been found (Supplementary Tab. S2). However, the descendants of P type domestic cattle did survive in two known modern cattle26 interpreted as descendants of a rare matrilinear introgression from wild aurochs26,27.
The evidence for cattle improvement/control/regimen makes particular sense in light of what is known about the Horgen culture in Switzerland. During this cultural-time period (3400–2800 BC) cattle were smaller but more robust than in earlier and later Swiss Neolithic cultures10,11. First evidence of using cattle as draught animals and of intentional breeding of, particularly, cows during this period is discussed10. Withers heights vary between 102.5 and 132 cm and the appearance of the first castrated animals with withers height around 130 cm has been suggested10. The bones of hunted aurochs were never frequently encountered and their number decreases further during Neolithic times from the Cortaillod period onwards (c. 3900 BC)32. Therefore, incorporation of European aurochs into domestic herds is possibly related to improvements of domestic cattle during the period of the “secondary products revolution” in the 4th Millennium BC36. The contribution of human intention and selection on early domestic herds has been questioned recently37, the Twann metarcarpus may, however, be evidence for genuine breeding during a time of innovation in animal husbandry and thus adds a further facet to the process of cattle domestication. It is probable that other, so far unnoticed, attempts also took place, as complete long bones are rarely found in the archaeological record and morphometric status identification of fragments is often difficult.
To further investigate the fate and legacy of P aurochs in domestic cattle, more ancient samples with unambiguous status identification need to be investigated. Furthermore, a genome will reveal the degree of domestication, phenotypic traits and other traits selected for in comparison to aurochs and modern cattle genomes. Together it might be even possible to clarify whether the Twann cow signifies an event of secondary domestication. Our results add to the growing body of novel insights from interdisciplinary research on early animal husbandry/management and the complex modes of human interaction with wild fauna.
Methods
Material
The Neolithic site of Twann – Bahnhof (Twann station) was excavated between 1975 and 1976 and today posesses UNESCO world heritage site status. In an excavated area of 2'300 m2, 13 settlement phases were found. Ten phases belong to the Cortaillod culture and three to the Horgen (layer MH and OH) or Port-Conty culture (layer UH), specifying the transition from Cortaillod to Horgen cultures. Dendrochronological dates place the ten Cortaillod phases between 3838 and 3532 BC, and the three Horgen phases between 3405 and 3072 BC. All layers and archaeological material have been preserved in a waterlogged condition. The complete metacarpus 2048.1 was a chance find during collection of faunal remains to study DNA preservation in waterlogged material. It belongs to the layer OH (obere Horgener Schicht = upper Horgen culture layer). The identification as cattle (Bos primigenius taurus) was done by H.R. Stampfli38. Most other bone remains in this layer are fragmented and the assemblage is interpreted as slaughter and/or kitchen waste (Supplementary note).
DNA extraction, PCR, and sequencing
Ancient DNA work was carried out according to accepted standards in aDNA research and as established at IPAS, Basel, CH4 and the Paleogenetics group in Mainz, D39.
Basel: Bone preparation, DNA extraction and PCR amplification
The outer surface of the bone was removed with sandpaper and a cube of c. 1 cm3 was cut with a Dremel® tool. The cube was ground with a mixer mill (Retsch MM2, Schieritz & Hauenstein, Allschwil, Switzerland). DNA extraction followed the User Developed Protocol: “Purification of total DNA from compact animal bone using the DNeasy® Blood & Tissue Kit” (Qiagen, Basel, Switzerland) for less than 100 mg. A mock control was performed with the sample. The extract was ultrapurified with water (molecular biology grade, Eppendorf, Allschwil, Switzerland) using 30 kD filter units (Amicon/Millipore, Zug, Switzerland). The final eluate was 200 μl.
Four partially overlapping targets of mitochondrial d-loop with different lengths covering nucleotide positions 15'903–16'312 (V00654) and two 27 bp segments of the zinc-finger genes in the X and Y chromosome (supplementary Table S1) were PCR amplified in 25 μl volumes containing 1.5 U AmpliTaq Gold, 1× GeneAmp PCR Gold buffer (150 mM Tris-HCl, 500 mM KCl, pH 8.0) and 2 mM MgCl2 (all Applied Biosystems, Hombrechtikon, Switzerland); 0.4 mM dNTP Mix (Promega, Dübendorf, Switzerland); 0.2 mM of each primer; 20 μg/μl BSA (bovine serum albumin, Roche, Basel, Switzerland), and 3–9 μl (5 μl for sexing PCRs) template DNA on a Mastercycler ProS (Eppendorf, Allschwil, Switzerland). The cycling conditions were: 12 min initial denaturation, followed by 50–70 cycles of denaturation at 95°C for 40 s, annealing at 50–58°C (55°C for sexing primers) for 30 s, and extension 72°C for 30 s, with a final extension of 60 s at 72°C. At least one non-template control was performed with all amplifications (Supplementary note). To overcome potential PCR inhibitors DNA extracts were diluted 1:10. MtDNA PCR products were cloned with the TOPO TA Cloning Kit (Invitrogen, Zug, Switzerland) following the manufacturer's protocol, except that the reaction volume was halved. One to four clones were sequenced and/or PCR products were directly sequenced by Microsynth (Balgach, Switzerland). Amplicons of the zinc finger loci were directly sequenced. All sequences obtained for 2048.1, either by cloning or by direct sequencing, are given in supplementary Fig. S1.
Sequences were aligned with BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html), using the Bos taurus reference genome V00654. A median joining network was built with NETWORK 4.6.1.240 using Bos primigenius sequence entries from Genbank (supplementary table S3). All sequences were truncated to positions 16'185–16'312 to include as many P type sequences as possible. A concatenated consensus sequence was deposited with Genbank (KJ101593).
Authenticity
Established standards in aDNA research at Integrative Prehistory and Archaeological Science (IPAS) were adhered to4,41. Briefly: all ancient DNA work (pre-PCR) was performed in dedicated, physically separated laboratories, following a strict one-way policy. Benches and tools were treated with bleach and UV irradiated, consumable plastic ware was UV-irradiated prior to use. No PCR products were observed in the negative controls (supplementary note). Each target was validated with two or three independent extractions and up to three PCR products.
From the site Twann Bahnhof several faunal remains were sampled and mtDNA preservation was confirmed (Supplementary note and Supplementary Tab. 4).
Mainz: DNA extraction, PCR, sequencing
The sample was processed in the ancient DNA facilities at the Institute of Anthropology, Mainz University (Germany), under strict rules for contamination prevention as described39.
Four cubes of total 0.89 g were incubated in sodium hypochlorite solution (<5%) for 15 min and subsequently rinsed with water, which had been osmotically purified and UV-irradiated for 8 hours. After UV-irradiation of the dried bone cubes for 45 min from two sides, they were powdered using a mixer mill (MM200, Retsch).
DNA was extracted using phenol-chloroform-isoamylalcohol (25:24:1; Roth), washed and concentrated using 50 kDa 15 ml Amicons (Millipore). A blank control was processed during extraction.
PCR and Sanger sequencing were performed as described42 using primers BosU8/BosL4 (5′- GTACATTATGTCAAATTCATTCTTGATAG- 3′/5′GATCCCTCTTCTCGCTCCG-3′) and BosU5/BosL5 (5′-TACCATGCCGCGTGAAACCA-3′/5′-TTCTTTCTTCAGGGCCATCTCA-3′). Amplicons cover positions 16'122 to 16'273 according to the reference sequence V00654. PCR blank controls were processed during each PCR run. In total eight PCR products per primer pair were successfully sequenced and used to create a consensus sequence by eye. Extraction and PCR blank controls did not show a band after PCR amplification.
Author Contributions
J.S. and A.S. conceived the work, J.S. performed measurements and archaeozoological work, J.E. performed aDNA analysis, A.S. supervised work. A.S., J.S., J.E. wrote the paper, all authors approved the final version of the paper.
Supplementary Material
Schibler Supplementary info
Acknowledgments
We thank the Archaeological Service of the Kanton Bern ADB for the sample. We thank Amelie Scheu for performing the independent verification and José Granado for help with lab work. We are grateful to Greger Larson and Keith Dobney for critical comments on the manuscript, and Ben Jennings for improving our English. The project was funded by Swiss National Science Foundation, project CR13/1-140638, and the University of Basel.
References
- Dobney K. & Larson G. Genetics and animal domestication: new windows on an elusive process. J. Zool. 269, 261–271 (2006). [Google Scholar]
- Larson G. & Burger J. A population genetics view of animal domestication. Trends Genet. 29, 197–205, 10.1016/j.tig.2013.01.003. (2013). [DOI] [PubMed] [Google Scholar]
- Larson G. et al. Current perspectives and the future of domestication studies. Proc. Nat. Acad. Sci. U.S.A. 111, 6139–6146, 10.1073/pnas.1323964111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumbaum A. et al. Ancient DNA, a Neolithic legging from the Swiss Alps and the early history of goat. J. Archaeol. Sci. 37, 1247–1251, 10.1016/j.jas.2009.12.025 (2010). [Google Scholar]
- Larson G. et al. Ancient DNA, pig domestication, and the spread of Neolithic into Europe. Proc. Nat. Acad. Sci. U.S.A. 104, 15276–15281 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. et al. Morphological and genetic evidence for early Holocene cattle management in northeastern China. Nat. Commun. 4, 2755, 10.1038/ncomms3755 (2013). [DOI] [PubMed] [Google Scholar]
- Benecke N. Der Mensch und seine Haustiere. 470 (Theiss, 1994). [Google Scholar]
- Stampfli H. R. Wisent, Bison bonasus (LINNÉ) 1758, Ur, Bos primigenius BOJANUS, 1827, und Hausrind Bos taurus (LINNÉ) 1758. In: Burgäschisee-Süd, Teil 3: Die Tierreste Vol. 2 (eds Boesseneck, J., Jéquier, J.-P. & Stampfli, H. R.) 117–196 (Acta Bernensia, 1963). [Google Scholar]
- Degerbøl M. & Fredskild B. The Urus (Bos primigenius Bojanus) and neolithic domesticated cattle (Bos taurus domesticus, LINNÉ) in Denmark. Det Kongelige Danske Videnskabernes Selskab Biologiske Skrifter 17, 1–227 (1970). [Google Scholar]
- Deschler-Erb S. & Marti-Grädel E. Viehhaltung und Jagd. Ergebnisse der Untersuchungen der handaufgelesenen Tierknochen. In: Die jungsteinzeitliche Siedlung Arbon Bleiche 3. Umwelt und Wirtschaft Vol. 12 Archäologie im Thurgau (eds Jacomet, S., Leuzinger, U. & Schibler, J.) Ch. 3, 158–231 (Huber & Co AG, 2004). [Google Scholar]
- Hüster-Plogmann H. & Schibler J. Archäozoologie. In: Ökonomie und Ökologie neolithischer und bronzezeitlicher Ufersiedlungen am Zürichsee Monographien der Kantonsarchäologie Zürich (eds Schibler, J. et al.) Ch. 2, 40–121 (Direktion der öffentlichen Bauten des Kantons Zürich, Hochbauamt, Abteilung Kantonsarchäologie, 1997). [Google Scholar]
- Edwards C. J. et al. Mitochondrial DNA analysis shows a Near Eastern Neolithic origin for domestic cattle and no indication of domestication of European aurochs. Proc. R. Soc. Lond. B 274, 1377–1385 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu A. et al. Ancient DNA provides no evidence for independent domestication of cattle in Mesolithic Rosenhof, Northern Germany. J. Archaeol. Sci. 35, 1257–1264 (2008). [Google Scholar]
- Mona S. et al. Population dynamics of the extinct European aurochs: genetic evidence of a north-south differentiation pattern and no evidence of post-glacial expansion. BMC Evol. Biol. 10, 83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lari M. et al. The complete mitochondrial genome of an 11,450 year-old aurochsen (Bos primigenius) from Central Italy. BMC Evol. Biol. 11, 32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achilli A. et al. The multifaceted origin of taurine cattle reflected by the mitochondrial genome. PLoS ONE 4, e5753, 10.1371/journal.pone.0005753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoni C. et al. Pig Domestication and Human-Mediated Dispersal in Western Eurasia Revealed through Ancient DNA and Geometric Morphometrics. Mol. Biol. Evol. 30, 824–832, 10.1093/molbev/mss261 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu A., Geörg C., Schulz A., Burger J. & Benecke N. in Population Dynamics in Prehistory and Early History - New Approaches Using Stable Isotopes and Genetics TOPOI 5 (eds Kaiser, E., Burger, J. & Schier, W.) 45–54 (De Gruyter, 2012). [Google Scholar]
- Warmuth V. et al. Reconstructing the origin and spread of horse domestication in the Eurasian steppe. Proc. Nat. Acad. Sci. U.S.A. 109, 8202–8206, 10.1073/pnas.1111122109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckli W. E., Niffeler U. & Gross-Klee E. Neolithikum. In: Die Schweiz vom Paläolithikum bis zum frühen Mittelalter Vol. 2 358 (Schweizerische Gesellschaft für Ur- und Frühgeschichte, Basel, 1995). [Google Scholar]
- Reimer P. J. et al. IntCal09 and Marine09 Radiocarbon Age Calibration Curves, 0–50,000 Years cal BP. Radiocarbon 51, 1111–1150 (2009). [Google Scholar]
- Bronk Ramsey C. Development of the radiocarbon calibration program OxCal. Radiocarbon 43, 1029–1058 (2001). [Google Scholar]
- von den Driesch A. A guide to the measurement of animal bones from archaeological sites. Vol. 1 (Harvard University, 1976). [Google Scholar]
- Matolcsi J. Historische Erforschung der Körpergrösse des Rindes auf Grund von ungarischem Knochenmaterial. J. Anim. Breed. Genet. 87, 89–137 (1970). [Google Scholar]
- Prummel W. & Niekus M. J. L. T. Late Mesolithic hunting of a small female aurochs in the valley of the River Tjonger (the Netherlands) in the light of Mesolithic aurochs hunting in NW Europe. J. Archaeol. Sci. 38, 1456–1467, 10.1016/j.jas.2011.02.009 (2011). [Google Scholar]
- Stock F. et al. Cytochrome b sequences of ancient cattle and wild ox support phylogenetic complexity in the ancient and modern bovine populations. Anim. Gen. 40, 694–700, 10.1111/j.1365-2052.2009.01905.x (2009). [DOI] [PubMed] [Google Scholar]
- Achilli A. et al. Mitochondrial genomes of extinct aurochs survive in domestic cattle. Curr. Biol. 18, 158 (2008). [DOI] [PubMed] [Google Scholar]
- Kyselý R. Aurochs and potential crossbreeding with domestic cattle in Central Europe in the Eneolithic period. A metric analysis of bones from the archaeological site of Kutná Hora-Denemark (Czech Republic). Anthropozoologica 43, 7–37 (2008). [Google Scholar]
- Götherström A. et al. Cattle domestication in the Near East was followed by hybridization with aurochs bulls in Europe. Proc. R. Soc. Lond. B 272, 2345–2350 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollongino R., Elsner J., Vigne J.-D. & Burger D. Y-SNPs do not indicate hybridization between European aurox and domestic cattle. PLoS One 3, e3418, 10.1371/journal.pone.0003418 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüster-Plogmann H., Schibler J. & Jacomet S. The significance of aurochs as a hunted animal in the Swiss Neolithic. Schriftenreihe des Neanderthalmuseums, Mettmann 151–160 (1999). [Google Scholar]
- Schibler J. & Steppan K.-H. Human impact on the habitat of large herbivores in Eastern Switzerland and South West Germany in the Neolithic. Archaeofauna 8, 87–99 (1999). [Google Scholar]
- Bonfiglio S. et al. The enigmatic origin of bovine mtDNA haplogroup R: sporadic interbreeding or an independent event of (Bos primigenius) domestication in Italy? PLoS One 5, e15760, 10.1371/journal.pone.0015760 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmer H. Neumühle-Riswicker Hirsche: Erste planmässige Zucht einer neuen Nutztierform. Naturwiss. Rundsch. 58, 255–261 (2005). [Google Scholar]
- Gautier A. La domestication: et l'homme créa ses animaux. (Errance, 1990). [Google Scholar]
- Sherratt A. in Pattern of the Past: Studies in honour of David Clarke (eds Hodder, I., Isaac, G. & Hammond, N.) 261–305 (Cambridge University Press, 1981). [Google Scholar]
- Marshall F. B., Dobney K., Denham T. & Capriles J. M. Evaluating the roles of directed breeding and gene flow in animal domestication. Proc. Nat. Acad. Sci. U.S.A., 10.1073/pnas.1312984110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfli H. R. Tierknochenfunde. In: Die Siedlungsreste der Horgener Kultur Vol. 7 Die Neolithischen Ufersiedlungen von Twann (ed Furger, A. R.) 141–160 (Staatlicher Lehrmittelverlag, 1980). [Google Scholar]
- Bramanti B. et al. Genetic discontinuity between local hunter-gatherers and Central Europe's first farmers. Science 326, 137–140 (2009). [DOI] [PubMed] [Google Scholar]
- Bandelt H.-J., Macaulay V. & Richards M. Median networks: Speedy construction and greedy reduction, one simulation, and two case studies from human mtDNA. Mol. Phylogenet. Evol. 16, 8–28 (2000). [DOI] [PubMed] [Google Scholar]
- Gilbert M. T. P., Bandelt H.-J., Hofreiter M. & Barnes I. Assessing ancient DNA studies. Trends Ecol. Evol. 20, 541–544 (2005). [DOI] [PubMed] [Google Scholar]
- Scheu A. Palaeogenetische Studien zur Populationsgeschichte von Rind und Ziege mit einem Schwerpunkt auf dem Neolithikum in Südosteuropa. Vol. 4 (Marie Leidorf GmbH, 2012). [Google Scholar]
- Chaix L. & Arbogast R. M. Holocene aurochs from Western Europe: osteometrical data. In: Archaeology and Biology of the Aurochs Vol. 1 (ed Weniger, G.-C.) 35–48 (Neanderthal Museum, 1999). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schibler Supplementary info