Abstract
Pancreatic paragangliomas are extremely rare with less than 20 cases ever described in the world literature. There is no detailed report of the vascular anatomy in this entity and its possible impact on patient management. We present a case of large pancreatic head paraganlioma in a 53-year-old woman. The tumour had a predominant arterial blood supply via both the hepatic artery and the superior mesenteric artery. Complex inflow was complemented by supplementary branches from the right renal artery. The arteriovenous communications within the lesion represented the most dangerous aspect of excision and the tumour removal was accompanied with a considerable blood loss. After pancreaticoduodenectomy, patient experienced transient elevation of liver function tests with no other identifiable cause than a change in portal haemodynamics. It is advisable that the precise knowledge of vascular anatomy in pancreatic head paraganglioma should be obtained prior to any intervention.
INTRODUCTION
The incidence of chromafine tissue tumours is about 1–2/100 000/year in the adult population [1, 2]. Paragangliomas represent an extra-adrenal form of the disease and account for approximately 10% of all pheochromocytomas. About 70% of paragangliomas are intra-abdominal, with various localizations being described, mostly adjacent to main vessels or retroperitoneal organs [3, 4]. Extra-adrenal pancreatic paragangliomas are exceptionally rare. There have been only 15 cases published up to 2011 [5]. Because paragangliomas have notoriously unpredictable behaviour and often metastasize late, surgical resection is a treatment of choice [5]. Critical appreciation of vascular anatomy may define operability, enable planning a surgical strategy and may prove extremely useful during the resection. In case of an inoperable tumour with malignant behaviour, chemotherapy or therapy with radionucleotides may be used [6, 7].
CASE REPORT
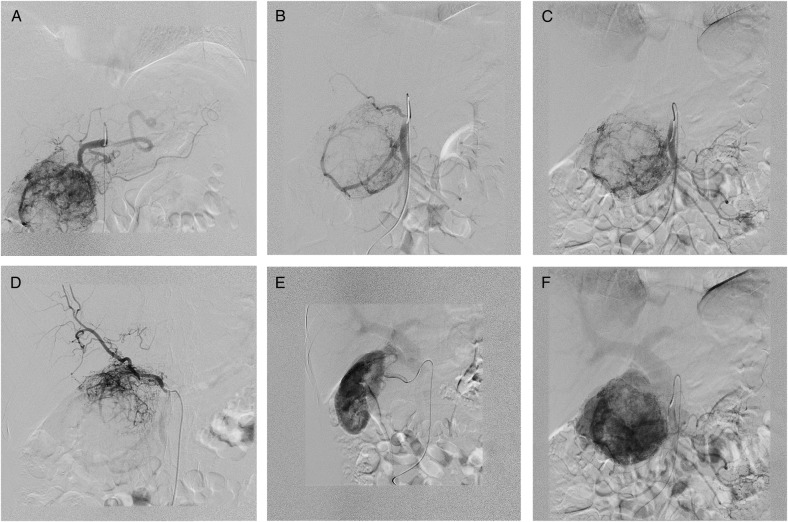
A 53-year-old, obese (body mass index 30.9 kg m−2), Caucasian woman was referred to our department with non-specific abdominal discomfort as the only presenting symptom. A large hypervascularized mass in the area of the pancreatic head was shown on abdominal ultrasonography and computed tomography (CT). There was neither lymphadenopathy nor distant spread found during pre-operative staging. Pre-operative imaging revealed an extremely hypervacularized tumour with abundant collateral vessels from the superior mesenteric artery (SMA), replaced right hepatic artery (HA), gastroduodenal artery (GDA) and right renal artery (RRA), with an early venous filling of the dilated superior mesenteric vein (SMV) and portal vein (PV) (Fig. 1A–C and Fig. 2A and B). The replaced right HA arising from SMA was crossing in between the dilated PV and overfilled tumour-draining veins (Fig. 1B). Abundant venous drainage was found. Extreme dilation of SMV and PV in both extra- and intra-hepatic course could be expected as numerous AV shunts within the tumour were present, but surprisingly, only a non-dilated, gracile splenic vein was found and no spleen enlargement was present (Fig. 1C). For better evaluation of vascular anatomy, selective digital substraction angiography (DSA) was performed. The upper portion of the tumour received main inflow via GDA and replaced right HA, the lower part via several branches of SMA (Fig. 3A and B). Capsular branch of RRA complemented tumour inflow (Fig. 3C).
Figure 1:
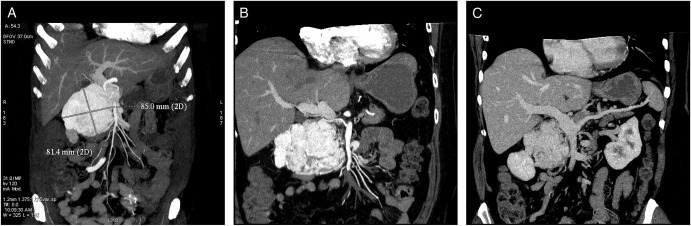
Cross-sectional CT imaging. (A) Hypervascularized tumour with early venous filling in the arterial phase of CT. (B) Variant subtle right hepatic artery originating from SMA, passing between the dilated PV and draining veins of a paraganglioma. (C) Isolated dilation of the PV with surprisingly gracile splenic vein without any spleen enlargement.
Figure 2:
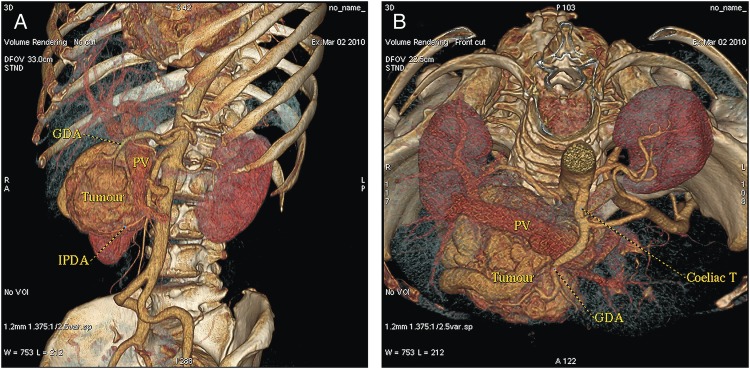
(A and B) CT volume rendering images with dilated PV and an early contrast filling of dilated peritumoral veins.
Figure 3:
DSA. (A) GDA supplying the upper portion of the paraganglioma. (B) SMA—tumour blood supply via both IPDA and replaced RHA. (C) Selective IPDA angiography. (D) Selective angiography via replaced RHA. (E) Complementary tumour blood supply via capsular branch of RRA draining into the portal system. (F) PV dilation in the venous phase of angiography.
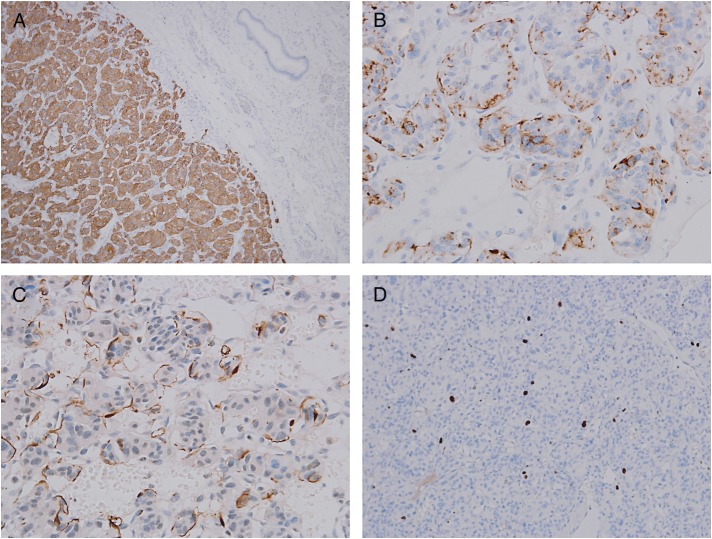
During the laparotomy a tumour with numerous, large pulsating veins running on its surface was found. After partial mobilization was completed, all main inflow and outflow vessels were secured prior to pancreaticoduodenectomy. After division of GDA and capsular branch of RRA, the tumour diminished to about one half of the original size. The neck of the pancreas was divided and dilated arterial and venous branches towards the tumour isolated. The major problem during this step turned out to be severe venous congestion caused by local AV shunts. The draining venous branches had to be divided first to gain access to hypertrophied feeding arteries at the very end of the dissection. Dilated, overfilled venous stumps had marked tendency to bleed on touch. We considered dissection too lengthy to clamp the main arterial stems (SMA and celiac trunk) for a prolonged period of time, and so a blood loss of 2000 ml during almost 9 h of surgery was tolerated. Histopathologically, the tumour characteristics typical of paraganglioma were present with chromogranin, synaptophysin, neuron-specific enolase and S-100 protein positivity in the immunohistochemical assay (Fig. 4A–D). Mitotic activity was low (1/30 HPF) with Ki-67 (MIB-1) 3–5%. The tumour had a strong fibrous capsule, no invasion to neighbouring tissue, lymphatic or blood vessels was found. Based on these findings, a benign variant of paraganglioma was suggested.
Figure 4:
Histopathological examination of resected specimen with different stains and sites. (A) Synaptophysin expression (100×). (B) Chromogranin expression (×400). (C) Sustentacular cells S100 immunostain (×400). (D) Low proliferation activity Ki-67 stain (×200).
The only abnormality in a post-operative period was an unexpected transient hepatal panel elevation with a peak on post-operative day 7 (Fig. 5). Although no invasive haemodynamic measurements were performed neither pre-operatively nor in the post-operative period, we speculate that this could have been an effect of changed liver inflow haemodynamics after the tumour excision. Extreme pre-operative SMV and PV dilation without splenic vein dilation in the presence of AV shunts could be a result of ‘a high flow–low resistance’ perfusion of the liver. After tumour removal, dramatic change in liver haemodynamics might occur. Pre-operatively, the large proportion of the arterial inflow consisted of arterial blood shunting directly into the PV from tumour feeding arteries. As both the left and the right HA were subtle, they provided for only non-substantial oxygen-rich hepatic inflow. Thus, tumour removal could lead to a critical decrease of oxygen delivery to the liver. Liver panel abnormality resolved spontaneously and never relapsed. The patient is now 49 months after pylorus preserving pancreaticoduodenectomy, the regular follow-up with positron emission tomography-computed tomography (PET/CT) has been performed and no recurrence has been observed so far.
Figure 5:
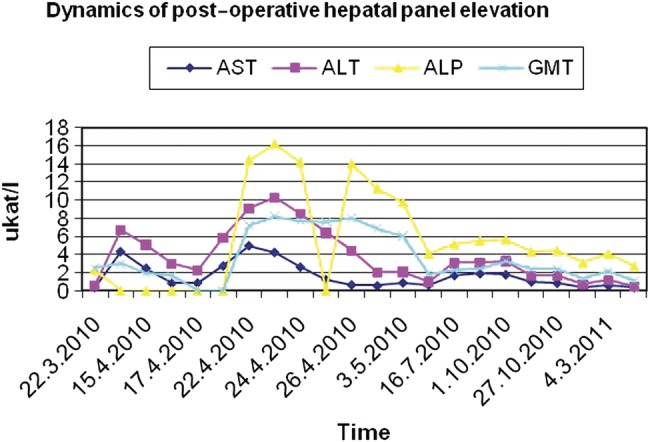
Transient post-operative elevation of hepatal panel.
DISCUSSION
The surgical removal of a paraganglioma represents the treatment of choice. A large tumour may require an extensive surgical approach, careful dissection and en bloc resection irrespective of whether it actually represents intraparenchymatous or only a perivascular, ‘organ adjacent’ paraganglioma. Special attention shoud be paid to the possibility of AV shunting within a tumour, either for the unestimated risk of a paradoxical embolism into the portal system (when attempting pre-operative embolization), or for possible intra-operative technical problems (bleeding caused by venous congestion or while manipulating the tumour). Suggested existence of a ‘high flow–low resistance’ hepatic inflow in the presence of significant AV shunts within the tumour in this localization may result in a significant change of hepatic perfusion after tumour removal. Even though this is the first report to describe such changes and no invasive haemodynamic measurements were carried out, we recommend the highest vigilance when similar situation occurs.
CONFLICT OF INTEREST
The authors declare that they have no competing interests. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
REFERENCES
- 1.Plouin PF, Gimenez-Roqueplo AP. Pheochromocytomas and secreting paragangliomas. Orphanet J Rare Dis. 2006;1:49. doi: 10.1186/1750-1172-1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chetrit M, Dube P, Royal V, Leblanc G, Sideris L. Malignant paraganglioma of the mesentery: a case report and review of literature. World J Surg Oncol. 2012;10:46. doi: 10.1186/1477-7819-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KY, Oh YW, Noh HJ, Lee YJ, Yong HS, Kang EY, et al. Extraadrenal paragangliomas of the body: imaging features. AJR Am J Roentgenol. 2006;187:492–504. doi: 10.2214/AJR.05.0370. [DOI] [PubMed] [Google Scholar]
- 4.Limaiem F, Bouraoui S, Bouslama S, Slama SB, Lahmar A, Mzabi-Regaya S. Retroperitoneal non-functioning paraganglioma: a case report. J Interdiscipl Histopathol. 2013;1:168–71. [Google Scholar]
- 5.Lightfoot N, Santos P, Nikfarjam M. Paraganglioma mimicking a pancreatic neoplasm. JOP. 2011;12:259–61. [PubMed] [Google Scholar]
- 6.Srirangalingam U, Khoo B, Walker L, MacDonald F, Skelly RH, George E, et al. Contrasting clinical manifestations of SDHB and VHL associated chromaffin tumours. Endocr Relat Cancer. 2009;16:515–25. doi: 10.1677/ERC-08-0239. [DOI] [PubMed] [Google Scholar]
- 7.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumours. Endocr Rev. 2004;25:458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]