Abstract
It has been assumed that most, if not all, signals regulating early development have been identified. Contrary to this expectation, we identified 28 candidate signaling proteins expressed during zebrafish embryogenesis, including Toddler, a short, conserved, and secreted peptide. Both absence and overproduction of Toddler reduce the movement of mesendodermal cells during zebrafish gastrulation. Local and ubiquitous production of Toddler promote cell movement, suggesting that Toddler is neither an attractant nor a repellent but acts globally as a motogen. Toddler drives internalization of G protein–coupled APJ/Apelin receptors, and activation of APJ/Apelin signaling rescues toddler mutants. These results indicate that Toddler is an activator of APJ/Apelin receptor signaling, promotes gastrulation movements, and might be the first in a series of uncharacterized developmental signals.
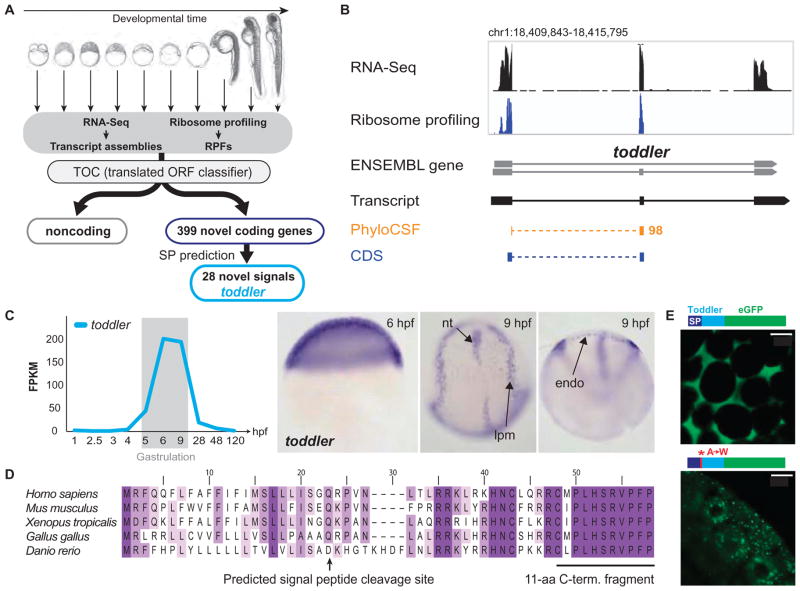
Many of the inductive events during early development are directed by a small number of signaling pathways whose agonists have been known for more than a decade (1). Therefore, it has been assumed that most, if not all, embryonic signals have been identified. However, the molecular control of some embryonic processes is still poorly understood. For example, it is largely unclear how cell migration is regulated during gastrulation or how cells coalesce into discrete tissues during organogenesis (2–5), suggesting that some of the involved signals are yet to be identified. Moreover, recent genomic studies have suggested that translation of short open reading frames (ORFs) and the generation of small peptides are much more pervasive than previously assumed (6, 7). To search for new candidate signaling molecules, we used the Translated ORF Classifier (TOC) (7) to examine zebrafish transcript annotations and ribosome profiling data sets (7–9) for non-annotated translated ORFs (Fig. 1A) (materials and methods in the supplementary materials). This analysis identified 700 novel protein-coding transcripts (399 loci) (supplementary data files S1 and S2), of which 81% (562 transcripts in 325 loci) shared nucleotide sequence alignments with other vertebrates (table S1). Notably, this approach identified 28 candidate signaling proteins (40 transcript isoforms) characterized by the presence of putative signal sequences and lack of predicted transmembrane domains (table S1). Ribosome profiling and phylogenetic analysis suggest that these RNAs can generate secreted peptides with lengths ranging from 32 to 556 amino acids (Fig. 1A, fig. S1, and table S1). Although these genes have not been identified previously or are annotated in the zebrafish Ensembl database as noncoding RNAs, the majority (24 of 28) appear to be conserved in other vertebrates (fig. S1 and table S1).
Fig. 1. Identification of the novel embryonic signal Toddler.
(A) Overview of the individual steps used to identify novel coding and noncoding transcripts. SP, signal peptide; RPFs, ribosome protected fragments. (B) Genomic features of toddler. Coverage tracks for RNA-Seq (black) and ribosome profiling (blue), and tracks outlining the highest scoring regions in PhyloCSF (orange). Note that both PhyloCSF (8) and ribosome profiling (7) predict toddler to be protein-coding. (C) Expression analysis of toddler transcripts during embryogenesis. toddler transcripts peak during gastrulation [RNA-Seq data (8)]. FPKM, fragments per kilobase of transcript per million mapped reads. RNA in situ hybridization reveals ubiquitous expression of toddler transcripts at the beginning of gastrulation [6 hours postfertilization (hpf)]; expression becomes restricted to mesendodermal cells toward the end of gastrulation (9 hpf). nt, notochord; lpm, lateral plate mesoderm; endo, endoderm. (D) Toddler is conserved in vertebrates. ClustalW2 multiple protein sequence alignment of Toddler peptide sequences from five vertebrates. Darker shading indicates higher percentage identity of the amino acid. The predicted signal peptide cleavage site and the highly conserved C-terminal 11–amino acid (aa) peptide fragment that was detected by mass spectrometry are indicated. (E) Toddler signal sequence drives secretion. Injection of mRNAs encoding C-terminal Toddler-eGFP fusion proteins reveals that the wild-type Toddler signal sequence drives secretion (extracellular localization of eGFP), whereas mutation of A→W in the signal peptide cleavage site causes Toddler-eGFP to remain intracellularly (top, wild-type Toddler ORF; bottom, A→W mutant Toddler ORF). Fusion protein diagrams are not drawn to scale. Scale bars, 20 μm.
Toddler Encodes a Short, Conserved, and Secreted Peptide
To test the functional potential of these candidate signals, we focused on a gene that we named toddler on the basis of the phenotype described below (Fig. 1B). Toddler (tdl) mRNA is expressed ubiquitously during late blastula and gastrula stages and becomes restricted to the lateral mesoderm, endoderm, and anterior and posterior notochord after gastrulation (Fig. 1C). Toddler is annotated as a non-coding RNA in zebrafish (ENSDARG00000094729), mouse [Gm10664; also called Ende (10)], and human (LOC100506013) (fig. S2) and is present in two lncRNA catalogs (9, 11); however, it contains a 58–amino acid ORF with a predicted signal sequence and high conservation in vertebrates, including human (Fig. 1D and fig. S3). Sequence comparisons with the highly conserved C-terminal portion did not identify homology to any other known proteins, raising the possibility that this gene encodes an uncharacterized embryonic signal.
Six lines of evidence indicate that toddler is translated and encodes a secreted peptide. First, phylogenetic comparisons of synonymous versus nonsynonymous codon changes reveal strong amino acid preservation in the toddler ORF (PhyloCSF score of 98 (8); see Fig. 1, B and D, and table S1). Second, previous ribosome profiling data in mouse (6) and zebrafish (7) indicate that the toddler ORF is protected by actively translating ribosomes in vivo (Fig. 1B). Third, mass spectrometric analysis of nontrypsinated protein extracts from embryos expressing toddler mRNA detected the 11–amino acid C-terminal Toddler peptide fragment that is predicted to be a convertase cleavage product (Fig. 1D and fig. S4). Fourth, enhanced green fluorescent protein (eGFP) fusion proteins containing the wild-type signal sequence of Toddler are found extracellularly, whereas signal peptide cleavage site mutants are retained in the cell (Fig. 1E). Fifth, as described below, extracellular injection of in vitro–synthesized Toddler peptide (C-terminal 21 amino acids) elicits the same gain-of-function phenotypes as excess of toddler mRNA. Sixth, wild-type but not frameshifted toddler mRNA rescues toddler mutants (see below), providing direct evidence that it is the peptide product rather than the RNA that is functional in vivo. Together, these findings identify Toddler as a short, conserved, and secreted peptide.
Toddler Is Essential for Embryogenesis
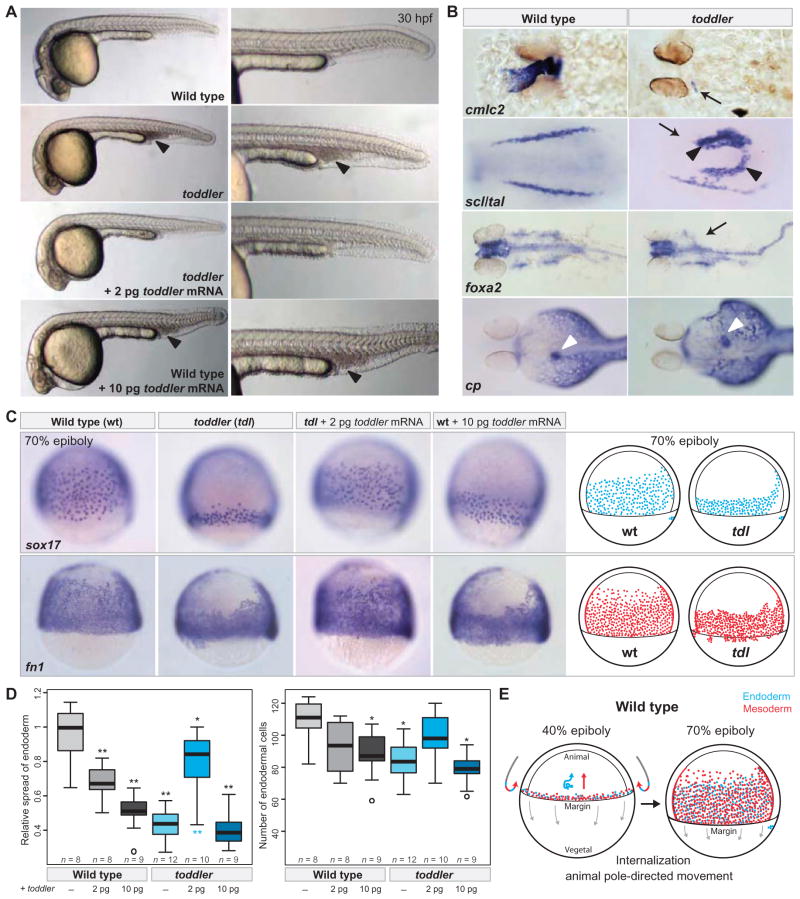
To disrupt toddler function, we generated mutants by TALEN-mediated mutagenesis (fig. S5 and materials and methods) (12, 13). Seven toddler alleles were recovered, each of which introduces a frameshift immediately after the signal peptide sequence (fig. S5, B and C). The vast majority of homozygous toddler mutants die between 5 and 7 days of development and display small or absent hearts, posterior accumulation of blood cells, malformed pharyngeal endoderm, and abnormal left-right positioning and formation of the liver (Fig. 2, A and B, and fig. S6). Penetrance and expressivity of toddler mutants vary, including occasional escapers that live to adulthood and rare instances of toddler mutants that display more severe defects in endoderm and mesoderm formation (fig. S7). Notably, the lethality of toddler mutants (survival, 0 of 25 animals) was rescued by injection of low amounts (2 pg) of wild-type (survival, 23 of 30 animals) but not frameshifted (survival, 0 of 32 animals) toddler mRNA (Fig. 2, A, C, and D). Embryonically rescued toddler mutants survived to adulthood and were fertile in the absence of any later source of Toddler peptide, indicating that zebrafish Toddler is only essential during early embryogenesis.
Fig. 2. Toddler is essential for embryogenesis.
(A) Morphological analysis of toddler mutants. TALEN-induced toddler null mutants (see fig. S5) lack a functional heart, have no blood circulation, and accumulate blood posteriorly (black arrowheads). Defects in toddler mutant embryos are rescued by low doses (2 pg) of toddler mRNA. Injection of higher doses of toddler mRNA (≥10 pg) causes phenotypes in wild-type embryos reminiscent of toddler loss-of-function mutants. Shown are lateral views of embryos of the indicated genotypes at 30 hpf. (B) Marker gene analysis in wild-type and toddler mutant embryos at 36 hpf (cmlc2), at the 8 to 10 somite stage (scl/tal), at 30 hpf (foxa2), and at 3 days postfertilization [ceruloplasmin (cp)]. Black arrows indicate lack of or reduced staining in toddler mutant embryos; black arrowheads indicate ectopic expression; white arrowheads point to the liver in wild-type (>70% on left side) and toddler mutant embryos (expression: 45% right, 15% medial, 40% none/nonspecific). (C) Toddler is required for movement of ventrolateral endoderm and mesoderm toward the animal pole. Both absence of Toddler (toddler) and overexpression of toddler mRNA (wild-type embryos + 10 pg of toddler mRNA) reduce the movement of endodermal (sox17) and mesodermal [ fibronectin 1 ( fn1)] cells toward the animal pole, as detected by in situ hybridization. All in situ images are lateral views of embryos at 70% epiboly (dorsal to the right). Illustrations of the observed endodermal (blue) and mesodermal (red) phenotypes in wild-type (wt) and toddler mutant (tdl) embryos are shown on the right. (D) Quantification of the endodermal defects at 70% epiboly. Left, relative spread of lateral endoderm along the animal-vegetal axis (that is, height of lateral band of sox17-expressing cells divided by the wild-type mean); right, number of endodermal cells within a lateral, fixed-size area. Gray, wild-type genomic background; cyan, toddler mutant genomic background. P values for pairwise comparisons with wild-type (black, top) or toddler mutant (cyan, bottom) were calculated on the basis of a standard Welch’s t test (*P < 0.01; **P < 0.00001). (E) Illustration of early gastrulation movements in wild-type zebrafish embryos. Mesodermal (red) and endodermal (blue) cells are induced and internalized at the margin (40% epiboly stage). Whereas internalized cells migrate toward the animal pole in either a directional (mesoderm) or random walk–like pattern (endoderm) (3, 15), epiboly movements are directed toward the vegetal pole (gray arrows). At 70% epiboly, mesodermal and endodermal cells have moved animally and cover most of the lateral side of the embryo.
Toddler Is Required for Normal Gastrulation Movements
To determine when Toddler function is required during early embryogenesis, we used a heat shock–inducible transgene. Induction of toddler expression during late blastula and early gastrula stages, but not at later times, rescued toddler mutants (fig. S8 and materials and methods).
The early requirement for Toddler, together with its expression peak during gastrulation (Fig. 1C), suggested that the later phenotypes originate from earlier defects. We therefore analyzed morphology and gene expression during blastula and gastrula stages and discovered that toddler mutant mesendodermal progenitors did not move properly toward the animal pole during gastrulation. Although ventral and lateral mesendodermal cells in wild-type embryos internalized at the margin and moved toward the animal pole (Fig. 2, C and E), these cells were closely packed and confined to a band near the margin in toddler mutant embryos (Fig. 2, C and D, and fig. S9). These defects were apparent by analysis of endodermal (sox17) and mesodermal (fibronectin1/fn1, spadetail/tbx16, fascin, draculin/drl) markers (Fig. 2C and fig. S9). In contrast, ectodermal (sox3), dorsal mesodermal (goosecoid/gsc, hgg1), and tail mesodermal (ntla) markers were largely unchanged in their expression domains (fig. S10). In addition to the ventrolateral movement defects, toddler mutants contained ~20% fewer endodermal cells at mid-gastrulation (Fig. 2, C and D, and fig. S9A). The initial expression of mesendodermal markers appeared unaffected (fig. S10B), suggesting that mesendodermal cells are specified normally in toddler mutant embryos but proliferate less. Notably, the toddler gastrulation phenotypes could be rescued by injecting low levels (2 pg) of toddler mRNA at the one-cell stage (Fig. 2, C and D, and fig. S9, A and C). These results reveal an important role for Toddler in the movement of ventral and lateral mesendodermal cells during gastrulation.
Toddler Promotes Endodermal and Mesodermal Cell Migration
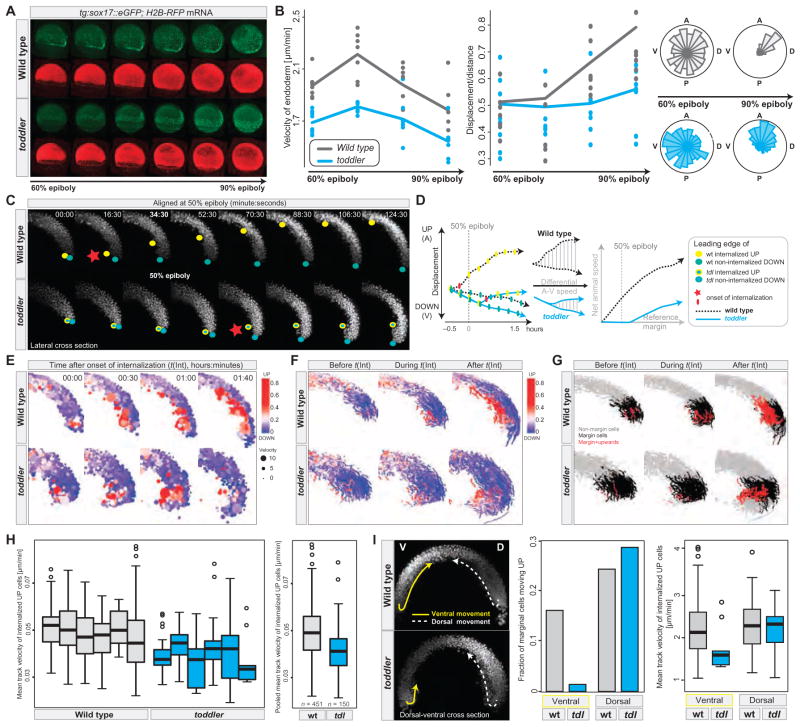
To determine how Toddler affects the movement of cells during gastrulation, we performed live cell imaging and followed cell trajectories in wild-type and toddler mutant embryos (movies S1 to S6). Toddler mutant endodermal cells [sox17::GFP (14)] displayed reduced movement toward the animal pole (Fig. 3A, fig. S11, and movies S1 and S2), migrated more slowly, and showed reduced net (start-to-end) displacement compared to wild-type cells (Fig. 3B and fig. S11). During early gastrulation, toddler mutant endodermal cells exhibited the characteristic random walk–like migration pattern observed in wild-type embryos (3, 15), but they migrated in a less directional fashion than their wild-type counterparts during later gastrulation (movie S1 and Fig. 3B).
Fig. 3. Abnormal gastrulation movements in toddler mutants.
(A and B) Analysis of endodermal cell migration in sox17::eGFP transgenic wild-type and toddler mutant embryos by confocal microscopy. Green, endodermal cells (marked by sox17::eGFP); red, nuclei [human histone2B-RFP (H2B-RFP) mRNA injection]. (A) Still images of maximum intensity projections of a time-lapse movie from 60 to 90% epiboly (movies S1 and S2). (B) Quantification of the average (median) velocity of endodermal cells (left), displacement versus distance travelled (middle), and directionality (rose-plots; right) in wild-type (gray) and toddler mutant (cyan) embryos. Each dot represents the average speed (or the ratio between displacement versus distance travelled) of all endodermal cells tracked within a single embryo during a 45-min time interval with respect to its previous position [speed = actual distance (micrometers)/time (min)]. Shown are the data for four consecutive 45-min time windows. Roseplots display the random movement of endodermal cells during early gastrulation and the more directional migration at later stages [animal (A), posterior (P), dorsal (D), ventral (V)]. (C to I) Analysis of early gastrulation movements in H2B-RFP mRNA injected wild-type and toddler mutant embryos by light-sheet microscopy (single-plane illumination microscopy). (C to H) Internalization and animal pole–directed movement of lateral mesendodermal cells are reduced in toddler mutants. Analyses are shown for lateral cross sections of a time-lapse movie (movie S4) of a wild-type–toddler mutant embryo pair, imaged in parallel at 90-s intervals within a single experiment. (C) Still images of maximum intensity projections of 40-μm lateral cross sections (20 z-slices) during the time of internalization (time in minutes:seconds). Movies were aligned at 50% epiboly (48:00). Leading edges of internalizing mesendodermal cells (yellow dots) and vegetally moving cells (green dots) highlight the opposing paths of cells during gastrulation. Red stars mark the onset of cell internalization. (D) Comparison of animally and vegetally directed migratory paths of the wild-type and mutant embryo pair shown in (C). Frame-to-frame displacements (plotted on the left) were used to derive the net animal pole–directed cell movement. Toddler mutants (cyan) show delayed onset of internalization and reduced step-to-step and net animal pole–directed movement. (E to G) Cell tracking and digital analysis of gastrulation movements. (E) Position, speed (dot size), and directionality [color-coded from blue (vegetal movement) to red (animal movement)] of tracked cells during and after the time of internalization [t(Int)]. Movies were aligned to the onset of internalization [t(Int) = 00:00; time in hours:minutes]. (F and G) Cell tracks before (t < −5 min), during (−5 min < t < 1 hour), and after (t > 1 hour) internalization in wild-type and toddler mutant embryos. In (F), tracks were color-coded on the basis of the total number of animal pole–directed (red) or vegetal pole–directed (blue) movements, normalized to the total number of frames per track. In (G), tracks were color-coded on the basis of their relative position and directionality with respect to the margin at the time of internalization (margin cells: cells located within 100 μm above the margin at the onset of internalization). Gray, nonmargin cells; black, margin cells; red, internalizing and upward-moving margin cells. (H) Quantification of the mean velocity of internalizing, animal pole–directed movement in wild-type and toddler mutant embryos. Mean track velocities were obtained from cell-tracking data derived from lateral cross sections of six wild-type (gray) and six toddler mutant (cyan) embryos, imaged in parallel in three independent experiments. Pooled wild-type and toddler mutant mean track velocities are plotted on the right (n = number of cell tracks). (I) Toddler mutant embryos are defective in ventrolateral but not dorsal internalization. (Left) Still image of maximum intensity projections of 40-μm dorsal-ventral cross sections (20 z-slices) of a wild-type–toddler mutant embryo pair 110 min after the onset of internalization. Arrows highlight the paths that the leading internalizing cells took on dorsal (D, dashed white line) and ventral (V, solid yellow line) sides of the embryo. Ventral movement toward the animal pole is severely reduced in the toddler mutant embryo, whereas dorsal internalization occurs normally. (Right) Quantification of the fraction and speed of internalizing marginal cells based on their positioning in the embryo (dorsal versus ventral) and genotype [wild type (gray) versus toddler mutant (cyan)] (see also movie S6).
To analyze the earliest steps of mesendoderm movement, we followed the paths of H2B-RFP–labeled nuclei by light-sheet microscopy in wild-type and toddler mutant embryos (movie S3 and fig. S12). Analysis of 10 wild-type and 11 toddler mutant embryos confirmed that the movement of ventrolateral but not dorsal internalizing cells toward the animal pole was impaired in toddler mutants (Fig. 3, C to I, figs. S12 to S14, and movies S3 to S6). Internalization of ventrolateral cells at the margin was delayed (Fig. 3, C and D, fig. S13A, and movies S4 and S5) and reduced (Fig. 3, E to G and I, fig. S13, and movies S3 to S6). Although internalization in wild-type embryos started about 30 min before embryos reached 50% epiboly, it often commenced only after the 50% epiboly stage in toddler mutants (Fig. 3, C and D, fig. S13A, and movies S4 and S5). Ventrolateral internalized cells moved more slowly (Fig. 3, H and I) and often piled up at the margin (Fig. 3, C and E, figs. S13 to S15, and movies S3 to S6). In addition, epiboly movements were often delayed in toddler mutants, particularly during the time of internalization (fig. S13, A and B). In rare cases, we observed an almost complete absence of animal pole–directed ventrolateral cell movements; in these embryos, ventral and lateral marginal cells instead moved vegetally (movies S3, S5, and S6), likely contributing to the ectopic accumulation of posteriorly located blood cells at later stages (Fig. 2, A and B). These results identify Toddler as a key signal that promotes the internalization and animal pole–directed movement of mesendodermal cells during gastrulation.
Overexpression of Toddler Phenocopies Toddler Mutants
In contrast to inducers of specific cell fates, many signals involved in cell migration or tissue morphogenesis share loss- and gain-of-function phenotypes. For example, both reduction and increase in Wnt/planar cell polarity signaling disrupt convergence and extension movements during gastrulation (2–5). To determine whether Toddler might share this feature, we carried out overexpression analyses. Injection of toddler mRNA at levels only five times higher (≥10 pg) than needed for rescue caused phenotypes in wild-type embryos that resembled toddler loss-of-function mutants, including gastrulation and heart defects (Fig. 2, A, C, and D, and fig. S9, A and C). Similar phenotypes were observed upon extracellular injection of an in vitro–synthesized Toddler peptide fragment (C-terminal 21 amino acids; fig. S16). These observations reveal that proper levels of Toddler are required for normal mesendodermal movement and provide further evidence of an important role for Toddler in cell migration.
Toddler Functions as a Motogen
Most genes encoding signals that attract or repel cells are expressed in specific domains (16), and ubiquitous production of such signals interferes with guided cell migration. In contrast, we find that toddler RNA is expressed ubiquitously (Fig. 1C and fig. S17A) and that ubiquitous expression of toddler mRNA upon injection at the one-cell stage promotes the normal movement of mesendodermal cells in toddler mutants (Fig. 2, C and D). To further test the role of Toddler in cell migration, we locally expressed Toddler in the vegetal or animal regions of toddler mutants. In both scenarios, localized Toddler production was able to promote the migration of mesendodermal cells and rescue toddler mutants (Fig. 4). Although more complex scenarios are formally possible [for example, local processing (17) and self-generated gradient formation (18, 19)], these results suggest that Toddler does not attract cells to or repel cells from specific sites. Instead, Toddler appears to act as a motogen (20–22)—a general promoter of mesendodermal cell migration.
Fig. 4. Toddler functions as a motogen.
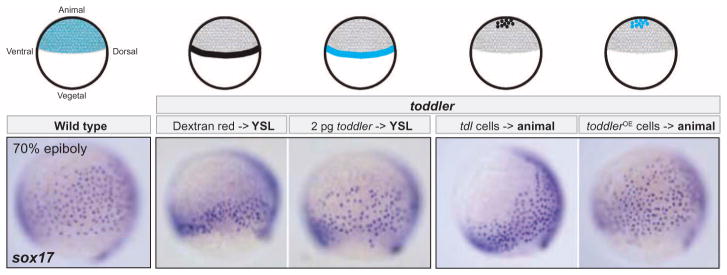
Ubiquitous or localized expression of Toddler promotes animal pole–directed endodermal cell migration in toddler mutant embryos. Toddler was expressed either vegetally from the yolk syncytial layer (YSL) (injection of toddler mRNA into the YSL) or animally from a toddler-overexpressing (OE) clone of cells transplanted into the animal pole. Dextran red injections into the YSL and transplantation of uninjected toddler mutant cells served as controls. Different treatments are illustrated on top; toddler expression domains are highlighted in cyan. All sox17 in situ hybridization images are lateral views of embryos at 70% epiboly (dorsal to the right).
Toddler Acts via APJ/Apelin Receptors
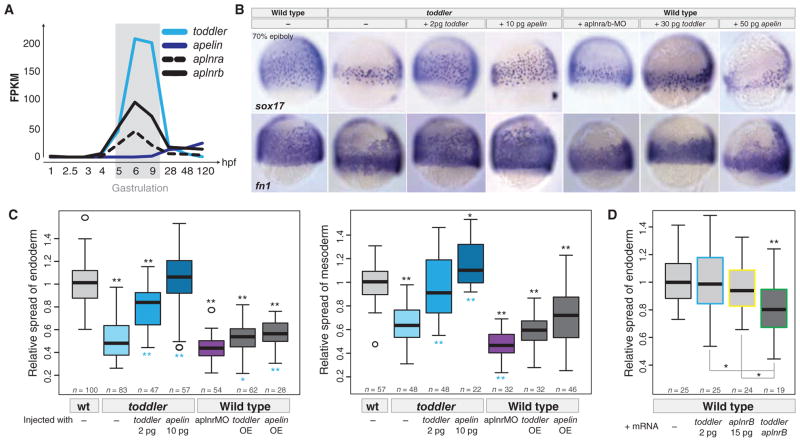
To identify candidate receptors for Toddler, we compared the toddler phenotype to previously described receptor mutant phenotypes. On the basis of the small size of Toddler peptide and the involvement of G protein signaling in gastrulation movements, we focused on G protein–coupled receptors (GPCRs) as candidate Toddler receptors (14, 23–30). Four observations raised the possibility that the G protein–coupled APJ/Apelin receptor might mediate Toddler signaling. First, loss of APJ/Apelin receptor signaling results in small hearts and affects lateral mesoderm migration in zebrafish (24–26), phenotypes reminiscent of some aspects of the toddler mutant phenotype. However, in contrast to the broad roles of Toddler in mesendoderm migration, APJ/Apelin receptor signaling had been specifically implicated in cardiovascular development (24–26, 31–36). Second, overexpression of Apelin, the only known ligand for the APJ/Apelin receptor (35–38), interferes with gastrulation movements in zebrafish (24–26). Third, the expression levels of Apelin receptors and Toddler peak during gastrulation (Fig. 5A), and Apelin receptors are expressed in mesendodermal cells [fig. S16A and (24, 25, 39)], the cell types affected in toddler mutants. Fourth, we found that Apelin is expressed only at the end of gastrulation [Fig. 5A and (24)], after the toddler and APJ/apelin receptor phenotypes (24, 25, 40) are apparent. These observations, together with the milder phenotypes observed in the absence of Apelin as compared to loss of APJ/Apelin receptors (24–26, 34, 36, 41–46), raised the hypothesis that Toddler might be the bona fide activator of APJ/Apelin receptor signaling during gastrulation. We tested three predictions of this model.
Fig. 5. Toddler acts via Apelin receptors.
(A) RNA-Seq–based expression levels of toddler, apelin, and apelin receptors (aplnra and aplnrb) during embryogenesis. (B) Genetic evidence for Toddler signaling via the Apelin receptor. Endodermal (sox17) and mesodermal [ fibronectin 1 ( fn1)] cell distributions were analyzed by in situ hybridization at 70% epiboly. Apelin receptor knockdown [aplnra/b morpholino (MO) injection] phenocopies toddler mutants, and Apelin production can rescue toddler mutants. Overexpression of Apelin causes phenotypes resembling toddler mRNA overexpression. (C) Quantification of the relative lateral spread of endoderm (left) and mesoderm (right). Quantifications are from multiple experiments (n = number of embryos per category). P values for pairwise comparisons with wild type (black, top) or toddler mutant (cyan, bottom) were calculated on the basis of a standard Welch’s t test (*P < 0.01; **P < 0.00001). (D) Synergistic effect of Toddler and Apelin receptor b on endodermal cell migration. Injection of toddler or aplnrb mRNA at low concentrations (2 and 15 pg, respectively) did not cause significant defects in animal pole–directed movement of endodermal cells (different batch of toddler mRNA than used in Fig. 2D). However, coinjection of both mRNAs reduced the extent of endoderm movement. Shown are the combined data of two independent experiments. P values for pairwise comparisons with wild type (top) or individual mRNA injections (bottom) were calculated on the basis of a standard Welch’s t test (*P < 0.01; **P < 0.00001).
First, we determined whether the absence of Apelin receptor function phenocopies toddler mutants. We reexamined aplnra and aplnrb double morphants (24–26) and found phenotypes that were highly similar to toddler mutants, including reduced movement of ventrolateral mesendoderm during gastrulation (Fig. 5, B and C). We also confirmed and extended previous analyses of the effects of Apelin overexpression (24–26) and found defects very similar to those caused by Toddler overexpression (Fig. 5, B and C). In addition, we observed that coexpression of Toddler and Apelin receptor at levels that individually did not cause major defects resulted in abnormal gastrulation movements reminiscent of Toddler and Apelin (24–26) overexpression phenotypes (Fig. 5D). These results reveal shared morphogenetic activities of the Apelin receptor and Toddler signaling pathways.
Second, we tested the epistatic relationship between Toddler and Apelin receptor signaling. The similarity of gain- and loss-of-function phenotypes precluded standard tests such as overexpression of Toddler in Apelin receptor mutants. Instead, we tested whether activation of Apelin receptor signaling can bypass the requirement for Toddler. Apelin mRNA injection into toddler mutant embryos restored normal mesendoderm migration (Fig. 5, B and C), cardiac development, and survival to adulthood. These results suggest that Toddler and Apelin activate the same signaling pathway.
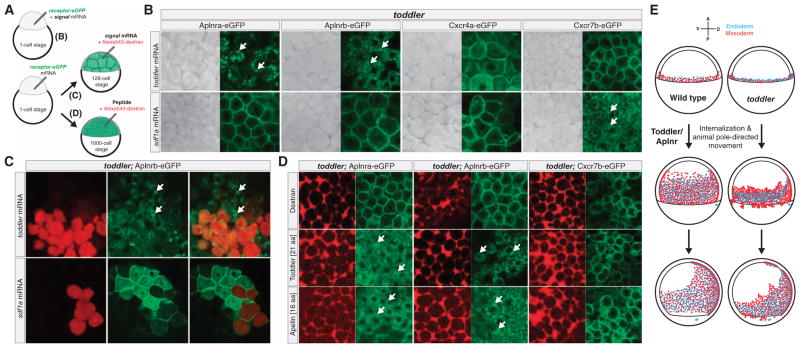
Third, we tested whether Toddler can drive the internalization of Apelin receptors (Fig. 6), a hallmark of activated GPCR signaling (47–50). We misexpressed toddler mRNA with eGFP-tagged Apelin receptor a or b and observed strong internalization of the receptors from the plasma membrane (Fig. 6B). This effect was specific because other signaling proteins (chemokines Sdf1a/Cxcl12a or Sdf1b/Cxcl12b) did not alter the distribution of membrane-bound Apelin receptors, nor did Toddler alter the distribution of other chemokine receptors (Cxcr4a-eGFP, Cxcr4b-eGFP, and Cxcr7b-eGFP) (Fig. 6B and fig. S18). Moreover, Toddler produced from a local clone of cells was sufficient to cause Aplnrb-eGFP internalization at a distance from the source, suggesting that secreted Toddler peptide can act on neighboring cells (Fig. 6C). This conclusion was further strengthened by the observation that extracellular injection of in vitro–synthesized C-terminal Toddler or Apelin peptides induced efficient internalization of Aplnr-eGFP (Fig. 6D). These results indicate that Toddler activates Apelin receptors.
Fig. 6. Toddler drives internalization of Apelin receptors.
(A) Schematic illustration of different treatments used to test for Toddler-mediated Apelin receptor internalization. (B) Test for signal-mediated internalization of eGFP-tagged receptors in zebrafish by coinjection of signal and receptor-eGFP mRNA into one-cell stage toddler mutant embryos. Receptor internalization was monitored by confocal microscopy. White arrows point to fluorescent foci of internalized receptors. In the absence of signal peptide overexpression, ectopically expressed receptors localize to the plasma membrane in pregastrulation toddler mutant embryos [see control Alexa543-dextran injections in (D)]. (C) Generation of a local source of Toddler or Sdf1a by injection of toddler or sdf1a mRNA (together with Alexa543-dextran as tracer) into a single cell at the 128-cell stage. Local expression of Toddler is sufficient to cause Aplnrb-eGFP internalization in cells that do not express toddler mRNA (non-red cells). (D) Extracellular injection of in vitro–synthesized C-terminal Toddler or Apelin peptide fragments is sufficient to drive internalization of Apelin receptors. (E) Model of the role of Toddler-Apelin receptor signaling in mesendodermal cell migration during zebrafish gastrulation. Left, wild type; right, toddler; top, 40% epiboly (mesendoderm specification and internalization); middle, 70% epiboly (animal pole–directed cell movement); bottom, 90% epiboly (dorsal convergence).
Discussion
Our study indicates that Toddler is an activator of APJ/Apelin receptor signaling, promotes gastrulation movements (see summary in Fig. 6E), and may be the first in a series of previously unknown developmental signals. While this study was under review, Toddler (named ELABELA) was independently reported to signal via APJ/Apelin receptors during endoderm differentiation and heart formation (51). The HUGO Gene Nomenclature Committee (HGNC) has recently designated the name Apela (apelin receptor early endogenous ligand) as the standardized symbol for Toddler/ELABELA/Ende. Our results lead to four major conclusions.
First, Toddler is a previously unrecognized signal that promotes cell movement during gastrulation. The rescue of toddler mutants by ubiquitous Toddler expression suggests that Toddler acts neither as a chemoattractant nor as a chemorepellent, but rather as a nondirectional signal to promote the internalization and movement of ventrolateral mesendodermal cells. Dorsal mesendoderm movement is largely unaffected in toddler mutants, consistent with the absence of Apelin receptor expression in this region and the role of other pathways in dorsal gastrulation movements (3). Both loss and overproduction of Toddler reduce cell movement, revealing that Toddler levels need to be tightly regulated to allow for normal gastrulation. It remains to be determined whether Toddler promotes motility by regulating cell shape, cellular protrusions, cell-substrate interactions, and cell-cell adhesion or through other means.
Second, Toddler-Apelin receptor signaling provides a long-sought link between mesendoderm induction and migration. Nodal signaling not only induces mesendoderm formation (52) but also activates the expression of Apelin receptors [fig. S17B and (39)]. Thus, Nodal-mediated induction of Apelin receptor expression might render cells competent to respond to Toddler and to become more motile (Fig. 6E). In this scenario, the activation of Apelin receptor expression in cells located at the margin at the end of the blastula stage would restrict the motogenic effects of Toddler and prevent ectopic and premature cell motility.
Third, Toddler is a novel agonist of APJ/Apelin receptor signaling, as evidenced by Toddler-induced internalization of Apelin receptors and rescue of toddler mutants by production of the known receptor agonist Apelin. Additionally, a fusion protein of alkaline phosphatase and Toddler binds to cells expressing Apelin receptors (51). Previous studies have implicated APJ/Apelin receptor signaling in a variety of biological processes, including the regulation of cardiovascular development and physiology, the control of fluid homeostasis, or even as a co-receptor for HIV infection (53, 54). Although Apelin has previously been the only known agonist of the APJ/Apelin receptor, genetic studies have found discrepancies between the roles of Apelin and its receptor in mouse (34, 36, 41, 45, 55) and zebrafish (24–26). For example, Apelin knockout mice are viable and fertile (45, 46, 56), whereas APJ/Apelin receptor mutant mice are born at sub-Mendelian ratios (34). Our studies suggest that both Toddler and Apelin can activate APJ/Apelin receptors and indicate that it is endogenous Toddler—not Apelin—that activates APJ/Apelin receptor signaling during zebrafish gastrulation. Analogously to the promise of Apelin in biomedical applications (53, 54), Toddler and its derivatives may take the place of Apelin in therapeutic contexts. Indeed, Toddler may also activate mammalian APJ/Apelin receptors because misexpression of zebrafish, mouse, and human Toddler induces similar overexpression phenotypes in zebrafish (fig. S19).
Fourth, our RNA-Seq and ribosome profiling data indicate that Toddler might just be one of several poorly characterized developmental signals that may have been missed in mutagenesis screens because of their small size. Applying similar genomic approaches to adult tissues might identify additional previously unknown signals that regulate physiological and behavioral processes.
Supplementary Material
Acknowledgments
We thank D. Richardson and C. Kraft from the Harvard Center for Biological Imaging for technical support; F. Merkle for providing human and mouse embryonic stem cell RNA; M. Lin for the initial PhyloCSF analysis; L. Solnica-Krezel, E. Raz, C. Houart, members of the 2013 MBL Zebrafish Course, and the Schier laboratory for helpful discussions; and S. Mango, W. Talbot, R. Losick, J. Farrell, and K. Rogers for comments on the manuscript. Obtaining the TALEN plasmids will require the completion of a Uniform Biological Material Transfer Agreement with the Massachusetts General Hospital. The Massachusetts General Hospital has applied for a patent that covers the FLASH method used to make the TALENs and J.K.J. is an inventor on this patent. J.K.J. has financial interests in Editas Medicine and Transposagen Biopharmaceuticals. J.K.J. is a member of the Scientific Advisory Board of Transposagen Biopharmaceuticals and is a co-founder and paid consultant of Editas Medicine and holds equity in both companies. J.K.J.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. This research was supported by NIH (A.F.S., A.P., and A.S.), Human Frontier Science Program (A.P. and E.V.), Howard Hughes Medical Institute (G.-L.C.), and the American Cancer Society (J.A.G.).
Footnotes
Author contributions: A.P. and A.F.S. conceived the study and wrote the paper. A.P. collected and analyzed the data, with contributions from A.F.S., M.L.N. (phenotypic characterization), E.V. (computational analyses), G.-L.C. (ribosome profiling), J.A.G., S.Z., D.R., S.Q.T., J.K.J. (TALEN-mediated mutagenesis), A.M., J.M., A.S. (mass spectrometry), and J.D. (MATLAB cell tracking).
References and Notes
- 1.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 5. Garland Science; New York: 2007. [Google Scholar]
- 2.Heisenberg CP, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Solnica-Krezel L, Sepich DS. Gastrulation: Making and shaping germ layers. Annu Rev Cell Dev Biol. 2012;28:687–717. doi: 10.1146/annurev-cellbio-092910-154043. [DOI] [PubMed] [Google Scholar]
- 4.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 5.Nowotschin S, Hadjantonakis AK. Cellular dynamics in the early mouse embryo: From axis formation to gastrulation. Curr Opin Genet Dev. 2010;20:420–427. doi: 10.1016/j.gde.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chew GL, et al. Ribosome profiling reveals resemblance between long non-coding RNAs and 5′ leaders of coding RNAs. Development. 2013;140:2828–2834. doi: 10.1242/dev.098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauli A, et al. Systematic identification of long noncoding RNAs expressed during zebrafish embryogenesis. Genome Res. 2012;22:577–591. doi: 10.1101/gr.133009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulitsky I, Shkumatava A, Jan CH, Sive H, Bartel DP. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell. 2011;147:1537–1550. doi: 10.1016/j.cell.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan AS, Hou J, Wei W, Hoodless PA. Expression of two novel transcripts in the mouse definitive endoderm. Gene Expr Patterns. 2010;10:127–134. doi: 10.1016/j.gep.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttman M, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyon D, et al. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sander JD, et al. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizoguchi T, Verkade H, Heath JK, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development. 2008;135:2521–2529. doi: 10.1242/dev.020107. [DOI] [PubMed] [Google Scholar]
- 15.Pézeron G, et al. Live analysis of endodermal layer formation identifies random walk as a novel gastrulation movement. Curr Biol. 2008;18:276–281. doi: 10.1016/j.cub.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Doitsidou M, et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/S0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 17.Haskel-Ittah M, et al. Self-organized shuttling: Generating sharp dorsoventral polarity in the early Drosophila embryo. Cell. 2012;150:1016–1028. doi: 10.1016/j.cell.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Venkiteswaran G, et al. Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. Cell. 2013;155:674–687. doi: 10.1016/j.cell.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donà E, et al. Directional tissue migration through a self-generated chemokine gradient. Nature. 2013;503:285–289. doi: 10.1038/nature12635. [DOI] [PubMed] [Google Scholar]
- 20.Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/S0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 21.Giacobini P, et al. Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration. J Neurosci. 2007;27:431–445. doi: 10.1523/JNEUROSCI.4979-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirfel G, Borm B, Rigort A, Herzog V. The secretory β-amyloid precursor protein is a motogen for human epidermal keratinocytes. Eur J Cell Biol. 2002;81:664–676. doi: 10.1078/0171-9335-00284. [DOI] [PubMed] [Google Scholar]
- 23.Nair S, Schilling TF. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science. 2008;322:89–92. doi: 10.1126/science.1160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng XXI, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell. 2007;12:391–402. doi: 10.1016/j.devcel.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Scott IC, et al. The G protein-coupled receptor Agtrl1b regulates early development of myocardial progenitors. Dev Cell. 2007;12:403–413. doi: 10.1016/j.devcel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Paskaradevan S, Scott IC. The Aplnr GPCR regulates myocardial progenitor development via a novel cell-non-autonomous, Gαi/o protein-independent pathway. Biol Open. 2012;1:275–285. doi: 10.1242/bio.2012380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, et al. Gpr125 modulates Dishevelled distribution and planar cell polarity signaling. Development. 2013;140:3028–3039. doi: 10.1242/dev.094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin F, et al. Gα12/13 regulate epiboly by inhibiting E-cadherin activity and modulating the actin cytoskeleton. J Cell Biol. 2009;184:909–921. doi: 10.1083/jcb.200805148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76:1075–1089. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 30.Parks S, Wieschaus E. The Drosophila gastrulation gene concertina encodes a Gα-like protein. Cell. 1991;64:447–458. doi: 10.1016/0092-8674(91)90652-F. [DOI] [PubMed] [Google Scholar]
- 31.D’Aniello C, et al. G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signaling pathway. Circulation. 2009;105:231–238. doi: 10.1161/CIRCRESAHA.109.201186. [DOI] [PubMed] [Google Scholar]
- 32.Wang IN, et al. Apelin enhances directed cardiac differentiation of mouse and human embryonic stem cells. PLOS One. 2012;7:e38328. doi: 10.1371/journal.pone.0038328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tempel D, et al. Apelin enhances cardiac neovascularization after myocardial infarction by recruiting Aplnr+ circulating cells. Circ Res. 2012;111:585–598. doi: 10.1161/CIRCRESAHA.111.262097. [DOI] [PubMed] [Google Scholar]
- 34.Kang Y, et al. Apelin-APJ signaling is a critical regulator of endothelial MEF2 activation in cardiovascular development. Circ Res. 2013;113:22–31. doi: 10.1161/CIRCRESAHA113.301324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inui M, Fukui A, Ito Y, Asashima M. Xapelin and Xmsr are required for cardiovascular development in Xenopus laevis. Dev Biol. 2006;298:188–200. doi: 10.1016/j.ydbio.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Charo DN, et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am J Physiol Heart Circ Physiol. 2009;297:H1904–H1913. doi: 10.1152/ajpheart.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatemoto K, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 38.Lee DK, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 39.Tucker B, et al. Zebrafish Angiotensin II Receptor-like 1a (agtrl1a) is expressed in migrating hypoblast, vasculature, and in multiple embryonic epithelia. Gene Expr Patterns. 2007;7:258–265. doi: 10.1016/j.modgep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Nornes S, Tucker B, Lardelli M. Zebrafish aplnra functions in epiboly. BMC Res Notes. 2009;2:231. doi: 10.1186/1756-0500-2-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scimia MC, et al. APJ acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488:394–398. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishida J, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 43.Roberts EM, et al. Abnormal fluid homeostasis in apelin receptor knockout mice. J Endocrinol. 2009;202:453–462. doi: 10.1677/JOE-09-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kidoya H, et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27:522–534. doi: 10.1038/sj.emboj.7601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuba K, et al. Impaired heart contractility in Apelin gene–deficient mice associated with aging and pressure overload. Circ Res. 2007;101:e32–e42. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 46.Sheikh AY, et al. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H88–H98. doi: 10.1152/ajpheart.00935.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans NA, et al. Visualizing differences in ligand-induced β-arrestin–GFP interactions and trafficking between three recently characterized G protein-coupled receptors. J Neurochem. 2001;77:476–485. doi: 10.1046/j.1471-4159.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee DK, Ferguson SSG, George SR, O’Dowd BF. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with β-arrestin. Biochem Biophys Res Commun. 2010;395:185–189. doi: 10.1016/j.bbrc.2010.03.151. [DOI] [PubMed] [Google Scholar]
- 49.Reaux A, et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–1096. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhou N, et al. Cell–cell fusion and internalization of the CNS-based, HIV-1 co-receptor, APJ. Virology. 2003;307:22–36. doi: 10.1016/S0042-6822(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 51.Chng SC, Ho L, Tian J, Reversade B. ELABELA: A hormone essential for heart development signals via the apelin receptor. Dev Cell. 2013;27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 53.Barnes G, Japp AG, Newby DE. Translational promise of the apelin–APJ system. Heart. 2010;96:1011–1016. doi: 10.1136/hrt.2009.191122. [DOI] [PubMed] [Google Scholar]
- 54.Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Kasai A, et al. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1717–1722. doi: 10.1161/ATVBAHA.108.163402. [DOI] [PubMed] [Google Scholar]
- 56.Kidoya H, Naito H, Takakura N. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood. 2010;115:3166–3174. doi: 10.1182/blood-2009-07-232306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.