Abstract
Fast-spiking (FS) interneurons provide the main route of feed-forward inhibition from cortex to spiny projection neurons in the striatum. A steep current-firing frequency curve and a dense local axonal arbor suggests that even small excitatory inputs could translate into powerful feed-forward inhibition, but such an arrangement is also sensitive to amplification of spurious synaptic inputs.
We show that a transient potassium (KA) current allows the FS interneuron to strike a balance between sensitivity to correlated input and robustness to noise, thereby increasing its signal to noise ratio (SNR). First, a compartmental FS neuron model was created to match experimental data from striatal FS interneurons in cortex-striatum-substantia nigra organotypic cultures. Densities of sodium, delayed rectifier and KA channels were optimized to replicate responses to somatic current injection. Spontaneous AMPA and GABA synaptic currents were adjusted to the experimentally measured amplitude, rise time, and inter-event interval histograms. Second, two additional adjustments were required to emulate the remaining experimental observations. GABA channels were localized closer to the soma than AMPA channels to match the synaptic population reversal potential. Correlation among inputs was required to produce the observed firing rate during up-states. In this final model, KA channels were essential for suppressing down-state spikes while allowing reliable spike generation during up-states. This mechanism was particularly important under conditions of high dopamine. Our results suggest that KA channels allow FS interneurons to operate without a decrease in SNR during conditions of increased dopamine, as occurs in response to reward or anticipated reward.
Keywords: computer model, up states, fast spiking interneuron, transient potassium current, dopamine
1. Introduction
The striatum is vital for the proper execution and selection of behavior, and disturbances in striatal dynamics give rise to both motor and cognitive disorders (Brown and Marsden 1990; DeLong 1990; Tekin and Cummings 2002). Of particular interest for our understanding of striatal dynamics is how striatal neurons integrate cortical inputs and participate in local striatal signal processing. In particular, periods of increased synaptic activity depolarize striatal spiny projection neurons (SP) into an ‘up-state’, which is the only time during which action potentials are generated (Wilson and Kawaguchi 1996). Though considerable attention has been given to how intrinsic properties of striatal neurons control up-states (e.g. Wilson 1993; Nisenbaum et al. 1996; Wilson and Kawaguchi 1996; Gruber et al. 2003), experimental findings on local synaptic transmission in the striatum (Plenz and Kitai 1998; Koos and Tepper 1999, 2002; Czubako and Plenz 2002; Tunstall et al. 2002; Blackwell et al. 2003; Guzman et al. 2003; Taverna et al. 2004; Koos et al. 2004) suggest that GABAergic circuits also play a fundamental role in modulating the spiking of SP neurons, which are the output neurons of the striatum (Plenz 2003; Tepper et al. 2004).
A major source of GABAergic synaptic input to SP neurons is from the fast spiking (FS) interneuron, through its dense, local axonal arbor (Kawaguchi 1993; Kawaguchi et al. 1995). FS interneurons receive glutamatergic inputs from cortico-striatal projection neurons; thus they provide feed-forward inhibition to striatal neurons (Plenz and Kitai 1998; Koos and Tepper 1999). In addition, FS interneurons receive GABAergic inputs from striatal interneurons and globus pallidus neurons. Despite the relatively small population of FS interneurons (1-5 %; Kita 1993), they may profoundly influence striatal activity because of their ability to fire at high rates (Koos and Tepper 1999; Plenz and Aertsen 1996; Nisenbaum and Berger 1992; Berke et al. 2004), dense axonal arborization and preferential innervation of SP neuron somata (Kubota and Kawaguchi 2000; Bennett and Bolam 1994).
These properties of FS interneurons suggest that small input signals may be translated into powerful inhibition; however, such an arrangement also is sensitive to ‘noise’, such as spurious synaptic inputs. This feedforward inhibitory circuit may require some filter mechanism that prevents irregular activation by a few random cortical inputs. Otherwise, inadvertent FS interneuron action potentials may suppress SP neuron firing, counteracting the selection mechanism of spiny projection neurons for cortical inputs, or disrupting the precise timing of action potentials, which control dendritic calcium dynamics (Kerr and Plenz 2002, 2004; Carter and Sabatini 2004).
Sensitivity to spurious synaptic inputs can be suppressed in several ways. For example, a very negative resting potential, as seen in spiny projection neurons, requires multiple synaptic inputs to coincide in time (spatial integration) to depolarize the neuron to spike threshold. Such a mechanism is unlikely to work in FS interneurons, as their resting potential is closer to spike threshold. Alternatively, a KA current necessitates multiple synaptic inputs over a more prolonged time period (temporal integration). FS interneurons exhibit a delay in spike generation in response to depolarization, which is suggestive of a KA current (found in fast spiking interneurons of the neocortex (Goldberg et al. 2003a, 2003b). In the present study, we use a computer model of an FS interneuron to assess the effect of KA currents on the selectivity of FS interneurons, by comparing spike generation during the up-state to spike generation during the down-state. The latter is representative of the sensitivity to spurious synaptic inputs because down states represent periods of low synaptic activity. We assessed the robustness of the effect to changes in intrinsic excitability and inhibitory synaptic inputs, as modulated by dopamine (Nicola et al. 2000; Bracci et al. 2002; Centonze et al. 2003)
2. Methods
2.1 Model
The compartmental model of an FS interneuron was created using the GENESIS simulation software (http://www.genesis-sim.org/GENESIS/) running on the Redhat Linux operating system. First, the morphology and passive properties were adjusted. Second, the voltage dependent channels were included (Table 1). Third, synaptic channels and input spike trains were incorporated (Table 2). Model responses to current injection and statistics of synaptic inputs were highly constrained by experimental measurements of synaptic inputs (Table 3).
Table 1.
Equations for the intrinsic currents. The state, x(V,t), of the different gating particles, m, n and h, are given by the equation: , where the expressions are given above for INa, IKv1.3 and IKv3.1/3.2. The transient potassium current is instead described using x∞(V) = αx(V)/(αx(V) + βx(V)), and τ(V) = 1/(αx(V) + βx(V)) or just considered as constant. All parameters are in SI units.
| Channel type |
αm, or m∞ for KA |
βm, or τm for KA |
αh, or h∞ for KA |
βh, or τh for KA |
||||
|---|---|---|---|---|---|---|---|---|
| Fast sodium current INa = ḡNa m3h(V − 0.045) |
|
|
|
|
||||
| Potassium current, Kv1.3 IKv 1.3 = ḡK13n4(V− (−0.09)) |
|
|
- | - | ||||
| Potassium current, Kv3.1/3.2 IKv3.13.2 = ḡK3132n2(V− (−0.09)) |
|
|
- | - | ||||
| Transient potassium current IKA = ḡKAm4h(V − (−0.09)) |
|
|
|
0.014 |
Table 2.
Densities of ion channel conductances in the different compartments. Units are in S/m2.
| Compartment | Na+ | Kv3.1/3.2 | Kv1.3 | KA |
|---|---|---|---|---|
| Soma | 1149 | 582 | 1.46 | 333 |
| Primary dendrite | 0 | 0 | 0 | 90 |
| Secondary/tertiary dendrite | 0 | 0 | 0 | 0 |
Table 3.
Synaptic currents modelled. Ion channels activated by synapses are described by: Isyn = Gmax · Gsyn(t)(V − Esyn) where Esyn is the reversal potential. Gsyn(t) is the synaptic conductance modeled as: where τ1 > τ2 and Amax is adjusted to approach unity at the peak. The units are in s, V and S.
| Synapse type | Esyn | Time Constants | Gmax | ||
|---|---|---|---|---|---|
| Inhibitory synapse | GABA | −0.060 | τ1 1.33 10−3 | τ2 4·10−3 | 1.131.10−9 |
| Excitatory synapse | AMPA | 0.000 | τ1 0.67·10−3 | τ2 2·10−3 | 0.754.10−9 |
2.1.1 Morphology
The branching structure of the fast spiking interneuron is a prototype of the morphology revealed by biocytin reconstructions in the acute slice (Kawaguchi 1993) and organotypic cultures (Plenz and Kitai 1998). The morphology contains three primary branches, 6 secondary branches, and 12 tertiary branches (Fig 1A), each subdivided into multiple, isopotential com-partments. Membrane resistivity=20,000 Ωcm2; axial resistivity = 300 Ωcm; membrane capacitance= 0.7 μF/cm2. These, passive properties were modified from commonly accepted values (Major et al. 1994; Spruston et al. 1994) to reproduce the input resistance and time constants previously measured (Blackwell et al. 2003). This branching structure was sufficient to reproduce the effect of electrotonic properties on distributed synaptic inputs (Fig 1B,C)
Figure 1.
Morphology, passive properties, and voltage dependent currents of FS interneuron model reproduce experimentally measured IV curves. (A) Morphology of FS interneuron. The 15 μm diameter soma has three primary dendrites, of 90 μm length × 1.5 μm diameter. Each primary branch bifurcates to form secondary branches of 148 μm length × 0.75 μm diameter. Each secondary branch bifurcates to form tertiary branches of 240 μm length × 0.5 μm diameter. Each branch is subdivided into compartments no more than 0.1 times the length constant, resulting in a total of 127 compartments for the cell. (B) Distribution of spike latencies for FS neurons (open squares) and model neuron (solid circle). (C) fI curve for FS neurons and model neuron.
2.1.2 Voltage-gated Channels
Action potentials were generated by the fast sodium current and delayed rectifier (Kv3.1/3.2 and Kv1.3) potassium currents in the soma (Erisir et al. 1999). Though transient potassium currents have not yet been identified in striatal FS neurons, a transient potassium current was included in the soma and primary dendrite compartments on the basis of the experimentally observed spike latency (Blackwell et al. 2003; Kawaguchi 1993; Koos and Tepper 2002), which is defined as the time between current injection and first spike. The voltage dependence was modified from that of transient potassium currents in spiny projection neurons (Akins et al. 1990; Nisenbaum et al. 1996; Tkatch et al. 2000) to produce the spike latency observed in experimental data (Blackwell et al. 2003). Maximal conductance of these three voltage dependent channels was optimized, using the simulated annealing routines in GENESIS, to produce spike latency, spike threshold, and frequency-current relationship similar to that measured in FS interneurons in vitro (Fig 1B,C; Blackwell et al., 2003). Note that the lack of an axon hillock and initial segment necessitates a high channel density for action potential initiation.
2.1.3 Synaptic Inputs
AMPA glutamatergic (Götz et al. 1997; Jahn et al. 1998; Stefani et al. 1998) and GABAergic (Salin and Prince 1996) synaptic channels were placed in the soma and dendrite compartments, resulting in 254 evenly distributed synaptic inputs. Each channel was activated by an independent Poisson distributed input train. As described in the results, this spatial distribution was corrected to better match characteristics of FS interneurons in triple co-cultures. The interspike interval of each of the 254 down-state Poisson trains was adjusted to 9 sec (frequency 0.11 Hz) to reproduce the experimentally observed down-state inter synaptic event interval (IEI) distribution for the population. The maximal synaptic conductance and distribution of the channels was adjusted to produce the same amplitude and rise time distribution measured experimentally (Blackwell et al. 2003). Table 4 illustrates that the mean amplitude, rise time and inter-event interval in the FS interneuron model is within the range found experimentally for synaptic inputs to FS interneurons in co-culture. In addition, simulated PSPs had the same skewed distribution as found experimentally (Figure 2A,B).
Table 4.
Comparison between model and experimental data. SD for experimental data is calculated from square root of the mean of the variances of the 6 FS neurons. Two aspects underly the difference in experimentally measured PSC IEI and PSP IEI. PSPs and PSCs were measured from partially overlapping subsets; the neurons unique to each subset had very different interevent intervals. Also, recordings in voltage clamp had more high frequency noise compared to recordings in current clamp; thus, slightly more synaptic events were detected in current clamp.
| Characteristic | Mean from Simulations ± (SD) | Measured Range (n=7) ± (SD) |
|---|---|---|
| PSP Amplitude | 2.77 ± 1.94 mV | 1.7 ± 1.33 mV |
| PSP Rise Time | 4.31 ± 1.32 ms | 5.4 ± 5.7 ms |
| PSP InterEvent Interval | 148 ± 143 ms | 65 ± 71 ms |
| PSC Amplitude | -17.4 ± 12.9 pA | -29 ± 29.7 pA |
| PSC Rise Time | 2.1 ± 0.7 ms | 1.0 ± 0.65 ms |
| PSC InterEvent Interval | 140 ± 143 ms | 131 ± 168 ms |
Figure 2.
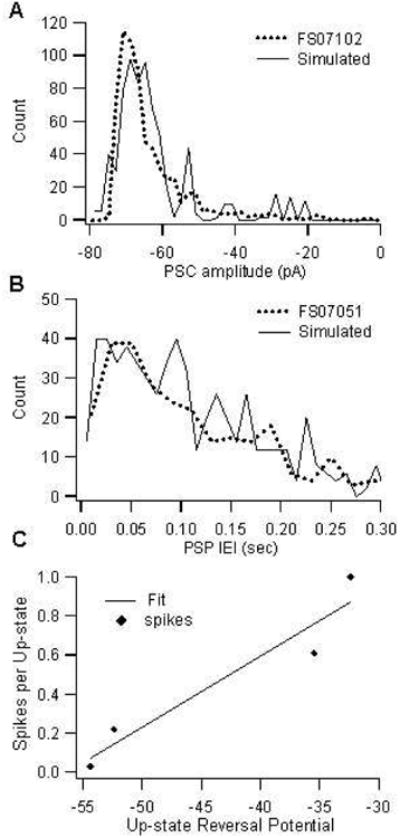
Synaptic characteristics of fast spiking interneuron model were similar to experimentally measured values (Blackwell et al. 2003). (A) Histogram of PSC amplitude from simulated neuron (solid line), and cell FS07102 (dashed line); simulated counts were scaled by 2 for comparison with measurements. (B) Histogram of inter-event interval of PSPs from simulated neuron (solid line), and cell FS07051 (dashed line). (C) Spikes per up-state is correlated with up-state reversal potential (R2=0.92).
Up-states were generated as transient periods of high synaptic input frequency of all AMPA and GABA synapses. Each of the 254 up-state Poisson trains had an interspike interval of 0.05 sec, compatible with experiment measurements (Blackwell et al. 2003), and activated the same set of synapses as the down-state Poisson trains. The duration of the up-states ranged from 50 ms to 400 ms (the range observed experimentally); down-state duration was set to 300 ms.
Most simulations were performed in current clamp using a time step of 0.01 msec. Additional voltage clamp simulations were performed at potentials between -70 mV and -20 mV using a 200 ms duration up-state to determine the reversal potential of the up-state charge. These simulations used a time step of 0.001 msec. Results were based on simulations of 200 up-states and down-states, each with a different set of synaptic inputs.
2.2 Signal to Noise Analysis
Ideally, signal to noise ratio (SNR) is quantified as spike rate in response to signal divided by spike rate in response to noise; however, in the striatum, signal synaptic inputs are not discriminable from noise synaptic inputs. Thus, under the assumption that important information is transmitted to the globus pallidus by the striatum during up-states, all up-state spikes are defined as signal spikes. Spikes during down-states (periods of low frequency synaptic inputs) are used as surrogates for noise spikes. The number of noise spikes often was zero, making signal to noise ratio (SNR) undefined; therefore, the SNR calculation was modified to be the ratio of up-state spikes to total spikes.
The number of up-state spikes, and the number of down-state spikes were measured for each combination of down-state activity, gKA, and up-state duration. The effect of these parameters on spike rates during both up-states and down-states was evaluated using the procedure LOGISTIC (which performs logistic regression on data with a limited number of ordinal response values) and GLM (which evaluates general linear models) in the statistical software SAS (SAS Institute, Gary, NC).
3 Results
This study addresses the issue of synaptic integration and the control of action potential generation. Recent in vivo findings show that neurons receive hundreds to thousands of inputs, producing up-states during which action potentials are generated. The present study uses theoretical techniques to address questions regarding synaptic inputs and action potential generation. In the first section, the development of a model highly constrained by electrophysiological data reveals some properties on synaptic inputs. In the second section, simulations evaluate the effect of the KA current on synaptic integration. In the third section, the interaction of dopamine and KA currents are addressed.
3.1 Adjustment of Population Synaptic Inputs and Spike Generation during Up-states
In the absence of precise anatomical data on synaptic inputs to striatal FS interneurons, we used electrophysiological data on spontaneous synaptic inputs during up-states in conjunction with model development to evaluate different distributions of synaptic inputs. Two characteristics, reversal potential of the population of post-synaptic currents (PSC) and mean number of spikes generated per up-state, were used simultaneously to constrain the distribution of synaptic inputs in the FS interneuron model. One characteristic was the up-state synaptic population reversal potential in FS interneurons, which ranged from -33 mV to -45 mV in triple co-cultures (after correction for junction potential of 14 mV) (Blackwell et al. 2003). The second characteristic used to constrain model synaptic input characteristics was mean number of spikes per up-state. Though a bimodal membrane potential distribution is not as prominent in FS neurons as in SP neurons (Plenz and Kitai 1998), FS neurons alternate between low synaptic activity states and high synaptic activity states. Furthermore, the high synaptic activity states are simultaneous with SP neuron up-states (n=4, (Plenz and Kitai 1998); n=2 (Blackwell et al. 2003)). Using synaptic activity as the indicator of up and down-states, we calculate that the number of spikes per down-state was 0, compared to a mean of 0.82 spikes per up-state (range of 0–2.25, N=6 FS interneurons). In addition, the number of spikes per up-state was highly correlated with the PSC reversal potential (Fig 2C, R2=0.92, N=4 FS interneurons).
The experimentally measured reversal potential was considerably lower than the -30 mV in the model with AMPA and GABA synapses evenly distributed. To lower the simulated reversal potential, GABA synaptic inputs from tertiary branches were re-distributed evenly among the soma, and primary and secondary dendritic branches. This spatial distribution, motivated by the spatial gradient of GABAergic inputs measured in hippocampal fast spiking interneurons (Pettit and Augustine 2000), produced a simulated reversal potential of -43 mV, which is within the range measured experimentally (Fig 3).
Figure 3.
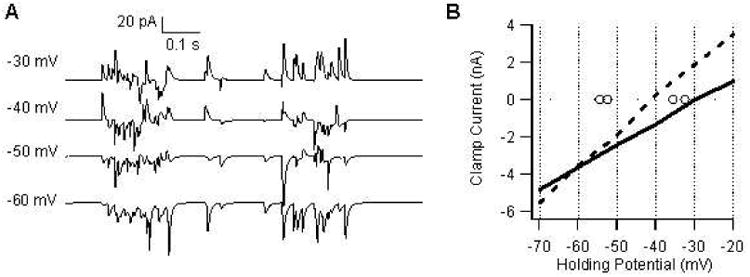
Reversal potential depends on distribution of synapses. (A) Simulated up-states in voltage clamp at potentials between −30 and −60 mV to demonstrate reversal of clamp current from inward to outward at a holding potential near −40 mV. The current is clearly inward at −60 mV, outward at −30 mV, and is a mixture of inward and outward at −40 mV. This pattern is similar to that observed experimentally (Blackwell et al., 2003). (B) Mean up-state current vs holding potential for equal distribution (solid line), or GABA redistributed toward soma (dashed line). The latter had a reversal potential of −43 mV, within the range of measured up-state reversal potentials (circles). Re-distributing the synaptic inputs lowers the reversal potential by approximately 12 mV.
Placing GABA synapses close to the soma rescued the reversal potential, but resulted in spontaneous spiking during up-states less frequently than measured experimentally (Figure 4A,C, mean rate = 0.17 per up-state). Several adjustments were possible to produce an increase in up-state spikes. One possibility was to increase the amount of glutamate current, either by an increase in frequency or amplitude of synaptic inputs. However, this type of solution raised the synaptic population reversal potential, and so was not acceptable. An alternative was to increase the correlation among the synaptic inputs as demonstrated by paired intracellular recordings (Plenz and Kitai 1998; Stern etal. 1998). Correlation was increased among all synaptic inputs, both GABA and AMPA, by activating randomly chosen inputs with the same Poisson spike trains (Rudolph and Destexhe 2001). The correlation parameter, c, is given by the equation where Ns is the number of independent Poisson spike trains, and N is the total number of synapses. This results in each spike train being assigned to approximately N/Ns synapses. As observed in Fig 4A,B, the increase in membrane potential fluctuations that accompanied the increase in correlation produced an increase in spike rate (mean rate = 0.35 spikes per upstate at correlation=0.49, Ns/N=0.3). The resulting spike rate is closer to the values observed experimentally (Fig 4C); and the correlation is similar to values used for synaptic inputs to cortical neurons (Rudolph and Destexhe 2001).
Figure 4.

Simulating the experimentally observed rate of up-state spiking required correlation among spike trains. (A) Change in up-state spike rate vs correlation. (B) Sample of up-state spike rate for uncorrelated (c=0.0) and correlated (c=0.5) Poisson input spike trains. The correlation parameter, c = f(N) (Ns = N + √c·(1−N)), where Ns is number of independent Poisson spike trains, and N is the number of synapses (Rudolph and Destexhe, 2001). (C) Sample of up-state spiking from data collected in Blackwell et al. (2003), cell FS07102, which had a mean spikes-per-up-state of 0.62. (D) Expanded view of a simulated (left) and experimental (right) up-state. The selected up-states are indicated with * in panels B, C.
These simulations imply that, given the experimental constraints of reversal potential and spontaneous firing rate during the up-state, GABA synaptic inputs are preferentially located close to the soma, and a modest correlation among all synaptic inputs is required during up-states.
3.2 Transient Potassium Currents Increase Signal to Noise Ratio
This optimized model, which replicates properties of up- and down-states of FS interneurons in triple cultures, was used to investigate the role of the KA current and background noise on signal processing. Specifically, we addressed how the delay to spike initiation, as produced by typical KA currents, influenced the signal to noise ratio (SNR) under different amounts of background activity. The role of the KA current was assessed quantitatively by performing simulations with the KA conductance (gKA) adjusted 20% higher and 20% or 40 % lower than the control value. SNR is calculated as the ratio of up-state spikes to the sum of down-state and up-state spikes. To evaluate the effect of down-state activity, simulations were performed with down-state Poisson train having ISIs of 0.012, 0.037, 0.11 (default), 0.33 and 1 Hz. Though spikes during the down-state do not influence firing of spiny projection neurons, down-state spikes are representative of the sensitivity to spurious synaptic inputs.
As noise was increased from 0.1 to 1 Hz, both up-state and down-state spike rates increased significantly, at all values of gKA (Table 5,6; Fig 5 and 6B,C). The number of up-state spikes doubled, but the number of down-state spikes increased several fold. The relative sensitivity of up-states and down-states to noise was captured in the SNR curves (Fig 6A), which demonstrate that SNR decreases at high noise. Fig 6A also demonstrates that high gKA is particularly important at high noise levels. The average up-state spike rate (the number of spikes divided by the up-state duration) increased as gKA decreased at all noise levels (Fig 5, 6B). In contrast during the down-state, the change in spike rate with gKA was seen only for higher noise levels (Fig 5, 6C). Therefore, the increase in down-state spike rate with noise was particularly prominent at lower gKA. The sensitivity to noise and gKA was the same for upstate durations from 50 to 400 ms, which covers the range of experimentally observed values in vitro (Blackwell et al. 2003; Table 2) and in vivo (Stern et al. 1998). These results demonstrate that KA is important because it increases SNR at high noise levels.
Table 5.
Results of statistical analysis evaluating the effect of noise and gKA on spike production for both down-states and several different up-state durations. Chi square and P value are from logistic regression. The noise by gKA interaction terms are not significant.
| Duration | Noise | gKA | noise*gKA |
|---|---|---|---|
| 50ms | 355 | 38 | 2.51 |
| <0.0001 | <0.0001 | 0.29 | |
| 100ms | 652 | 105 | 3.31 |
| <0.0001 | <0.0001 | 0.19 | |
| 200ms | 988 | 163 | 1.36 |
| <0.0001 | <0.0001 | 0.51 | |
| 400ms | 1740 | 387 | 7.85 |
| <0.0001 | <0.0001 | 0.02 | |
| downstate | 448 | 858 | 0.19 |
| <0.0001 | <0.0001 | 0.91 |
Table 6.
Effect of duration on up-state spike rate (number of spikes divided by duration) was assessed using general linear models. The duration by gKA interaction term was significant because duration modulated spike rate only for gKA =60%.
| Noise | gKA | noise*gKA | duration | duration*gKA | |
|---|---|---|---|---|---|
| F value | 182 | 471 | 10.3 | 0.67 | 7.91 |
| P value | <0.0001 | <0.0001 | <0.0001 | 0.41 | <0.0001 |
Figure 5.
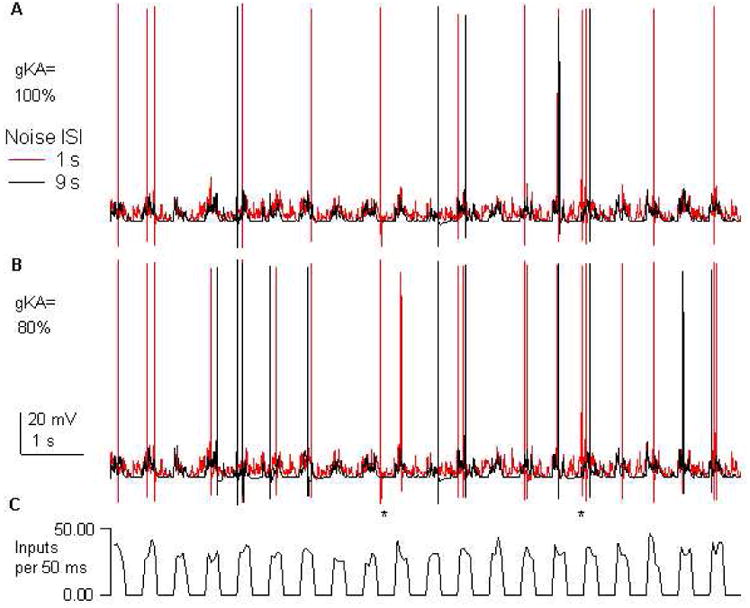
Effect of noise and gKA on spiking during up-states. Simulated traces of membrane potential for (A) gKA=100% and (B) gKA=80% of control values. Black traces show responses with noise frequency=0.11 Hz, the default value. Red traces show responses to high noise, frequency=1 Hz. The same random pattern of up-state synaptic inputs are repeated for each condition. (C) Up-states are created with an increase in synaptic inputs. With down-state activity in the middle of the experimentally measured frequencies (noise freq=0.11 Hz), approximately half of the 200 ms duration up-states produce action potentials, and no down-state spikes are observed. When the down-state activity is increased (noise freq=1 Hz), the number of up-state spikes increases. When gKA is reduced to 80% of its control value, there is also a small increase in the number of down-state spikes (indicated by *).
Figure 6.
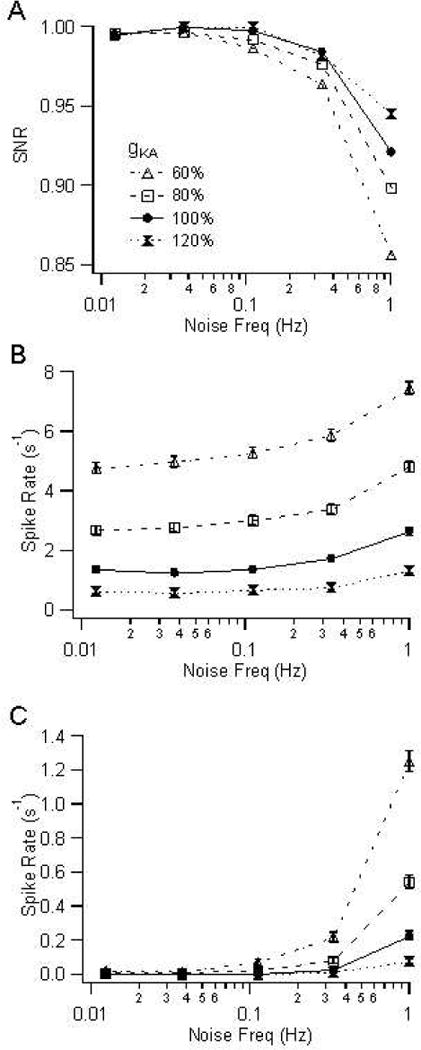
Effect of noise and gKA on (A) SNR, defined as up-state spike rate / (up-state spike rate + down-state spike rate), (B) up-state spike rate, and (C) down-state spike rate.
To demonstrate that properties attributed to the KA current are not due to a general decrease in potassium currents, simulations were repeated in models with 80% gKA and a compensatory increase in the Kv3.1/3.2 conductance. The compensatory value was determined by using the simulated annealing parameter optimization routine, which adjusted the Kv3.1/3.2 conductance to produce the best match to the experimentally measured FI curves. This increase in the Kv3.1/3.2 current did not compensate for the decrease in the gKA value in terms of effect on SNR. Thus the ability to suppress down-state and up-state spikes is specific to the KA current.
The ability of the KA current to suppress down-state spikes was robust to variation in unconstrained parameters. High values of gKA prevented an increase in down-state spikes in FS model neurons in which (A) synaptic inputs were uncorrelated, (B) GABA and AMPA synapses were evenly distributed, and (C) PSCs had slower rise times and decay times. Thus, the result is not contingent on particular characteristics of synaptic inputs.
NMDA currents were not included in the original model because the NMDA contribution is very small in FS interneurons in the cortex, and because the linear relationship between up-state charge and holding potential (Blackwell et al 2003) suggests that the NMDA contribution is small also in striatal FS interneurons. Nonetheless, robustness of our results was further explored with additional simulations performed in two additional models with NMDA channels. In one model, the addition of NMDA currents with a distribution similar to the AMPA channels decreased, but did not eliminate, the correlation needed to produce spiking during up-state periods. In a second model, with both NMDA channels and KA channels in all dendritic compartments, inclusion of KA channels on distal dendrites counteracted the effect of NMDA channels on excitability. In both of these models, the presence of NMDA channels did not change the role of KA currents in improving SNR: both models exhibits higher SNR with higher values of gKA.
3.3 KA Currents Increase SNR during High Levels of Dopamine
Neuromodulation, especially that produced by dopamine, is an important aspect of striatal function (Nicola et al. 2000; Gruber et al. 2003). Dopamine release is increased in response to reward (Schultz 2002), and dopamine modifies the characteristics of FS interneurons (Bracci et al. 2002), producing a small depolarization, and decreasing the amplitude of GABA synap-tic inputs. The effect of dopamine on the model was simulated as a 2 mV depolarization (produced by increasing the leak reversal potential), and a 20% reduction in amplitude of all GABA synaptic conductances (Centonze et al. 2003). This effect of dopamine caused a decrease in the SNR (Fig 7A) for higher noise values. The reduced SNR is attributed to an increase in down-state spike frequency seen at higher noise levels. Dopamine also produced a 1 Hz increase in up-state spike frequency at the control value of gKA, and a 2 Hz increase in up-state spike frequency with gKA = 80%, though this had little effect on SNR. The decrease in SNR was smaller for gKA = 100%, showing that gKA minimizes the reduction in SNR during times of elevated dopamine. These results demonstrate that multiple factors influence a neuron's function. Under low noise or low dopamine conditions, a reduced gKA may be optimal, but under high dopamine conditions, a larger value of gKA may be needed for suppressing spikes in response to spurious synaptic inputs.
Figure 7.
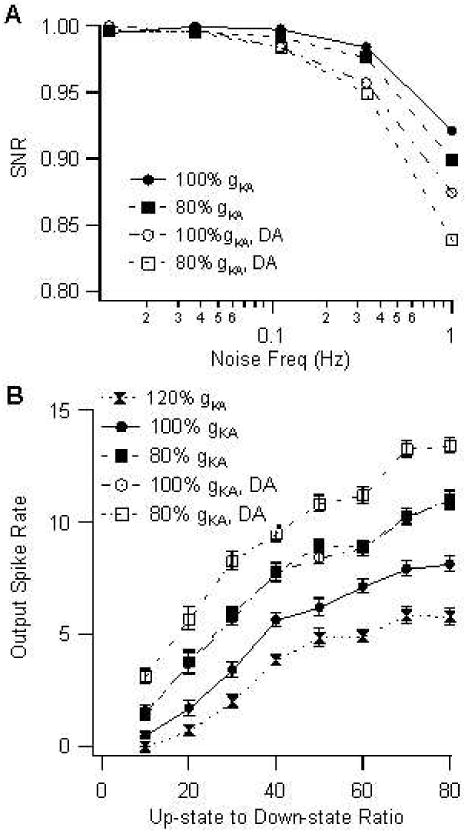
Sensitivity to noise (A) and change in input frequency (B) during simulated dopamine modulation of FS interneuron model (+2mV depolarization, GABA reduced to 80%). (A) SNR as a function of noise frequency; (B) Sensitivity of output spike rate to change in up-state frequency. Down-state frequency is held constant at the control value; up-state frequency is the ratio indicated on the abscissa times the down-state frequency of 0.11 Hz. The slope of this curve, calculated using linear regression, is greatest for gKA = 80% of control and under conditions of increased dopamine.
The SNR analysis evaluates sensitivity of the FS interneuron model to noise at a single up-state (signal) input frequency. A different aspect of information processing is sensitivity to changes in input frequency for a given noise level; in other words, how well can FS interneurons detect a change in cortical activity level. A large change in output spike rate in response to a change in input frequency indicates high sensitivity. Thus, the effect of gKA and dopamine was evaluated for up-state input frequencies ranging from 1 Hz to 8 Hz, while keeping the down-state frequency constant at the control value of around 0.1 Hz. This set of up-state input frequencies yielded ratios of up-state to down-state synaptic frequency from 10 to 80, which encompassed the experimentally observed range of 11 to 72 (Blackwell et al. 2003). Fig 7B illustrates the FS interneuron output spike rate as a function of up-state input frequencies. Either a reduction in gKA or the presence of dopamine increased the sensitivity of the FS interneuron to changes in synaptic input frequency during the up-state.
In summary, in the FS interneuron the control level of gKA conveys robustness to down-state synaptic inputs (noise), whereas the increase in dopamine increases sensitivity to changes in up-state synaptic inputs (signal).
4 Discussion
We developed a compartmental model of a striatal fast spiking interneuron to investigate whether synaptic inputs during the down-state adversely affect signal detection during up-states. Furthermore, we examined how up-state firing is controlled by transient potassium currents that produce delays in action potential generation in response to depolarization. Key features of the model, such as morphology, intrinsic currents, and synaptic currents, were matched to experimentally obtained data from cortex-striatum-substantia nigra cultures. Overall, results showed that the KA current serves an important role to enhance signal detection by suppressing action potentials in response to synaptic noise. Thus, this study quantitatively tested and confirmed previously proposed ideas on the role of the KA current (Nisenbaum and Wilson 1995; Wilson 1995).
Reproducing the experimentally observed spike latency and high firing frequency required inclusion of three different potassium channels in the model. Equations for the two delayed rectifier currents were those describing Kv3.1/3.2 and Kv1.3 currents in fast spiking interneurons of the cortex (Erisir et al. 1999). The Kv1.3 and Kv3.1/3.2 have slightly different effects on the spiking behaviour and are not interchangable; both were needed to obtain the best fit to experimental data. The Kv3.1/3.2 potassium current is a faster activating current than Kv1.3, but requires larger depolarizations than the former. It also deactivate quickly, which, given its prominent expression in cortical FS interneurons, may explain the high firing frequency of these neurons (Erisir et al. 1999). The high conductance assigned to the Kv3.1/3.2 channel may be a consequence of using parameters obtained from homomeric channels. Specifically, neurons in globus pallidus and hippocampus have Kv3.4 subunits, which co-assemble with Kv3.1 subunits (Baranauskas et al. 2003). The heterotrimeric channels have a lower activa-tion voltage, but similar kinetics. It is possible that if activation potential were made smaller consistent with heterotrimeric channels, the optimized conductance in the model would be lower. The parameter modifications made to the KA currents are consistent with the change in kinetics expected if these currents had been recorded at body temperature. A reduction in the voltage dependence of activation is expected from theoretical considerations, and has been demonstrated for delayed rectifier potassium currents (Tiwari and Sikdar 1999). The two fold reduction in the time constant of activation represents a conservative Q10 of 2.0 (Huguenard et al. 1991). Nonetheless, experimental verification of the model requires experimental measurements of potassium currents in striatal FS interneurons.
Though model parameters were adjusted to those of FS interneurons in co-cultures, many of the properties are similar to that measured in slice preparations. The down-state membrane is similar to that of Koos and Tepper (Koos and Tepper 2002), but higher than that in Kawaguchi (Kawaguchi 1993); spike width is slightly wider than that reported previously, but AHP amplitude is comparable to that reported in Kawaguchi. Latency is not listed in either reference, but traces reveal latencies of 25-50 ms, within the range of those measured in co-cultures. It is not possible to compare synaptic inputs, because these have not been quantified for FS interneurons in slice.
Validating the model supported a number of anatomical findings regarding the spatial distribution of synapses on striatal FS interneurons (Bevan et al. 1998). GABAergic inputs to FS neurons are from NADPH interneurons, other striatal FS interneurons, and GP projection neuron collaterals. FS interneurons preferentially target the soma (Kubota and Kawaguchi 2000). Similarly, GABAergic synapses from the Globus Pallidus predominantly target soma and proximal dendrites. A biased localization of GABAergic synapses also is found in fast spiking interneurons of neocortex and hippocampus (Pettit and Augustine 2000; Gulyas et al. 1999). Consistent with these findings, simulations show that matching both the population reversal potential and the up-state spike rate measured experimentally required placement of GABA synapses close to the soma as compared to glutamatergic synapses in the model neuron. Various spatial distributions have been suggested to serve different functions. For example, GABAergic inputs to the soma might be involved in suppression and timing of action potentials, while GABAergic inputs on distal dendrites might be important for integration of synaptic inputs (Reyes et al. 1998).
After adjusting for amplitude, time course and IEI of synaptic inputs during down-states, the correlation among synaptic inputs had to be increased to match experimentally measured spike rates during up-states. Such correlation among synaptic inputs has been demonstrated experimentally. For example, membrane potential fluctuations during up-states reveal correlated synaptic inputs in both in vivo and in vitro conditions in striatum (Stern et al. 1998; Plenz and Kitai 1998). This requirement of correlated inputs was not eliminated by the addition of NMDA currents to the model. Our correlation used is in the same range as that used in a neocortical pyramidal cell model (Ho and Destexhe 2000; Rudolph and Destexhe 2001) to reproduce spontaneous in vivo like subthreshold membrane potential activity and spike rate during up-states. Rather than depolarizing the cell, correlated synaptic inputs produce an increase in membrane potential fluctuations that boosts the rate of action potential generation (Salinas and Sejnowski 2000).
The ability of FS interneurons to profoundly influence striatal activity implies that FS interneurons need to be highly selective in their responses to synaptic inputs. Thus, in the fully adjusted model, we explored the KA current as a possible mechanism for creating input specificity in FS interneurons. We demonstrated that the ability of KA currents to suppress responses to random synaptic inputs while allowing responses to correlated synaptic inputs created such input specificity. In other words, a strong KA current results in a better SNR in high noise conditions by preferentially suppressing down-state spikes.
These findings are robust with respect to changes in synaptic input characteristics and channel distribution. The same effect of the KA current is observed when synaptic currents have slower rise times, and when GABA synapses are evenly distributed over all dendritic branches (results not shown). Similarly, the presence of NMDA channels does not change the role of KA currents in improving SNR, though it makes the FS interneuron more excitable. Inclusion of KA channels on distal dendrites counteracts the effect of NMDA channels on excitability, in accordance with previous simulations (Wilson 1995), but does not eliminate the ability of KA channels to improve SNR. This robustness includes conditions of elevated dopamine, in that FS interneurons exhibit input specificity when GABA amplitude is reduced, as observed with dopamine receptor activation (Bracci et al. 2002; Centonze et al. 2003). More importantly, this effect is specific to the KA current and cannot be achieved with the Kv3.1/3.2 current, probably due to its lower activation voltage. Kv3.1/3.2 is activated following a spike, whereas KA is activated prior to spike generation due to its lower activation threshold. Our results suggest that KA channels allow FS interneurons to operate without a decrease in SNR during conditions of increased dopamine, as occurs in response to reward or anticipated reward.
Another mechanism to increase input specificity is to increase background synaptic activity (Bernander etal. 1991), which lowers the gain of neurons (Ho and Destexhe 2000; Chance et al. 2002). Gain is the change in output firing frequency with a change in input synaptic frequency. If the gain is too high, a small increase in input frequency may saturate the neuron's output, preventing the neuron from accurately signaling larger changes in input frequency. Background activity improves input specificity by lowering the overall conductance of the neuron, which necessitates large input signals to reliably depolarize the neuron to spike threshold (Bernander et al. 1991). In addition, background noise makes the neuron responsive to lower values of signal input, allowing the neuron to spike when a small signal is coincident with background excitatory input (Ho and Destexhe 2000; Chance etal. 2002).
In contrast to the synchronous excitatory synaptic input used as the signal by other studies, striatal up-states are relatively long periods of increased, relatively asynchronous, excitatory and inhibitory synaptic inputs. As such, the up-state itself is similar to a large increase in background synaptic activity; thus, the gain during the up-state is already low (Fig 7B). Consequently, a small increase in down-state activity does not improve input specificity (Fig 6), and a large increase in down-state activity may make the gain too low, hindering the ability of the neuron to reliability signal changes in the input signal, or the presence of an up-state.
We performed additional model simulations under conditions of increased dopamine to assist interpretation of a recent experimental finding on the effect of dopamine on FS interneurons. That study showed that dopamine depolarizes the FS interneuron, and decreases the amplitude of GABA IPSCs onto the FS interneuron. Our simulations show that dopamine increases the gain of the FS interneuron, allowing more reliable up-state spike generation, while maintaining a high SNR (Fig 7). This increase in gain improves input sensitivity since the FS interneuron's firing rate (< 10 Hz) is still significantly below its peak firing rate (200 Hz). This mechanism may make the FS interneuron more responsive to input stimuli when they are associated with reward or anticipated reward, which causes an increase in dopamine release.
These simulation results can be rephrased in terms of a set of experimentally testable predictions: (1) FS interneurons have KA currents: though they exhibit a delay to spike generation during depolarization, which is reminiscent of a KA current, KA currents have not been identified in these neurons; (2) Partial pharmacological blockade of the KA current will increase firing rate both in the down-state and in the up-state without a significant decrease in SNR, unless high noise conditions prevail; (3) Under high synaptic input noise conditions, an increased level of dopamine will increase the firing rate in the up-state and even more in the down-state thereby decreasing the SNR level.
What are the implications of these observations in terms of FS interneuron function in local striatal circuits? The effect of FS interneurons on SP neuron up- and down-states is difficult to analyze with the present FS neuron model without an SP neuron model to receive FS neuron inputs. Experimentally, FS interneurons in the striatum have been shown to fire in two different modes. They fire bursts of action potential during slow-wave sleep (Berke et al. 2004), epilepsy (Slaght et al. 2004), and in response to suprathreshold current injections (Kawaguchi 1993; Plenz and Kitai 1998; Koos and Tepper 1999). On the other hand, FS interneurons have been found to fire predominantly single spikes during wakefulness (Berke et al. 2004) and in organotypic cortex-striatum-substantia nigra co-cultures (Plenz and Kitai 1999). While burst firing of FS interneurons provides powerful inhibition for prolonged periods of time to the striatal microcircuit, the functional importance of single spike firing is poorly understood. It has been shown in the acute slice that single spikes from FS interneurons can significantly delay action potentials in SP neurons (Koos and Tepper 1999). This is important, because the timing of action potentials during the up-state controls intracellular calcium influx through NMDA receptors in SP neurons (Kerr and Plenz 2002, 2004). Our result from the present study, that correlated, high frequency synaptic input is required to produce a spike in FS interneurons, implies that single spikes carry information on the underlying synaptic inputs. The single spike provides a temporally precise, short-lasting inhibition in the feed forward circuit formed by the FS interneuron in the striatum. Though these spikes occur irregularly, they are not induced by noise; thus, the single FS interneuron spikes that strongly influence SP neuron activity are predominantly produced by correlated, high frequency synaptic input. Further elucidating how FS interneurons modulate SP neuron dynamics requires simulations using networks of striatal neurons.
Acknowledgments
J. Hellgren Kotaleski was supported by Knut and Alice Wallenbergs Foundation and the Swedish Science Council (project number K2004-33XD-15059-01A). K.T. Blackwell was supported by NSF grant IBN-0077509. D. Plenz was supported by the intramural research program of the NIMH, NIH. We are grateful to Rory Kirchner who helped to develop the initial model.
References
- Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990;344:240–242. doi: 10.1038/344240a0. [DOI] [PubMed] [Google Scholar]
- Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of kv3.1 channels in fast-spiking neurons. Nat Neurosci. 2003;6:258–266. doi: 10.1038/nn1019. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum. Neuroscience. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrain-ment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Bernander O, Douglas RJ, Martin KA, Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A. 1991;88:11569–73. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;18:9438–52. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KT, Czubayko U, Plenz D. Quantitative estimate of synaptic inputs to striatal neurons during up- and down-states in vitro. J Neuroscience. 2003;23:9123–9132. doi: 10.1523/JNEUROSCI.23-27-09123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci E, Centonze D, Bernardi G, Calabresi P. Dopamine excites fast-spiking interneurons in the striatum. J Neurophysiol. 2002;87:2190–2194. doi: 10.1152/jn.00754.2001. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in parkinson's disease: from description to theory. Trends Neurosci. 1990;13:21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin A, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Cal-abresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neuroscience. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Czubako U, Plenz D. Fast synaptic transmission between striatal spiny projection neurons. Proc Natl Acad Sci U S A. 2002;99:15764–9. doi: 10.1073/pnas.242428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard C. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiology. 1999;82:2476–2489. doi: 10.1152/jn.1999.82.5.2476. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R, Tamas G. Ca2+ imaging of mouse neocortical interneurone dendrites: contribution of Ca2+-permeable ampa and nmda receptors to sub-threshold Ca2+ dynamics. J Physiol. 2003a;551:67–78. doi: 10.1113/jphysiol.2003.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R, Tamas G. Ca2+ imaging of mouse neocortical interneurone dendrites: Ia-type K+ channels control action potential backpropagation. J Physiol. 2003b;551:49–65. doi: 10.1113/jphysiol.2003.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz T, Kraushaar U, Geiger J, Lübke J, Berger T, Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci. 1997;17:204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Solla SA, Surmeier DJ, Houk JC. Modulation of striatal single units by expected reward: a spiny neuron model displaying dopamine-induced bistability. J Neurophysiol. 2003;90:1095–1114. doi: 10.1152/jn.00618.2002. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the ca1 area of the rat hippocampus. J Neurosci. 1999;19:10082–97. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Hernandez A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci. 2003;23:8931–40. doi: 10.1523/JNEUROSCI.23-26-08931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N, Destexhe A. Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. J Neurophysiol. 2000;84:1488–1496. doi: 10.1152/jn.2000.84.3.1488. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Coulter DA, Prince DA. A fast transient potassium current in thalamic relay neurons: kinetics of activation and inactivation. J Neurophysiol. 1991;66:1304–1315. doi: 10.1152/jn.1991.66.4.1304. [DOI] [PubMed] [Google Scholar]
- Jahn K, Bufler J, Franke C. Kinetics of AMPA-type glutamate receptor chan-nels in rat caudate- putamen neurones show a wide range of desensitization but distinct recovery characteristics. Eur J Neurosci. 1998;10:664–672. doi: 10.1046/j.1460-9568.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends in Neuroscience. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. J Neurosci. 2002;22:1499–512. doi: 10.1523/JNEUROSCI.22-05-01499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Action potential timing determines dendritic calcium during striatal up-states. J Neurosci. 2004;24:877–885. doi: 10.1523/JNEUROSCI.4475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H. Gabaergic circuits of the striatum. Prog Brain Res. 1993;99:51–72. doi: 10.1016/s0079-6123(08)61338-2. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. Neuroscience. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM, W CJ. Comparison of ipscs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Depence of gabaergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G, Larkman AU, Jonas P, Sakmann B, Jack JJB. Detailed passive cable models of whole-cell recorded CA3 pyramidal neurons in rat hippocampal slices. J Neurosci. 1994;14:4613–4638. doi: 10.1523/JNEUROSCI.14-08-04613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Berger TW. Functionally distinct subpopulations of striatal neurons are differentially regulated by GABAergic and dopaminergic inputs–I. In vivo analysis. Neuroscience. 1992;48:561–578. doi: 10.1016/0306-4522(92)90402-n. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci. 1995;15:4449–463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ, Foehring RC, Surmeier DJ. Isolation and characterization of a persistent potassium current in neostriatal neurons. J Neurophysiology. 1996;76:1180–1194. doi: 10.1152/jn.1996.76.2.1180. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Augustine GJ. Distribution of functional glutamate and gaba receptors on hippocampal pyramidal cells and interneurons. J Neurophysiol. 2000;84:28–38. doi: 10.1152/jn.2000.84.1.28. [DOI] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Plenz D, Aertsen A. Neural dynamics in cortex-striatum co-cultures-II. spatiotem-poral characteristics of neuronal activity. Neuroscience. 1996;70:893–924. doi: 10.1016/0306-4522(95)00405-x. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Up and down states in striatal medium spiny neurons si-multaneously recorded with spontaneous activity in fast-spiking interneurons studied in cortex-striatum-substantia nigra organotypic cultures. J Neurosci. 1998;18:266–283. doi: 10.1523/JNEUROSCI.18-01-00266.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–85. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rudolph M, Destexhe A. Do neocortical pyramidal neurons displey stochastic resonance? J Comput Neurosci. 2001;11:19–42. doi: 10.1023/a:1011200713411. [DOI] [PubMed] [Google Scholar]
- Salin PA, Prince DA. Spontaneous GABAA receptor-mediated inhibitory currents in adult rat somatosensory cortex. J Neurophysiol. 1996;75:1573–1588. doi: 10.1152/jn.1996.75.4.1573. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. J Neurosci. 2000;20:6193–6209. doi: 10.1523/JNEUROSCI.20-16-06193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Slaght SJ, Paz T, Chavez M, Deniau JM, Mahon S, Charpier S. On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy. J Neurosci. 2004;24:6816–6825. doi: 10.1523/JNEUROSCI.1449-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Jaffe DB, Johnston D. Dendritic attenuation of synaptic po-tentials and currents: the role of passive membrane properties. Trends Neuroscience. 1994;17:161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Stefani A, Chen Q, Flores-Hernandez J, Jiao Y, Reiner A, Surmeier DJ. Physiological and molecular properties of AMPA/kainate receptors expressed by striatal medium. Dev Neurosci. 1998;20:242–252. doi: 10.1159/000017318. [DOI] [PubMed] [Google Scholar]
- Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simul-taneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–8. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- Taverna S, van Dongen YC, Groenewegen HJ, Pennartz CM. Direct physiological evidence for synaptic connectivity between medium-sized spiny neurons in rat nucleus accumbens in situ. J Neurophysiol. 2004;91:1111–21. doi: 10.1152/jn.00892.2003. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsy-chiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. Gabaergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tiwari JK, Sikdar SK. Temperature dependent conformation changes in a voltage-gated potassium channel. European Biophys J. 1999;28:338–354. doi: 10.1007/s002490050216. [DOI] [PubMed] [Google Scholar]
- Tkatch T, Baranauskas G, Surmeier DJ. Kv4.2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci. 2000;20:579–588. doi: 10.1523/JNEUROSCI.20-02-00579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol. 2002;88:1263–1269. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. The generation of natural firing patterns in neostriatal neurons. Prog Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Dynamic modification of dendritic cable properties and synaptic transmission by voltage-gated potassium channels. J Comput Neurosci. 1995;2:91–115. doi: 10.1007/BF00961882. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]



