Abstract
Introduction
Auditory verbal hallucinations (AVH) in schizophrenia (SZ) have been proposed to result from abnormal local, interregional and interhemispheric integration of brain signals in regions involved in language production and perception. This abnormal functional integration may find its base in morphological abnormalities. Structurally, AVHs have been frequently linked to abnormal morphology of the superior temporal gyrus (STG), but only a few studies investigated the relation of hallucination presence with both whole-brain gray matter (GM) and white matter (WM) morphometry.
Methods
Using a unified voxel-based morphometry–DARTEL approach, we investigated correlates of AVH presence in 51 schizophrenia patients (20 non-hallucinating [SZ −], 31 hallucinating [SZ +]), and included 51 age and sex matched healthy participants. Effects are reported at p < .05 FWE corrected.
Results
Patients showed lower GM volume of the left STG than controls, irrespective of AVH presence. In addition, SZ + showed lower GM volume of the left inferior frontal and right parahippocampal gyrus, and higher WM volume of the left postcentral and superior parietal lobule than controls. Finally, volume of the putamen was lower in SZ + compared to SZ −. No effects on corpus callosum morphometry were observed. Delusion severity, general positive and negative symptomatology illness duration, and medication status could not explain the results.
Discussion
Results suggest that STG GM abnormalities underlie the general susceptibility to experience psychotic symptoms and that additional abnormalities in a network of medial temporal, ventrolateral, putaminal, and parietal regions related to verbal memory and speech production may specifically increase the likelihood of experiencing AVH. Future studies should clarify the meaning of morphometry abnormalities for functional interregional communication.
Keywords: Voxel based morphometry, Auditory verbal hallucinations, Schizophrenia, Superior temporal gyrus, Inter-hemispheric connectivity, Positive and negative syndrome scale
Highlights
-
•
We studied whole-brain voxel-based morphometry of gray (GM) and white-matter (WM).
-
•
We compared schizophrenia patients with and without hallucinations to controls.
-
•
We showed lower GM-volume of the superior temporal gyrus in all patients.
-
•
Low volume in GM and WM in language areas related to hallucinations.
-
•
The superior temporal gyrus is not alone in increasing the risk of hearing voices.
1. Introduction
Auditory verbal hallucinations (AVHs) are the predominant type of hallucinations experienced by patients with schizophrenia and are characterized by complex auditory phantom perceptions of verbal or non-verbal content (Andreasen and Flaum, 1991). They have been proposed to result from abnormal inter-regional and inter-hemispheric integration of brain processes (Stephan et al., 2009) in regions associated with language and memory processing, including the inferior frontal, parahippocampal, and temporo-parietal gyri (Curcic-Blake et al., 2012; Dierks et al., 1999; Diederen et al., 2010, 2012; Jardri et al., 2011; Vercammen et al., 2010). The abnormal functional integration of frontal-temporal language production and perception areas (Curcic-Blake et al., 2012) may link to local cortical gray matter (GM) abnormalities and abnormalities in the pathways that connect these language perception and production areas, i.e. the white matter (WM) tracts.
The superior temporal gyrus (STG) appears to be the most consistently reported GM abnormality associated with AVH as shown in a number of manual tracing studies specifically studying the relation between STG morphometry and AVH (e.g. (Barta et al., 1990; Onitsuka et al., 2004; Rajarethinam et al., 2000; Sun et al., 2009)), a finding that is supported by two recently performed meta-analyses based on voxel based morphometry (VBM) studies related to AVH severity (Modinos et al., 2013; Palaniyappan et al., 2012). However, only a limited number of independent VBM studies investigated the relations of AVH presence and whole-brain morphometry in samples with an adequate size and distribution of severity (see (Modinos et al., 2013)). Moreover, only one study investigated effects of AVH presence while controlling for general disease related factors by including a healthy control group (Shapleske et al., 2002). This study however did not find support for the specific involvement of STG morphology in AVH. Finally, the majority of studies failed to control for other symptoms of reality distortion, including delusions. Therefore, further whole brain studies are needed before a definite conclusion on the specific and restricted involvement of the STG in the structural pathology underlying hallucinations can be drawn.
Next to the STG, AVH severity or occurrence has been related to morphometric abnormalities of other cortical regions associated with language and self-referential processing, including lateral and medial temporal-, insular-, inferior frontal-, and medial parietal regions (Shapleske et al., 2002; Garcia-Marti et al., 2008; Modinos et al., 2009; Nenadic et al., 2010; O'Daly et al., 2007; Shin et al., 2005). Moreover, AVHs have been proposed to result from hampered interregional communication that finds its base in white matter (WM) volume and diffusion abnormalities, thought to reflect the propensity to transfer information between regions and hemispheres. Abnormalities in WM related to hallucinations have been observed in the temporal, parietal, and frontal regions (O'Daly et al., 2007; Shin et al., 2005; Hubl et al., 2004; Lee et al., 2009; Shergill et al., 2007) and corpus callosum (Hubl et al., 2004; Knochel et al., 2012; Makris et al., 2010; Mulert et al., 2011; Plaze et al., 2011). However, both GM and WM abnormalities have been reported with large variability, that could result from variable clinical characteristics (e.g. including only patients with persistent AVHs (Garcia-Marti et al., 2008; Modinos et al., 2009; O'Daly et al., 2007) or inner vs. outer AVH experience (Plaze et al., 2011) or methodological issues such as small (sub)sample size (< 20) (Garcia-Marti et al., 2008; Mulert et al., 2011; Plaze et al., 2011; Neckelmann et al., 2006)). The use of different imaging modalities and analysis procedures (e.g. different segmentation/parcellation strategies), and not taking into account total brain volume and severity on other symptom dimensions, including delusional behavior may further contribute to inconsistent results.
We aimed to test for both GM and WM relations with AVH presence, while controlling for delusion severity, total positive symptomatology, total negative symptomatology, chronicity of symptoms and type of medication used. We investigated whole-brain morphometry in 51 schizophrenia patients (20 non-hallucinating patients and 31 hallucination patients, matched for severity on other symptom dimensions (i.e. negative and general symptomatology) and included a sample of 51 healthy individuals, matched for age and sex. We used a unified voxel-based morphometry approach following the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL; (Ashburner, 2007)), and hypothesized lower GM volume of the STG, inferior frontal gyrus (IFG), parahippocampal gyrus, and medial parietal regions specifically related to AVH occurrence. With respect to WM, we expected abnormal morphometry of the corpus callosum and temporo-parietal region to be associated with presence of hallucinations.
2. Methods
2.1. Sample characteristics
Structural MRI data of 102 participants who participated in neuroimaging studies at our center between January 2008 and December 2010 were pooled for the current analysis (See Table 1 for clinical characteristics). Patients were included when complete Positive and Negative Symptoms Scale (PANSS; (Kay et al., 1987)) data were available from studies conducted at our center (van der Meer et al., 2013a; Liemburg et al., in preparation; vd Velde et al, in preparation) and when data was not included in a previous report on GM correlations of AVH severity (Modinos et al., 2009). Studies ran between 2008 and 2010 at the Neuroimaging Center at the University Medical Center Groningen (UMCG) and used the same T1-3D imaging sequence. All patients (n = 51) met DSM-IV criteria for schizophrenia. Diagnosis was confirmed using the Schedules for Clinical Assessment in Neuropsychiatry or MINI-international neuropsychiatric interview (Sheehan et al., 1998). Forty-one patients were using stable doses of antipsychotic medication (classical antipsychotic agents [n = 3]: haloperidol, perphenazine; Atypical antipsychotic agents [n = 38]: risperidone, clozapine, olanzapine, quetiapine, and/or aripiprazol) and ten patients were not using antipsychotic medication at time of inclusion. Healthy controls (n = 51) were recruited through advertisements and were screened to have no Axis-I diagnosis lifetime. Written informed consent was obtained from all participants prior to participation to the studies, after full explanation of the experimental procedure. All studies obtained institutional review board approval (UMCG) and were carried out in accordance with the Declaration of Helsinki. None of the current structural data has been published as a separate report previously; but summary results for GM volumes for part of the sample (N = 33) were included in a recent meta-analysis (Modinos et al., 2013).
Table 1.
Clinical characteristics.
SZ: Schizophrenia patients; HC: healthy controls; SZ −: non-hallucinating patients; SZ +: hallucinating patients; PANSS: positive and negative syndrome scale; P3: PANSS positive subscale, item 3, hallucinatory behavior; P1: PANSS positive subscale, item 1, delusions.
| Between-group |
Between group (inl HC) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SZ | HC | X2(d) | F | p | SZ − | SZ + | X2d | F | p | |||
| N | 51 | 51 | 20 | 31 | ||||||||
| Gender (males/female) | n | 44/7 | 37/14 | 2.16 | .14 | 17/3 | 27/4 | 3.03 | .22 | |||
| Age | in years; mean (sd) | 34.04 (11.40) | 36.14 (10.93) | .90 | .35 | 35.00 (9.69) | 33.42 (12.50) | .23 | .63 | |||
| range | 18–57 | 18–58 | 20–54 | 18–57 | ||||||||
| Education | in years; mean (sd) | 12.21 (2.87) | 12.64 (2.66) | .05 | .83 | 12.05 (2.59) | 12.32 (3.08) | .09 | .76 | |||
| Handedness (right/left) a | n | 44/4 | 48/3 | .007 | .71 | 19/1 | 25/3 | .75 | .69 | |||
| Gray matter totals | in mm3; mean (sd) | 759.31 (8.93) | 744.45 (85.32) | .004 | .95c | 731.23 (69.32) | 777.43 (80.47) | 2.09 | .13c | |||
| White matter totals | in mm3; mean (sd) | 503.57 (62.16) | 495.33(62.37) | .14 | .71c | 479.76 (55.73) | 518.93 (62.05) | 3.39 | .038c | |||
| csf totals | in mm3; mean (sd) | 504.95 (62.59) | 497.05 (62.57) | .11 | .75c | 481.78 (56.00) | 519.90 (62.88) | 3.18 | .046c | |||
| Gray + white matter totals | in mm3; mean (sd) | 1262.88 (112.70) | 1239.79 (133.24) | .022 | .88c | 1210.99 (102.72) | 1296.36 (107.39) | 3.15 | .047c | |||
| 1 × 2 ANOVA | ||||||||||||
| Age of onset b | in years; mean (sd) | 25.20 (8.19) | – | 24.18 (5.750 | 25.85 (9.46) | .43 | .52 | |||||
| Illness duration b | in years; mean (sd) | 8.77 (8.71) | – | 10.41 (7.91) | 7.74 (9.16) | .98 | .33 | |||||
| PANSS positive symptoms | mean (sd) | 11.48 (4.19) | – | 10.84 (4.82) | 11.87 (3.78) | .71 | .41 | |||||
| (minus P3) | range | 7–25 | – | 1–2 | 3–5 | |||||||
| PANSS negative symptoms | mean (sd) | 14.37 (4.18) | – | 12.65 (4.46) | 14.84 (3.98) | .99 | .33 | |||||
| range | 7–24 | – | 7–24 | 9–22 | ||||||||
| PANSS generalized psychopathology | mean (sd) | 29.84 (7.29) | – | 27.40 (7.16) | 31.42 (7.05) | 3.91 | .054 | |||||
| range | 18–49 | – | 18–49 | 20–45 | ||||||||
| PANSS P3 (hallucinations) | mean (sd) | 2.55 (1.47) | – | 1.35 (.49) | 3.90 (.83) | 153.73 | < .001 | |||||
| range | 1–5 | – | 1–2 | 3–5 | ||||||||
| PANSS P1 (delusions) | mean (sd) | 2.90 (1.45) | – | 2.25 (1.68) | 2.74 (1.32) | 1.36 | .25 | |||||
| range | 1–6 | – | 1–6 | 1–5 | ||||||||
| Classical antipscyhotic use | n | 3 | 1 | 2 | 2.22 | 33 | ||||||
| Atypical antipsychotic use | n | 38 | 17 | 21 | ||||||||
| No medication | n | 10 | 2 | 8 | ||||||||
Handedness information was not available of 3 patients.
RELIABLE information on age of onset and thus illness duration was missing of 7 patients (3 SZ − and 4 SZ +).
Corrected for age & gender.
2.2. Symptom profile assessment
Current symptomatology (for the week prior to scanning) was assessed using the semi-structured PANSS interview (Kay et al., 1987), rated by two trained interviewers. The PANSS has good inter-rater reliability and validity (Kay et al., 1988). The interviewers obtained a consensus score for each item on all three subscales (positive symptoms (P), negative symptoms (N), and general psychopathology (G)) on a seven point scale indicating severity of the symptom in the last week (1 = absent;2 = minimal;3 = mild;4 = moderate;5 = moderate severe;6 = severe;7 = extreme). For the purpose of studying volume as a function of hallucination presence, we chose the P3 (positive subscale, item 3, hallucinatory behavior) score as indicator of hallucination severity. Unfortunately, no other measure that reliably quantifies intensity, frequency or content of hallucinations was available for all patients. Nevertheless, the P3 item of the PANSS has shown good convergent validity with the Psychotic Symptom Rating Scale (r = .47; (Haddock et al., 1999); and Hamilton Program for Schizophrenia Voices Questionnaire (HPSVQ; r = .66; (Van Lieshout and Goldberg, 2007)), especially with the factors ‘frequency’, ‘duration’, ‘disruption’, and ‘beliefs about the origin of the voices’ (Kim et al., 2010; Steel et al., 2007). Because AVHs and delusions could both be considered symptoms of reality distortion and we aimed to specifically test for effects of AVH presence, we controlled post-hoc for delusion severity. Item P1 (positive subscale, item 1, delusions) has shown high correlations (r = .88) with the Delusions Severity subscale of the PSYRATS (Schneider et al., 2011) and was used to objectify delusion severity.
2.3. MRI data acquisition
Imaging data were acquired using a Philips 3-tesla Intera Magnetic Resonance system (Best, The Netherlands), equipped with a standard SENSE-8 channel head coil. For each subject, anatomical images were obtained using a sagittal 3D gradient-echo T1-weighted sequence (TR = 9 ms, TE = 3.5 ms; matrix 256x256; voxel size: 1x1x1mm; 170 slices, duration: 4 min 11 s).
2.4. Data analysis
Demographic and clinical data were analyzed with SPSS 16.0 (IBM SPSS Statistics, m=nd). Age and sex were entered as nuisance variables in each analysis.
2.4.1. Preprocessing of imaging data
Imaging data were analyzed using Voxel Based Morphometry (VBM), following the DARTEL approach (Ashburner, 2007) using Statistical Parametric Mapping software (SPM8; www.fil.ion.ucl.ac.uk/spm) implemented in Matlab 7.10.0.499 (The Mathworks inc, Natick, MA). VBM-DARTEL preprocessing included: 1) manual reorientation of the images to the center point of the anterior commissure; 2) segmentation of the images into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) using the standard unified segmentation option implemented in SPM8. Image segmentation included an intensity non-uniformity correction to account for smooth intensity variations caused by gradient distortions (setting: very light regularization); 3) applying the DARTEL approach for registration, normalization, and modulation; 4) further normalization of the GM and WM images to MNI space; and 5) smoothing of the GM and WM images with an 8 mm full width at half maximum Gaussian kernel to increase signal to noise ratio. In the resulting images, each voxel represented an absolute amount of brain volume, equivalent to the brain volume per unit prior to normalization. To achieve maximal sensitivity, to optimize voxel residual smoothness estimation and to exclude false positives in non-GM (or non-WM) tissue, voxel-wise comparisons were masked using a comparison-specific explicit optimal threshold GM (WM) mask created using the Masking toolbox (//www0.cs.ucl.ac.uk/staff/g.ridgway/masking/).
2.4.2. Statistical modeling
Data were analyzed in the context of the General Linear Model for GM and WM images separately. We performed an ANCOVA to test for the main effect of group with group (3; healthy controls, no hallucinations [SZ −; P3 < 3], hallucinations [SZ +; P3 ≥ 3]) as random factor, the GM (WM) density maps as dependent factor, and age and sex as covariates. Total parenchyma (GM + WM totals; globals) were entered by means of proportional scaling. Patients with a P3 score of 1 or 2 were classified as having no hallucinations (SZ −), whereas patients with a P3 score of 3 and higher were classified as ‘hallucinators’ (SZ +). To test for the possible confounding influence of other clinical variables, we extracted volumes of clusters showing a main effect of group at p < .005 and exported these to SPSS. In a first post-hoc linear regression model (method enter), we tested whether P1 (delusions) and positive symptoms (minus P3 & P1) and total negative symptomatology explained any additional variance or could explain the effects instead. In a second model, we entered years since onset of first psychotic episode (i.e. chronicity) to the model. In a third model, type of anti-psychotic used were stepwise entered into the model by means of 3 dummy variables coding for either use of 2nd generation antipsychotic use (n = 38), classic anti-psychotics (n = 3), or no medication use (n = 10). Unfortunately, accurate information on dose and duration of medication use was not available of all patients, excluding the possibility to control for medication-status using dose-equivalences (e.g. chlorpromazine-equivalences or the olanzapine-equivalences). Hallucinations yes/no, age, sex, and GM + WM totals were first entered to all the models.
2.4.3. Statistical thresholding
Significance for demographical and clinical data was set at p < .05, two-tailed.
Main effect of Group (HC, SZ −, SZ +) were explored using an F-tests at p < .005 uncorrected. Post-hoc T-tests had to meet p < .05 Family Wise Error (FWE) corrected at the cluster level to be considered significant using a voxel-wise threshold of p < .005 uncorrected. Non-stationarity correction was applied to account for the non-uniformity of the smoothness of VBM data (http://fmri.wfubmc.edu/cms/software#NS).
Multiple comparison correction of effects occurring in our a priori regions of interest was restricted to the spatial extent of a composite ROI covering these regions and was constructed based on imaging reports that relate to hallucinations as outlined in the introduction. The composite ROI encompassed the bilateral IFG, bilateral temporal parietal areas, the posterior cingulate cortex (PCC), and the parahippocampal regions. The following labels of the Anatomic Automatic Labeling system implemented in the WFU Pickatlas (http://www.nitrc.org/projects/wfu_pickatlas/) were used: Frontal_Inf_Oper_L, Frontal_Inf_Tri_L, Frontal_Inf_Oper_R, Frontal_Inf_Tri_R, Temporal_Sup_L, Parietal_Inf_L, Temporal_Sup_R, and Parietal_Inf_R, Heschl's_gyrus_L, Heschl's_gyrus_R, Cingulum_Post_L, Cingulum_Post_R, Parahippocampal_L, and Parahippocampal_R labels. As defining ROIs for WM regions is less straightforward, we used bilateral lobar labels covering the whole lobule implemented in the WFU pickatlas to define the correction area for white matter effects (i.e. bilateral whole temporal lobe for temporal effects, bilateral whole parietal lobe for parietal effects, bilateral whole frontal lobe for frontal effects and bilateral whole limbic lobe for limbic effects including the posterior cingulate cortex). Effects occurring outside these ROIs had to meet cluster wise p < .05 FWE whole-brain correction (following non-stationarity correction).
3. Results
3.1. Sample descriptive
Patients did not differ from HC in age, years of education, handedness, sex, and total GM, total WM, or total GM + WM volume (Table 1). Hallucinating patients (SZ +) did not differ from non-hallucinating patients (SZ −) in age, years of education, handedness, sex distribution, age of onset of the disorder, total positive symptomatology (minus P3), total negative symptomatology, total general psychopathology, delusion severity, and medication status (Table 1). However, SZ + had higher WM and WM + GM matter volume than SZ − (post-hoc t-test: WM: p = .035; GM + WM: p = .042; all Bonferroni corrected), but not compared to HC.
3.2. VBM results: gray matter
A main effect of group was observed in the left STG extending into the inferior parietal lobule (IPL), the left inferior frontal gyrus (IFG), the bilateral parahippocampal gyrus, bilateral putamen, the medial frontal gyrus, the precentral gyrus, and the right lingual gyrus (See the Appendix 1c, Table A-I for a complete overview and details of the main effects). Plotting of parameter estimates guided the post-hoc t-tests in these regions and showed that patients were characterized by lower volume of the STG, an effect that was independent of hallucination presence (See Fig. 1a).
Fig. 1.
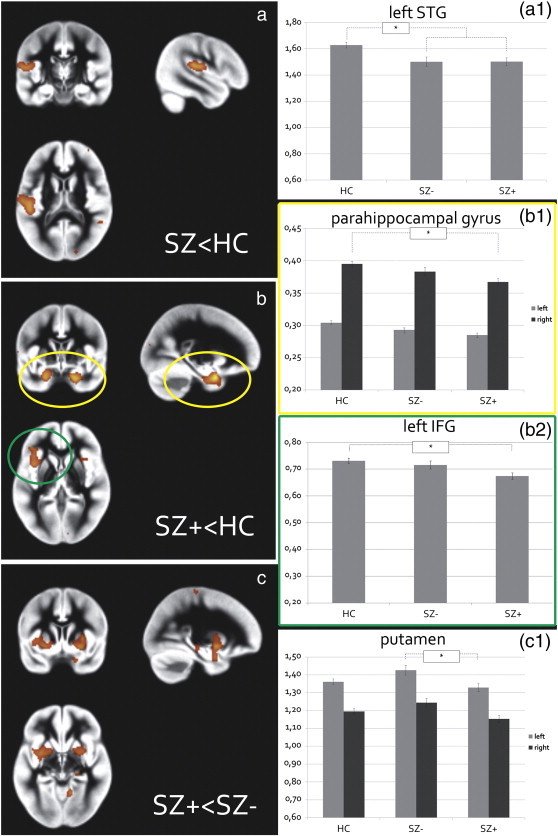
Gray matter volumetric effects.
a) Patients (both hallucinating [SZ +] and non-hallucinating patients [SZ −]) showed lower volume of the left superior temporal gyrus (STG) than healthy controls [HC], extending into the inferior parietal lobule, no difference in STG volume was observed between SZ + and SZ −(see plot A1).
b) SZ + showed lower volume than HC of the parahippocampal gyri (of which only the right parahippocampal effect was significant; see plot B1) and left inferior frontal gyrus (IFG: see plot B2). Although volumes appeared lower in SZ − compared to HC, results were non-significant.
c) SZ + showed lower volume of the bilateral putamen than SZ −, although only the left side survived whole brain correction for multiple comparisons.
All effects are displayed at p < .005 uncorrected. Plots A1, B1, B2, and C1 represent the mean GM volume of the clusters (corrected for total brain volume, age, and gender) revealed by the F-test testing for the main effect of group. The y-axis denotes mean volume in ml.
Compared to HC, SZ + showed lower volume of the left IFG and right parahippocampal gyrus (Fig. 1b). The left parahippocampal gyrus was observed just subthreshold in this comparison (k = 836, Z = 3.93, MNI coordinate: [z = − 21 y = 3 z = − 29], pFWE_ROI = .085). No significant volumetric differences in these regions were observed between SZ + and SZ −, and between SZ − and HC.
SZ + showed lower volume of the left putamen than SZ −, an effect that was observed just sub-threshold in the right putamen (k = 1765, Z = 3.89, MNI-coordinate: [z = 29 y = 5 z = − 11], pFWE_WholeBrain = .19). As Fig. 1c1 shows, an inverted u-shape is suggested in the relation of putaminal volume and hallucination presence, as SZ − appeared to show higher putaminal volume than HC. However, this difference was not significant. Finally within our composite ROI, SZ − showed lower volume of the bilateral PCC compared to SZ +, although this effect did not reach significance (k = 591, Z = 3.35, MNI coordinates: [x = − 8 y = − 36 z = 31] / [x = 9 y = − 40 z = 28], pFWE_ROI = .27).
Results are listed in Table 2.
Table 2.
between group comparisons of regional gray and white matter volume.
L/R: left/right hemisphere; BA: Brodmann area; k: cluster size at p < .005, uncorrected, MNI: Montreal Neurological Institute; p (whole brain): whole brain FWE corrected p-value at the cluster level after applying non-stationarity correction; p(ROI): FWE corrected p-value at the cluster level for the extent of the composite ROI after applying non-stationarity correction.
| Gray matter between-group comparisons SZ < HC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinate |
|||||||||||
| L/R | Region | BA | k (whole brain) | k (ROI) | x | y | z | Z-score | T-value | p (whole brain) | p (ROI) |
| L | Superior temporal gyrus | 41 | 2194 | 883 | − 50 | − 28 | 15 | 3.78 | 3.94 | .036 | .043 |
| Transverse temporal gyrus | 42 | − 60 | − 12 | 10 | 3.31 | 3.42 | |||||
| Posterior insula | 13 | − 44 | − 9 | 18 | 2.64 | 2.70 | |||||
| SZ + < SZ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinate |
||||||||||
| L/R | Region | BA | k (whole brain) | x | y | z | Z-score | T-value | p (whole brain) | p (ROI) |
| L | Putamen | – | 2146 | − 33 | 2 | − 2 | 3.47 | 3.59 | .048 | – |
| Putamen | – | − 29 | 2 | − 9 | 3.47 | 3.59 | ||||
| Putamen | – | − 24 | 9 | − 11 | 3.44 | 3.56 | ||||
| SZ + < HC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinate |
|||||||||||
| L/R | Region | BA | k (whole brain) | k (ROI) | x | y | z | Z-score | T-value | p (whole brain) | p (ROI) |
| R | Uncus/parahippocampal gyrus | 28/35 | 1427 | 707 | 21 | 6 | − 29 | 4.55 | 4.81 | .12 | .044 |
| L | Inferior frontal gyrus | 47 | 1369 | 548 | − 42 | 35 | 0 | 3.86 | 4.02 | .06 | .019 |
| Insula | 13 | − 41 | 11 | 7 | 3.34 | 3.45 | |||||
| Inferior frontal gyrus | 47 | − 41 | 24 | 0 | 3.20 | 3.29 | |||||
| White matter between-group comparisons SZ − < SZ + | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinate |
||||||||||
| L/R | Region | k (whole brain) | x | y | z | Z-score | T-value | p (whole brain) | p (ROI) | |
| L | postcentral lobule | 518 | 492 | − 30 | − 46 | 61 | 3.91 | 4.09 | .10 | .029 |
| superior parietal lobule | − 18 | − 51 | 61 | 3.58 | 3.72 | |||||
| medial parietal lobule/precuneus | − 9 | − 58 | 52 | 3.06 | 3.14 | |||||
3.2.1. Effects of confounders
Volumes of the significant clusters per participant were exported to SPSS to test for confounders. Within patients, volume of the STG was unrelated to P3 score (continuous), both across SZ + and SZ − and within SZ + (SZ: β = 0.027, p = .78; SZ +: β = 0.13, p = .35). Also, STG volume was unrelated to Positive symptomatology (minus P1 & P3), Negative symptomatology, years since disease onset, and type of medication (all β < .14, p > .28).
Volume of the IFG and parahippocampal gyrus within SZ + was not further correlated with hallucination severity, and volume was unrelated to P1, Positive psychopathology (minus P1 & P3), Negative symptomatology, age of onset and medication used (all β < |.45|, p > .16). Finally, the difference between SZ + and SZ − in putaminal volume was unaffected when clinical and medication confounders were stepwise added to the model.
3.3. VBM results: white matter
A main effect of group was observed on WM of the left superior and inferior parietal lobule, the superior temporal lobule, and the right middle frontal lobule (See the appendix, Table A-1 for a complete overview and details of the main effects).
Post-hoc t-test showed that SZ + had higher WM than SZ − in the postcentral gyrus extending into the superior parietal lobule and medial precuneus (See Table 2 and Fig. 2a) and subthreshold in the IPL (k = 447, Z = 3.82, MNI coordinates: [x = − 29 y = − 46 z = 40], pFWE_ROI = .11) and PCC (k = 209/66, Z = 3.42/3.19, MNI coordinates: [x = − 29 y = − 46 z = 40], pFWE_ROI = .21). The relations of WM volume in the superior and inferior parietal lobule were characterized by (inverted) U-shapes (See Fig. 2), as SZ − appeared to have lower WM of the superior parietal lobule than HC (k = 65, Z = 3.56, MNI-coordinates: [x = − 26 y = − 40 z = 60]) but higher volume of the IPL than HC (k = 183, Z = 3.33, MNI coordinates: [x = − 48 y = − 46 z = 39]. However, these comparisons did not reach significance (pFWE_ROI = .45 and pFWE_ROI = .27, respectively).
Fig. 2.
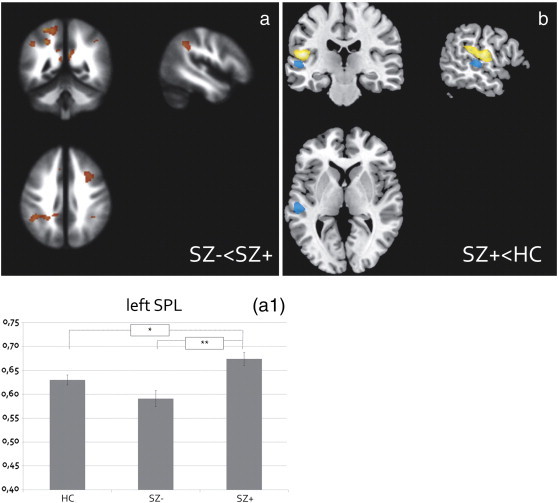
White matter volumetric effects.
a) Patients (both hallucinating [SZ +]) showed higher postcentral and superior parietal lobule white matter (WM), extending into the medial parietal lobule and inferior parietal lobule, as compared to non-hallucinating patients [SZ −] (see plot A1).
b) SZ + showed higher superior temporal lobule as compared to HC, adjacent to the gray matter (GM) cluster that was lower in volume in patients compared to HC, irrespective of hallucination presence. The GM cluster is depicted in yellow, the WM cluster is depicted in blue.
All effects are displayed at p < .005 uncorrected. Plot A1 represents the mean WM volume of the postcentral/superior parietal lobule cluster (corrected for total brain volume, age, and gender) revealed by the F-test testing for the main effect of group. The y-axis denotes mean volume in ml.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Compared to HC, SZ + had higher WM of the superior temporal lobe, adjacent to the GM cluster that was observed in SZ − and SZ + compared with HC (See Fig. 2b). However, the WM effect did not reach significance (k = 307, Z = 4.07, MNI coordinates: [x = − 60 y = − 27 z = 3], pFWE_ROI = .23).
Finally, no effects of hallucination presence were observed in the corpus callosum, also not at p < .005 uncorrected.
3.4. Post-hoc sensitivity analysis
Patients that demonstrated questionable hallucinatory symptomatology scored a ‘2’ on the PANSS-P3 item. Because of the uncertainty in hallucinating behavior, these patients may obscure the relation between regional brain volume and absence/presence of AVHs. Therefore, we post-hoc repeated the analysis on the volumes associated with AVH-presence after excluding seven 2-scoring patients using SPSS. However, excluding these seven patients did not influence the observations. If any, it made the association with putaminal GM-volume and SPL WM-volume stronger. Details on these analyses and results can be found in Appendix-1b.
4. Discussion
In this study, we investigated GM and WM morphometry related to AVH occurrence in 51 patients with schizophrenia (20 without hallucinations [SZ −]; 31 with hallucinations [SZ +]) and 51 healthy controls. Results indicate that lower volume of the left superior temporal gyrus (STG), including the primary auditory cortex is a robust abnormality in patients with schizophrenia, which seems unrelated to hallucination presence or severity (confirmed by whole-brain continuous linear regression analyses; see Appendix-1c and Fig. A-1). Abnormal low volumes of the left inferior frontal gyrus (IFG) and parahippocampal gyrus were however specifically related to the presence of hallucinations. Our results further indicate that the putamen plays a role in the structural pathology underlying hallucinations, where low volume is characteristic of SZ + as compared with SZ −. Finally, higher absolute whole-brain WM and relative WM volume of the left postcentral and parietal lobule was specifically associated with occurrence of hallucinations. All effects were unrelated to delusion severity, total positive symptomatology (minus hallucinations and delusions), negative symptomatology, general psychopathology, age of onset of the disorder or medication status. No effect on corpus callosum morphometry was observed. Together our results suggest that morphological abnormalities in a structural network encompassing the IFG, parahippocampal gyrus and putamen in addition to the disease general STG abnormalities may underlie the experience of hallucinations.
The medial temporal gyrus, inferior frontal gyrus and putamen abnormalities specific to hallucinations, together with the disease-generic STG abnormality, strikingly resembles the network of brain regions recently described in meta-analyses of functional studies on voice hearing in both psychotic and non-psychotic people (Jardri et al., 2011; van et al., 2013). Also, this set of morphological abnormalities supports the corticostriatal model proposed by Hoffman et al. (Hoffman et al., 2011a; Hoffman and McGlashan, 2001). In this model, increased putamen-IFG connectivity is proposed to be specific for SZ +, whereas increased STG-putamen connectivity is common for SZ patients irrespective of hallucination presence. We speculate that this hyperconnectivity may result from lower cortical volume owing to a lower propensity to exert inhibitory control in the communication between regions. Lower cortical gray matter may represent a lower glial count, lower cel size, or low density of neurons in the putamen, both excitatory and inhibitory. Lower glial and neuronal density may represent a loss of inhibitory activity, causing disinhibition from one region to another and higher connectivity between regions. Along this line of reasoning, the lower IFG and putaminal volume in SZ + patients, may result in a lack of down-regulation of the IFG over the putamen, leading to a more pronounced hyperconnectivity between these regions in hallucinating patients (SZ +). However, one might also argue that higher volume leads to a higher propensity to communicate and thus to higher connectivity, indicating a need for future studies to focus on structure–function relations. Our current results reflect the commonness of STG abnormalities for SZ patients and specificity of putaminal and IFG abnormalities for hallucinating patients. Moreover, we found abnormal parahippocampal morphometry to be more abnormal in hallucinating than in non-hallucinating patients as compared with controls. The parahippocampal gyrus has, within the context of voice hearing, been linked to memory processing and retrieving content from memory (Diederen et al., 2010) and abnormality in these processes may corrupt narratives in language (Hoffman et al., 2011b). However, this hippocampal region has also been linked to generation of conscious preceptions (Behrendt, 2013), binding of information from different sensory association cortices (Behrendt, 2013), behavioral inhibition and emotion regulation (Sjoerds et al., 2013; Gray and McNaughton, 2003), and attentional regulation over sensory inputs (Behrendt, 2010), and thereby may play an important role in the occurrence and content of hallucinations. Moreover, functional connectivity patterns of the hippocampus was shown to differentiate those experiencing auditory from those experiencing auditory and visual hallucinations (Amad et al., 2013), further suggesting the importance of the hippocampus for integrating signals from multiple sensory cortices for conscious experiencing (Behrendt, 2010).
Finally, the importance of this regions for experiencing hallucinations, for altered consciousness, and abnormal integration of information processed in cortical areas for accurate sensory representations, and finally note that differential connectivity of this region may link to the content of hallucinations (i.e. with or without visual hallucinations present). The left IFG has been linked to both voice hearing and voice hearing detection in functional neuroimaging studies (Diederen et al., 2012; van et al., 2013). Our results support the role of the left IFG in the primary pathology of hallucinations. Next to its undisputable role in language production, the IFG has been linked to attentional and inhibitory control and the selection of goal-appropriate behavior, underlying adequate emotion and behavioral regulation (Ochsner et al., 2012; van der Meer et al., 2013b). Finally, the putamen has been linked to the initiation and execution of speech (Price, 2010; Argyropoulos et al., 2013), integration of auditory and visual information (von Saldern and Noppeney, 2013), and with the conscious perception of auditory stimuli (Mhuircheartaigh et al., 2010), underlining the significance of the putamen for speech perception, production and multimodal integration that all have importance for experiencing hallucinations.
As GM volume of the STG was unrelated to current hallucination presence and severity, positive symptomatology in general, and duration of illness, we suggest that abnormalities in certain portions of the STG may not be truly specific to AVHs, but rather related to the schizophrenia disease process in general. The amount of studies that related schizophrenia to STG pathology is abundant (Sun et al., 2009; Honea et al., 2005) and places the STG at a prominent position within the structural pathology underlying the occurrence of psychotic symptoms, although its specific relevance for hallucinations has been reported as well (Palaniyappan et al., 2012; Neckelmann et al., 2006; Gaser et al., 2004). Discrepancies in results could be explained by differences in sample size (Neckelmann et al., 2006), number of hallucinating patients (Gaser et al., 2004), or a non-optimal distribution of hallucination severity to study correlations in a linear fashion (Nenadic et al., 2010). It is important to note that we do not refute the importance of the STG for hallucinations, but propose that the likelihood for experiencing hallucinations is increased by the specific involvement of a defective network of medial temporal, putaminal and lateral prefrontal regions in addition to general disease-related STG pathology.
An intriguing open issue is how structural variation influences functional integration. In attempts to link function to structure it became apparent that relations are not always mapped one to one: functional relations may exist in the absence of structural connections whereas a fair amount of information is transferred via indirect structural connections (Honey et al., 2009). In the specific case of schizophrenia patients, researchers have noted that function-structure relations are stronger in patients (van den Heuvel et al., 2013), which would suggest that our volumetric results predict decreased connectivity between these regions, in line with previous work of our group (Curcic-Blake et al., 2012; Vercammen et al., 2010). However, structure-function relations have only been studied using diffusion tensor imaging data. So far, the predictive value of GM morphometry for functional connectivity has, to our knowledge, not been studied in schizophrenia, although interesting relations have been shown in major depressive disorder (van Tol et al., 2013). We here hypothesize that decreased volume of the IFG and parahippocampal gyrus and putamen in SZ + together with a disease-generic abnormality in the STG hampers the direct connectivity between these regions, leading to a disintegration of signal and eventually to the experience of hallucinations.
Related to WM abnormalities, we found no associations with corpus callosum volume, but relations between AVH and total WM volume and relative WM volume of the postcentral and superior parietal lobule were observed, and partly included the arcuate fasciculus. This WM tract has been repeatedly linked to AVH in schizophrenia in diffusion tensor imaging studies. Our result of increased WM volume fits the previous observations of higher fractional anisotropy (FA) (Hubl et al., 2004; Abdul-Rahman et al., 2012) and higher volume of the arcuate fasciculus (Hubl et al., 2010). As higher FA and volume most likely reflects a higher propensity to transmit signals between regions (Witelson, 1995), increased WM in the region connecting this post-central gyrus and medial parietal regions may increase the likelihood that information is transmitted between temporo-parietal cortical regions associated with voice hearing (Diederen et al., 2012; Jardri et al., 2011; Kuhn and Gallinat, 2010), the generation of inner speech (Shergill et al., 2003), and self-referential processing (e.g. the medial precuneus/PCC (Whitfield-Gabrieli and Ford, 2012). However, conflicting results have been reported as well. Decreased arcuate fasciculus FA in patients with a history of hallucinations as compared to patients without such history (Catani et al., 2011), compared to healthy controls (de Weijer et al., 2011), or compared non-psychotic voice-hearers (de Weijer et al., 2013) has been reported as well. However, these results may not specifically apply to the posterior parts of the AF (Catani et al., 2011), and specificity of effects for hallucinating vs. non-hallucinating patients were not explicitly studies (de Weijer et al., 2011, 2013). Moreover, we could not support lower FA in the posterior parts of the AF in a subsample of the current sample (Curcic-Blake et al., 2013). The absence of a relation between hallucinations and volume of the corpus callosum does not support a central role of this structure in the structural pathology underlying AVH. This is consistent with earlier studies (Rossell et al., 2001; Rotarska-Jagiela et al., 2008; Serpa et al., 2012), although abnormal diffusion abnormalities have been observed related to hallucinations (Hubl et al., 2004; Knochel et al., 2012; Makris et al., 2010; Mulert et al., 2011). However, current macroscopic observations can not fully exclude the possibility that microscopic abnormalities in interhemispheric communication through the corpus callosum underlies hallucinations.
Some limitations should be mentioned. Although medication use did not differ between hallucinating and non-hallucinating patients and we investigated if use (yes/no) affected the observed effects, a possible effect of dose and duration of usage of antipsychotic medication on our results cannot be ruled out (Ho et al., 2011). Unfortunately, dose-equivalences of for example chlorpromazine could not be calculated because reliable dose information was not available of all patients. Moreover, we employed only one measure of hallucination severity; thereby we had limited information on frequency, content, duration, and form of the hallucinations. Although the majority of patients experienced auditory–verbal hallucinations as expressed during the interview, use of the PANSS gives no specific information on content of hallucinations and therefore current results may not be fully specific for AVH, but relate to the general presence of hallucinations. Moreover, next to reflecting current propensity to hallucinate (last week), the PANSS P3 item may additionally reflect efficacy of treatment or levels of experienced stress in the past week. Caution should therefore be taken when interpreting our results as reflecting trait-or state hallucinations. Nevertheless, in various studies the P3 item has been shown to be a valid measure of AVH presence (Kim et al., 2010; Steel et al., 2007), and to be highly correlated with more extensive assessments, such as the Psychotic Symptoms Rating Scale (PSYRATS; (Haddock et al., 1999) and with the factor AVH hallucination duration of the Schizophrenia Voices Questionnaire (HPSVQ; (Kim et al., 2010; Steel et al., 2007).
4.1. Conclusions
In sum, results from this study suggest that structural abnormalities in a cortical network including the medial temporal lobe, inferior frontal gyrus and putamen in addition to disease-common abnormal superior temporal morphology increases the likelihood for experiencing hallucinations. Abnormal WM morphometry of the postcentral and parietal lobule may further promote interregional communication subserving voice hearing and self-referential processing. Future studies should focus on the meaning of GM and WM morphological abnormalities for integration of functional networks associated with AVH.
Acknowledgments
This work was supported by a European Science Foundation EURYI grant (NWO no. 044035001) awarded to A.A. All authors deny any conflicts of interest or commercial associations in connection with the submitted manuscript. We would like to thank Leonie Bais, Edith Liemburg, Jorien van der Velde, Marte Swart, Erna van 't Hag, Dick Smid, and Peter Croeze for their help in data collection and data management.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by a European Science Foundation EURYI grant (NWO no. 044035001) awarded to A.A.
Contributor Information
Marie-José van Tol, Email: m.j.van.tol@umcg.nl.
Lisette van der Meer, Email: l.vandermeer@lentis.nl.
Richard Bruggeman, Email: r.bruggeman@umcg.nl.
Gemma Modinos, Email: gemma.modinos@kcl.ac.uk.
Henderikus Knegtering, Email: h.knegtering@lentis.nl.
André Aleman, Email: a.aleman@umcg.nl.
Appendix A. Supplementary data
Supplementary material
References
- Abdul-Rahman M.F., Qiu A., Woon P.S., Kuswanto C., Collinson S.L., Sim K. Arcuate fasciculus abnormalities and their relationship with psychotic symptoms in schizophrenia. PLoS One. 2012;7:e29315. doi: 10.1371/journal.pone.0029315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amad A., Cachia A., Gorwood P., Pins D., Delmaire C., Rolland B., Mondino M., Thomas P., Jardri R. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol. Psychiatry. 2013 doi: 10.1038/mp.2012.181. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Andreasen N., Flaum M. Schizophrenia — the characteristic symptoms. Schizophr. Bull. 1991;17:27–49. doi: 10.1093/schbul/17.1.27. [DOI] [PubMed] [Google Scholar]
- Argyropoulos G.P., Tremblay P., Small S.L. The neostriatum and response selection in overt sentence production: an fMRI study. NeuroImage. 2013;82C:53–60. doi: 10.1016/j.neuroimage.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barta P.E., Pearlson G.D., Powers R.E., Richards S.S., Tune L.E. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am. J. Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Behrendt R.P. Contribution of hippocampal region CA3 to consciousness and schizophrenic hallucinations. Neurosci. Biobehav. Rev. 2010;34:1121–1136. doi: 10.1016/j.neubiorev.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Behrendt R.P. Hippocampus and consciousness. Rev. Neurosci. 2013;24:239–266. doi: 10.1515/revneuro-2012-0088. [DOI] [PubMed] [Google Scholar]
- Catani M., Craig M.C., Forkel S.J., Kanaan R., Picchioni M., Toulopoulou T., Shergill S., Williams S., Murphy D.G., McGuire P. Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biol Psychiatry. 2011;70(12):1143–1150. doi: 10.1016/j.biopsych.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Curcic-Blake B., Liemburg E., Vercammen A., Swart M., Knegtering H., Bruggeman R., Aleman A. When broca goes uninformed: reduced information flow to Broca's area in schizophrenia patients with auditory hallucinations. Schizophr. Bull. 2012;39:1087–1095. doi: 10.1093/schbul/sbs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcic-Blake B., Nanetti L., van der Meer L., Cerliani L., Renken R., Pijnenborg G.H., Aleman A. Not on speaking terms: hallucinations and structural network disconnectivity in schizophrenia. Brain Struct. Funct. 2013 doi: 10.1007/s00429-013-0663-y. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- de Weijer A.D., Mandl R.C., Diederen K.M., Neggers S.F., Kahn R.S., Hulshoff Pol H.E., Sommer I.E. Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr. Res. 2011;130:68–77. doi: 10.1016/j.schres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- de Weijer A.D., Neggers S.F., Diederen K.M., Mandl R.C., Kahn R.S., Hulshoff Pol H.E., Sommer I.E. Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non-psychotic individuals. Hum. Brain Mapp. 2013;34:626–634. doi: 10.1002/hbm.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen K.M., Neggers S.F., Daalman K., Blom J.D., Goekoop R., Kahn R.S., Sommer I.E. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am. J. Psychiatry. 2010;167:427–435. doi: 10.1176/appi.ajp.2009.09040456. [DOI] [PubMed] [Google Scholar]
- Diederen K.M., Daalman K., de Weijer A.D., Neggers S.F., van G.W., Blom J.D., Kahn R.S., Sommer I.E. Auditory hallucinations elicit similar brain activation in psychotic and nonpsychotic individuals. Schizophr. Bull. 2012;38:1074–1082. doi: 10.1093/schbul/sbr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks T., Linden D.E., Jandl M., Formisano E., Goebel R., Lanfermann H., Singer W. Activation of Heschl's gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Marti G., Aguilar E.J., Lull J.J., Marti-Bonmati L., Escarti M.J., Manjon J.V., Moratal D., Robles M., Sanjuan J. Schizophrenia with auditory hallucinations: a voxel-based morphometry study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:72–80. doi: 10.1016/j.pnpbp.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Gaser C., Nenadic I., Volz H.P., Buchel C., Sauer H. Neuroanatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb. Cortex. 2004;14:91–96. doi: 10.1093/cercor/bhg107. [DOI] [PubMed] [Google Scholar]
- Gray J.A., McNaughton N. 2nd ed. Oxford University Press; New York: 2003. The Neuropsychology of Anxiety, An enquiry into the function of the septo-hippocampal system. [Google Scholar]
- Haddock G., McCarron J., Tarrier N., Faragher E.B. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychol. Med. 1999;29:879–889. doi: 10.1017/s0033291799008661. [DOI] [PubMed] [Google Scholar]
- Ho B.C., Andreasen N.C., Ziebell S., Pierson R., Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.E., McGlashan T.H. Neural network models of schizophrenia. Neuroscientist. 2001;7:441–454. doi: 10.1177/107385840100700513. [DOI] [PubMed] [Google Scholar]
- Hoffman R.E., Fernandez T., Pittman B., Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol. Psychiatry. 2011;69:407–414. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.E., Grasemann U., Gueorguieva R., Quinlan D., Lane D., Miikkulainen R. Using computational patients to evaluate illness mechanisms in schizophrenia. Biol. Psychiatry. 2011;69:997–1005. doi: 10.1016/j.biopsych.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R., Crow T.J., Passingham D., Mackay C.E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Honey C.J., Sporns O., Cammoun L., Gigandet X., Thiran J.P., Meuli R., Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D., Koenig T., Strik W., Federspiel A., Kreis R., Boesch C., Maier S.E., Schroth G., Lovblad K., Dierks T. Pathways that make voices—White matter changes in auditory hallucinations. Arch. Gen. Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Hubl D., Dougoud-Chauvin V., Zeller M., Federspiel A., Boesch C., Strik W., Dierks T., Koenig T. Structural analysis of Heschl's gyrus in schizophrenia patients with auditory hallucinations. Neuropsychobiology. 2010;61:1–9. doi: 10.1159/000258637. [DOI] [PubMed] [Google Scholar]
- IBM SPSS Statistics, IBM corporation, New York, USA.
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (Panss) for Schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Opler L.A., Lindenmayer J.P. Reliability and Validity of the Positive and Negative Syndrome Scale for Schizophrenics. Psychiatry Res. 1988;23:99–110. doi: 10.1016/0165-1781(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Jung H.Y., Hwang S.S., Chang J.S., Kim Y., Ahn Y.M., Kim Y.S. The usefulness of a self-report questionnaire measuring auditory verbal hallucinations. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:968–973. doi: 10.1016/j.pnpbp.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Knochel C., Oertel-Knochel V., Schonmeyer R., Rotarska-Jagiela A., van de Ven V., Prvulovic D., Haenschel C., Uhlhaas P., Pantel J., Hampel H., Linden D.E. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. NeuroImage. 2012;59:926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- Kuhn S., Gallinat J. Quantitative Meta-Analysis on State and Trait Aspects of Auditory Verbal Hallucinations in Schizophrenia. Schizophr. Bull. 2010;38:779–786. doi: 10.1093/schbul/sbq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Yoshida T., Kubicki M., Bouix S., Westin C.F., Kindlmann G., Niznikiewicz M., Cohen A., McCarley R.W., Shenton M.E. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a Diffusion Tensor Imaging study. Schizophr. Res. 2009;108:33–40. doi: 10.1016/j.schres.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg, E.J., Dlabac-De Lange, J.J., Bais, L., Knegtering, H., van Osch, M.J.P., Renken, R.J., Aleman, A. Neural correlates of apathy and planning performance in patients with schizophrenia. In preparation. [DOI] [PubMed]
- Makris N., Seidman L.J., Ahern T., Kennedy D.N., Caviness V.S., Tsuang M.T., Goldstein J.M. White matter volume abnormalities and associations with symptomatology in schizophrenia. Psychiatry Res. Neuroimaging. 2010;183:21–29. doi: 10.1016/j.pscychresns.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhuircheartaigh R.N., Rosenorn-Lanng D., Wise R., Jbabdi S., Rogers R., Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J. Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G., Vercammen A., Mechelli A., Knegtering H., McGuire P.K., Aleman A. Structural covariance in the hallucinating brain: a voxel-based morphometry study. J. Psychiatry Neurosci. 2009;34:465–469. [PMC free article] [PubMed] [Google Scholar]
- Modinos G., Costafreda S.G., van Tol M.J., McGuire P.K., Aleman A., Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: a quantitative meta-analysis of voxel-based morphometry studies. Cortex. 2013;49:1046–1055. doi: 10.1016/j.cortex.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Mulert C., Kirsch V., Whitford T.J., Alvarado J., Pelavin P., McCarley R.W., Kubicki M., Salisbury D.F., Shenton M.E. Hearing voices: a role of interhemispheric auditory connectivity? World J. Biol. Psychiatry. 2011;13:153–158. doi: 10.3109/15622975.2011.570789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckelmann G., Specht K., Lund A., Ersland L., Smievoll A.I., Neckelmann D., Hugdahl K. MR morphometry analysis of grey matter volume reduction in schizophrenia: association with hallucinations. Int. J. Neurosci. 2006;116:9–23. doi: 10.1080/00207450690962244. [DOI] [PubMed] [Google Scholar]
- Nenadic I., Smesny S., Schlosser R.G., Sauer H., Gaser C. Auditory hallucinations and brain structure in schizophrenia: voxel-based morphometric study. Br. J. Psychiatry. 2010;196:412–413. doi: 10.1192/bjp.bp.109.070441. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Daly O.G., Frangou S., Chitnis X., Shergill S.S. Brain structural changes in schizophrenia patients with persistent hallucinations. Psychiatry Res. Neuroimaging. 2007;156:15–21. doi: 10.1016/j.pscychresns.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Onitsuka T., Shenton M.E., Salisbury D.F., Dickey C.C., Kasai K., Toner S.K., Frumin M., Kikinis R., Jolesz F.A., McCarley R.W. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am. J. Psychiatry. 2004;161:1603–1611. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L., Balain V., Radua J., Liddle P.F. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr. Res. 2012;137:169–173. doi: 10.1016/j.schres.2012.01.038. [DOI] [PubMed] [Google Scholar]
- Plaze M., Paillere-Martinot M.L., Penttilae J., Januel D., de Beaurepaire R., Bellivier F., Andoh J., Galinowski A., Gallarda T., Artiges E., Olie J.P., Mangin J.F., Martinot J.L., Cachia A. “Where Do Auditory Hallucinations Come From?”-A Brain Morphometry Study of Schizophrenia Patients With Inner or Outer Space Hallucinations. Schizophr. Bull. 2011;37:212–221. doi: 10.1093/schbul/sbp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. Vol. 1191. 2010. The anatomy of language: a review of 100 fMRI studies published in 2009; pp. 62–88. (Year in Cognitive Neuroscience 2010). [DOI] [PubMed] [Google Scholar]
- Rajarethinam R.P., DeQuardo J.R., Nalepa R., Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr. Res. 2000;41:303–312. doi: 10.1016/s0920-9964(99)00083-3. [DOI] [PubMed] [Google Scholar]
- Rossell S.L., Shapleske J., Fukuda R., Woodruff P.W.R., Simmons A., David A.S. Corpus callosum area and functioning in schizophrenic patients with auditory–verbal hallucinations. Schizophr. Res. 2001;50:9–17. doi: 10.1016/s0920-9964(00)00070-0. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A., Schoenmeyer R., Oertel V., Haenschel C., Vogeley K., Linden D.E.J. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. NeuroImage. 2008;39:1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Schneider S.D., Jelinek L., Lincoln T.M., Moritz S. What happened to the voices? A fine-grained analysis of how hallucinations and delusions change under psychiatric treatment. Psychiatry Res. 2011;188:13–17. doi: 10.1016/j.psychres.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Serpa M.H., Schaufelberger M.S., Rosa P.G., Duran F.L., Santos L.C., Muray R.M., Scazufca M., Menezes P.R., Busatto G.F. Corpus callosum volumes in recent-onset schizophrenia are correlated to positive symptom severity after 1year of follow-up. Schizophr. Res. 2012;137:258–259. doi: 10.1016/j.schres.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Shapleske J., Rossell S.L., Chitnis X.A., Suckling J., Simmons A., Bullmore E.T., Woodruff P.W.R., David A.S. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb. Cortex. 2002;12:1331–1341. doi: 10.1093/cercor/12.12.1331. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shergill S.S., Brammer M.J., Fukuda R., Williams S.C.R., Murray R.M., McGuire P.K. Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br. J. Psychiatry. 2003;182:525–531. doi: 10.1192/bjp.182.6.525. [DOI] [PubMed] [Google Scholar]
- Shergill S.S., Kanaan R.A., Chitnis X.A., O'Daly O., Jones D.K., Frangou S., Williams S.C.R., Howard R.J., Barker G.J., Murray R.M., McGuire P. A diffusion tensor imaging study of fasciculi in schizophrenia. Am. J. Psychiatry. 2007;164:467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Shin S.E., Lee J.S., Kang M.H., Kim C.E., Bae J.N., Jung G. Segmented volumes of cerebrum and cerebellum in first episode schizophrenia with auditory hallucinations. Psychiatry Res. 2005;138:33–42. doi: 10.1016/j.pscychresns.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Sjoerds Z., van Tol M.J., van den Brink W., van der Wee N.J., van Buchem M.A., Aleman A., Penninx B.W., Veltman D.J. Family history of alcohol dependence and gray matter abnormalities in non-alcoholic adults. World J. Biol. Psychiatry. 2013;14(8):565–573. doi: 10.3109/15622975.2011.640942. [DOI] [PubMed] [Google Scholar]
- Steel C., Garety P.A., Freeman D., Craig E., Kuipers E., Bebbington P., Fowler D., Dunn G. The multidimensional measurement of the positive symptoms of psychosis. Int. J. Methods Psychiatr. Res. 2007;16:88–96. doi: 10.1002/mpr.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Maller J.J., Guo L., Fitzgerald P.B. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res. Rev. 2009;61:14–32. doi: 10.1016/j.brainresrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O., Collin G., Scheewe T., Mandl R.C., Cahn W., Goni J., Hulshoff Pol H.E., Kahn R.S. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- van der Meer L., de Vos A.E., Stiekema A.P., Pijnenborg G.H., van Tol M.J., Nolen W.A., David A.S., Aleman A. Insight in schizophrenia: involvement of self-reflection networks? Schizophr. Bull. 2013;39(6):1288–1295. doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L., Groenewold N.A., Pijnenborg M., Aleman A. Psychosis-proneness and neural correlates of self-inhibition in theory of mind. PLoS One. 2013;8:e67774. doi: 10.1371/journal.pone.0067774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde J., Gromann P.M., Swart M., de Haan L., Wiersma D., Bruggeman R., Krabbendam L., Aleman A. Gray matter, an endophenotype for schizophrenia? A voxel-based morphometry study in siblings of patients with schizophrenia. 2013 doi: 10.1503/jpn.140064. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout R.J., Goldberg J.O. Quantifying self-reports of auditory verbal hallucinations in persons with psychosis. Can. J. Behav. Sci. 2007;39:73–77. [Google Scholar]
- van Tol M.J., Li M., Metzger C.D., Hailla N., Horn D.I., Li W., Heinze H.J., Bogerts B., Steiner J., He H., Walter M. Local cortical thinning links to resting state disconnectivity in major depressive disorder. Psychol. Med. 2013 doi: 10.1017/S0033291713002742. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- van L.R., Diederen K.M., Koops S., Begemann M.J., Sommer I.E. The influence of stimulus detection on activation patterns during auditory hallucinations. Schizophr. Res. 2013;145:27–32. doi: 10.1016/j.schres.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Vercammen A., Knegtering H., den Boer J.A., Liemburg E.J., Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol. Psychiatry. 2010;67:912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- von Saldern S., Noppeney U. Sensory and striatal areas integrate auditory and visual signals into behavioral benefits during motion discrimination. J. Neurosci. 2013;33:8841–8849. doi: 10.1523/JNEUROSCI.3020-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Witelson S.F. Neuroanatomical bases of hemispheric functional specialization in the human brain: possible developmental factors. In: Kitterle F.L., editor. Hemispheric Communication: Mechanisms and Models. Lawrence Erlbaum Associates, Inc.; New Yersey: 1995. pp. 61–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


