Abstract
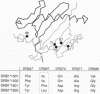
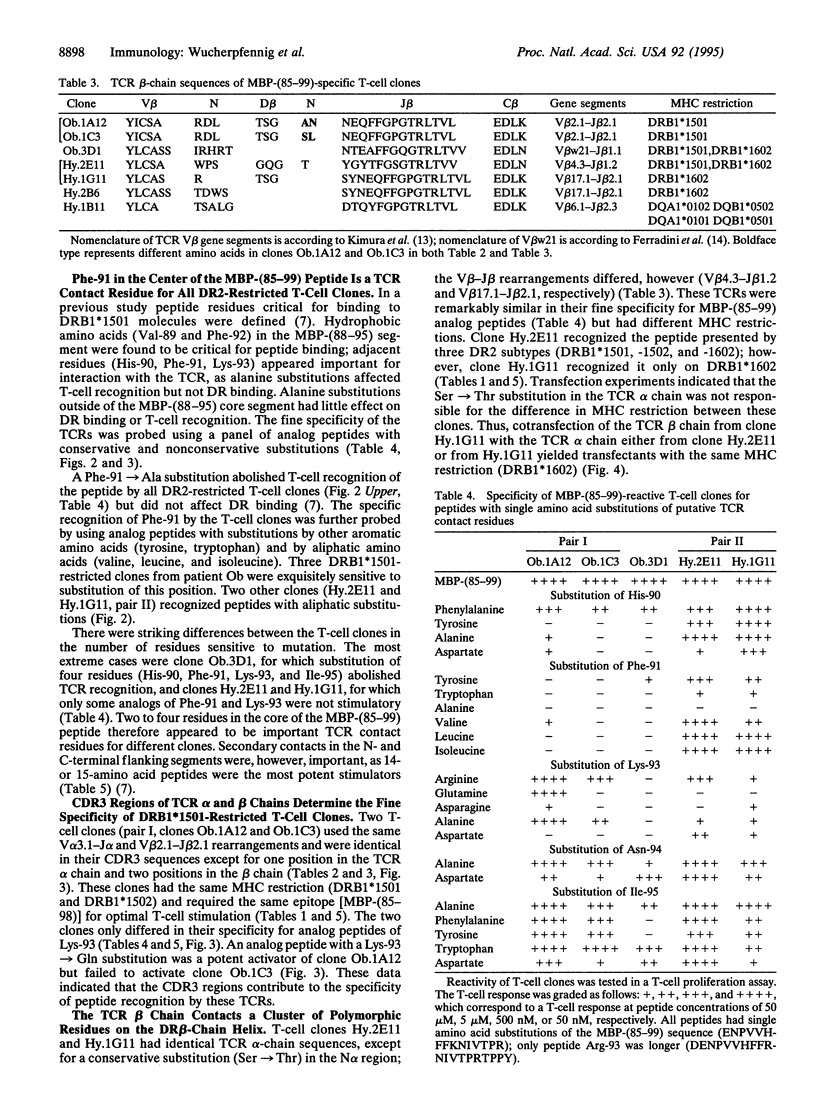
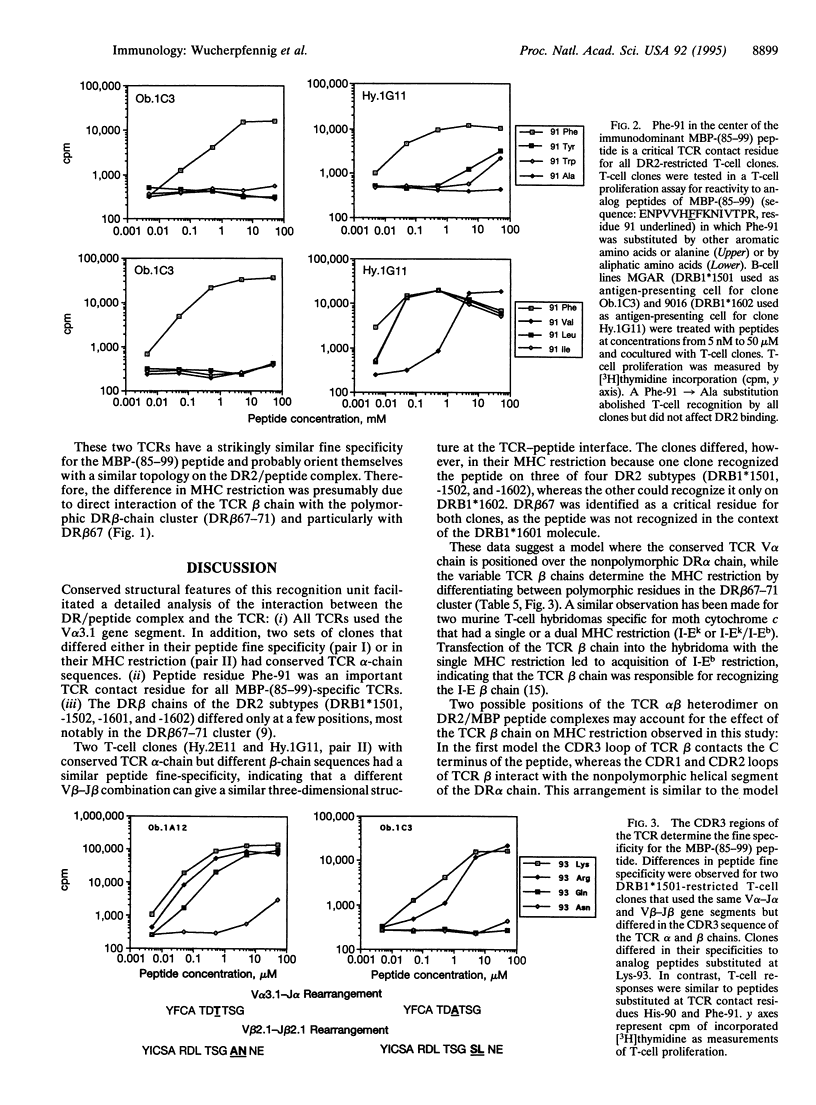
T-cell receptors (TCRs) recognize peptide bound within the relatively conserved structural framework of major histocompatibility complex (MHC) class I or class II molecules but can discriminate between closely related MHC molecules. The structural basis for the specificity of ternary complex formation by the TCR and MHC/peptide complexes was examined for myelin basic protein (MBP)-specific T-cell clones restricted by different DR2 subtypes. Conserved features of this system allowed a model for positioning of the TCR on DR2/peptide complexes to be developed: (i) The DR2 subtypes that presented the immunodominant MBP peptide differed only at a few polymorphic positions of the DR beta chain. (ii) TCR recognition of a polymorphic residue on the helical portion of the DR beta chain (position DR beta 67) was important in determining the MHC restriction. (iii) The TCR variable region (V) alpha 3.1 gene segment was used by all of the T-cell clones. TCR V beta usage was more diverse but correlated with the MHC restriction--i.e., with the polymorphic DR beta chains. (iv) Two clones with conserved TCR alpha chains but different TCR beta chains had a different MHC restriction but a similar peptide specificity. The difference in MHC restriction between these T-cell clones appeared due to recognition of a cluster of polymorphic DR beta-chain residues (DR beta 67-71). MBP-(85-99)-specific TCRs therefore appeared to be positioned on the DR2/peptide complex such that the TCR beta chain contacted the polymorphic DR beta-chain helix while the conserved TCR alpha chain contacted the nonpolymorphic DR alpha chain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Ferradini L., Roman-Roman S., Azocar J., Michalaki H., Triebel F., Hercend T. Studies on the human T cell receptor alpha/beta variable region genes. II. Identification of four additional V beta subfamilies. Eur J Immunol. 1991 Apr;21(4):935–942. doi: 10.1002/eji.1830210412. [DOI] [PubMed] [Google Scholar]
- Hong S. C., Chelouche A., Lin R. H., Shaywitz D., Braunstein N. S., Glimcher L., Janeway C. A., Jr An MHC interaction site maps to the amino-terminal half of the T cell receptor alpha chain variable domain. Cell. 1992 Jun 12;69(6):999–1009. doi: 10.1016/0092-8674(92)90618-m. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Esser U., Fazekas de St Groth B., Reay P. A., Davis M. M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992 Jan 16;355(6357):224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Kimura N., Toyonaga B., Yoshikai Y., Du R. P., Mak T. W. Sequences and repertoire of the human T cell receptor alpha and beta chain variable region genes in thymocytes. Eur J Immunol. 1987 Mar;17(3):375–383. doi: 10.1002/eji.1830170312. [DOI] [PubMed] [Google Scholar]
- Marsh S. G., Bodmer J. G. HLA class II nucleotide sequences, 1992. Hum Immunol. 1992 Sep;35(1):1–17. doi: 10.1016/0198-8859(92)90090-a. [DOI] [PubMed] [Google Scholar]
- Martin R., Jaraquemada D., Flerlage M., Richert J., Whitaker J., Long E. O., McFarlin D. E., McFarland H. F. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol. 1990 Jul 15;145(2):540–548. [PubMed] [Google Scholar]
- Ota K., Matsui M., Milford E. L., Mackin G. A., Weiner H. L., Hafler D. A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990 Jul 12;346(6280):183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Pette M., Fujita K., Wilkinson D., Altmann D. M., Trowsdale J., Giegerich G., Hinkkanen A., Epplen J. T., Kappos L., Wekerle H. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7968–7972. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Germain R. N. Predictable acquisition of a new MHC recognition specificity following expression of a transfected T-cell receptor beta-chain gene. Nature. 1987 Sep 17;329(6136):256–259. doi: 10.1038/329256a0. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994 Mar 17;368(6468):215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Valli A., Sette A., Kappos L., Oseroff C., Sidney J., Miescher G., Hochberger M., Albert E. D., Adorini L. Binding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T cells from multiple sclerosis patients. J Clin Invest. 1993 Feb;91(2):616–628. doi: 10.1172/JCI116242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Sette A., Southwood S., Oseroff C., Matsui M., Strominger J. L., Hafler D. A. Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med. 1994 Jan 1;179(1):279–290. doi: 10.1084/jem.179.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Zhang J., Witek C., Matsui M., Modabber Y., Ota K., Hafler D. A. Clonal expansion and persistence of human T cells specific for an immunodominant myelin basic protein peptide. J Immunol. 1994 Jun 1;152(11):5581–5592. [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]