Genetic, molecular, pathological and biomarker studies suggest the amyloid precursor protein (APP) derivative β-amyloid (Aβ) plays an important role in the pathogenesis of Alzheimer's disease (AD). Generation of 40–42 amino acid-Aβ is contingent upon sequential proteolytic processing of APP by β-secretase-1 (BACE1) and γ-secretase (Vassar et al., 1999). Cleavage of APP at one of two β-sites by BACE1 generates truncated C-terminal (C99 or C89) fragments that are subsequently cleaved by γ-secretase to release Aβ peptides. Though the majority of Aβ peptides are 40 aa (Aβ40) in length, mutations in the genes encoding APP and the γ-secretase catalytic components presenilin-1 (PS1) and presenilin-2 increase production of 42-amino-acid species (Aβ42), which are capable of rapid nucleation (Jarrett et al., 1993). These genetic mutations ultimately cause early onset, familial forms of AD (FAD) by elevating the Aβ42/Aβ40 ratio and exacerbating Aβ42 deposition in select forebrain regions, including the hippocampal complex and neocortex (Scheuner et al., 1996).
For over a quarter of a century, reports have indicated that non-neuronal cells express APP (Card et al., 1988) and secrete putative Aβ peptides (Busciglio et al., 1993), but it is widely believed that forebrain excitatory neurons produce the majority of Aβ in an activity-dependent manner (Kamenetz et al., 2003). In short, NMDAR-dependent neuronal activity is thought to increase APP endocytosis from the membrane via a clathrin-dependent mechanism, rendering it more susceptible to amyloidogenic processing within the endosomal system by BACE1. Intracellular Aβ monomers and oligomers, the latter of which possess their own neurotoxic properties, are then released into the extracellular milieu (for review, see O'Brien and Wong, 2011), where they aggregate into larger fibrils and plaques and act as proinflammatory lesions, stimulating the activation of microglia and astrocytes. Reactive glial cells cluster around Aβ deposits, indicative of a phagocytic response that may have both beneficial and harmful effects on surrounding neurons. The deleterious effects of reactive glia on the AD brain are thought to stem from Aβ-mediated release of proinflammatory molecules, including reactive oxygen species, interleukins, prostaglandins, cytokines, and complement factors. However, a recent report in The Journal of Neuroscience (Veeraraghavalu et al., 2014) suggests that astrocytes and microglia may play an additional role in AD pathobiology by contributing directly to cortical and hippocampal Aβ load.
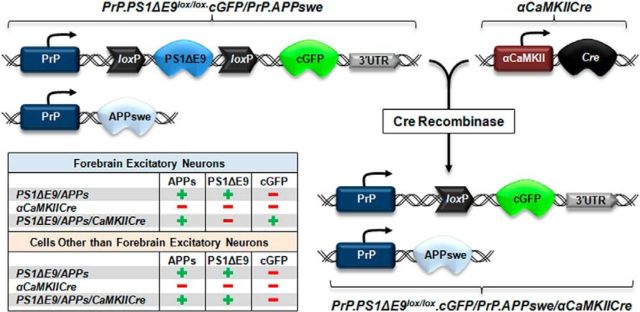
To examine glial-derived effects on overall brain Aβ load, Veeraraghavalu and colleagues (2014) used three previously characterized transgenic murine lines to exclusively direct production of human Aβ to non-neuronal cells (Fig. 1). In the first transgenic line, the ubiquitously expressed murine prion protein (PrP) promotes expression of FAD-linked PS1ΔE9 cDNA flanked by two loxP sites and a downstream copepod green fluorescent protein (cGFP) reporter (PrP.PS1ΔE9lox/lox.cGFP). The deletion of exon 9 (ΔE9) from PS1 renders the protein resistant to endoproteolysis and alters the Aβ40/Aβ42 ratio. (Thinakaran et al., 1996). Crossing this line with mice expressing Cre recombinase driven by the excitatory neuron-specific promotor αCaMKII (αCaMKIICre) generates a second line of mice in which the mutant PS1ΔE9 is removed, but cGFP continues to be expressed in excitatory neurons located within the hippocampus, cortex, striatum, septum, and amygdala (Fig. 1). Thus, subsequent enhancement of Aβ production is limited to cells devoid of αCaMKII in these areas. However, it is important to note that interneurons, in addition to glia, may provide an alternative source of Aβ in these mice.
Figure 1.
Illustration depicting generation of the APPswe/PS1ΔE9flox/αCaMKIICre transgenic line. Mice harboring mutated PS1ΔE9 cDNA flanked by two loxP sites and a downstream cGFP reporter were crossed with mice expressing αCaMKII-driven Cre recombinase. The table indicates cell-type-specific transgene expression in the models used by Veeraraghavalu and colleagues (2014). 3′-UTR, 3′-untranslated sequences of the PrP gene.
In PrP.PS1ΔE9lox/lox.cGFP mice, recombination-mediated reductions in PS1ΔE9 levels and subsequent cGFP expression occurred concomitantly, beginning at 6 weeks of age. Silencing and appearance of PS1ΔE9 and cGFP expression, respectively, were not observed at earlier ages because transcription of αCaMKII mRNA is developmentally restricted. Previous reports have indicated that expression of the excitatory neuron-specific, serine-threonine kinase αCaMKII increases approximately tenfold during the third week of life (Burgin et al., 1990) and stabilizes shortly thereafter. By 7 months of age, PS1ΔE9flox/αCaMKIICre bigenic animals displayed a nearly 80% reduction in human PS1ΔE9 levels relative to age-matched PS1ΔE9flox mice. Excision of the human PS1ΔE9 cassette also elevated the accumulation of murine N-terminal fragmented PS1, indicative of successful recombination and recovery of endogenous PS1 expression. Moreover, PS1ΔE9 deletion was restricted to excitatory neurons as cGFP signal did not colocalize with the astrocyte-specific antigens glial fibrillary acidic protein and S100 calcium binding protein B (Veeraraghavalu and Sisodia, 2013).
A third transgenic strain used by Veeraraghavalu and colleagues (2014) was generated by breeding the PS1ΔE9flox line with mice overexpressing APP mutations (K595N, M596L) linked to FAD (APPswe) (Veeraraghavalu et al., 2014). Bigenic APPswe/PS1ΔE9flox mice were then crossed with αCaMKIICre mice to generate trigenic animals expressing PS1ΔE9 in non-neuronal cells, cGFP in forebrain excitatory neurons, and APPswe ubiquitously (APPswe/PS1ΔE9flox/αCaMKIICre) (Fig. 1).
APPswe mice do not display plaque pathology until 18–20 months of age. However, Veeraraghavalu and colleagues (2014) show that global incorporation of mutated PS1 with APPswe (APPswe/PS1ΔE9flox) increases Aβ42 production and exacerbates plaque deposition as early as 5–7 months of age. When mutated PS1 was restricted to non-neuronal cells (APPswe/PS1ΔE9flox/αCaMKIICre), cortical and hippocampal Aβ load was negligible and indistinguishable from that in age-matched APPswe mice. In line with this finding, Aβ load was significantly reduced in 8–9-month-old APPswe/PS1ΔE9flox/αCaMKIICre mice relative to age-matched APPswe/PS1ΔE9flox mice. However, by age 10–12 months, APPswe/PS1ΔE9flox/αCaMKIICre mice displayed robust increases in soluble and insoluble AB42 as well as plaque burden relative to age-matched APPswe mice. Therefore, 10–12-month-old APPswe/PS1ΔE9flox/αCaMKIICre mice were largely indistinguishable from APPswe/PS1ΔE9flox mice in terms of forebrain Aβ load, despite the difference in the sources of Aβ42 production (i.e., non-neuronal versus neuronal).
In addition to the in vivo findings, Veeraraghavalu and colleagues (2014) found that astrocytes and microglia isolated from young APPswe/PS1ΔE9flox mice produced and secreted Aβ in a γ-secretase-dependent manner. Although it appears that astrocytes, microglia, and neural progenitor cells harvested from APPswe/PS1ΔE9flox mice express notable levels of human APP and Aβ40/42, how these levels compare to those produced by excitatory forebrain neurons remains unknown. A side-by-side comparison of the levels of neuron- and glia-derived Aβ species (monomer, dimer, trimer, oligomer, etc.) and other APP fragments would be beneficial in enhancing understanding of APP processing in non-neuronal cells.
Previous reports have shown that murine lines expressing the APP Swedish mutation alone (Tg2576) exhibit progressive impairments in spatial reference memory beginning at 9 months of age, and co-expression of mutated PSEN1 with APPswe expedites these impairments by ∼3 months. It would be interesting to ascertain whether excision of excitatory–neuronal PS1ΔE9, which Veeraraghavalu and colleagues (2014) suggest accounts for ∼80% of brain PS1ΔE9 at 7 months of age, is able to delay the appearance or slow the progression of behavioral impairments in APPswe/PS1ΔE9flox/αCaMKIICre mice relative to age-matched APPswe/PS1ΔE9flox mice. Such data would support the development of specialized presenilin-1 inhibitors as potential therapeutic targets in AD. It is also possible that non-neuronal-derived Aβ species possess enhanced nucleation capacity, as amyloid burden rapidly increases between 8–9 and 10–12 months in APPswe/PS1ΔE9flox/αCaMKIICre mice. If that were the case, perhaps APPswe/PS1ΔE9flox/αCaMKIICre mice would show exacerbated cognitive impairments relative to APPswe/PS1ΔE9flox mice between the ages of 8 and 15 months. Such a finding could potentially implicate non-neuronal cells as the primary source of neurotoxic Aβ in the aged brain. Conversely, aggregation of glial-derived soluble Aβ oligomers and fibrils into plaques may provide a beneficial mechanism for sequestering more soluble forms of Aβ from the neuropil. Insertion of the APPswe and PS1ΔE9 transgenes under the control of microglia- or astrocyte-specific promotors from birth would allow analysis of Aβ load independent of a contribution from excitatory neurons, ultimately leading to a greater understanding of AD gliobiology. Since interneuron-derived Aβ may constitute a portion of the total Aβ load seen in their model, interneuron-specific promotion of the transgenes may also account for Aβ production independent of excitatory neurons. Regardless, such ideas highlight several areas for future research based on the experiments of Veeraraghavalu et al. (2014).
Three important conclusions can be drawn from the data reported by Veeraraghavalu and colleagues (2014). First, excitatory neurons contribute greatly to early amyloid seeding in APPswe/PS1ΔE9flox mice, reinforcing the notion of activity-dependent Aβ production. Second, non-neuronal cells possess the cellular machinery to synthesize and cleave APP and secrete Aβ40/42 in a manner similar to neurons, supporting the need for a greater understanding of glial function in AD. Third, cells other than excitatory forebrain neurons contribute greatly to hippocampal and cortical Aβ load in aged APPswe/PS1ΔE9flox/αCaMKIICre mice, likely by constitutively synthesizing de novo Aβ peptides, which coalesce onto existing seeds or generate new seeds. In light of these findings, it is important to investigate the role of non-neuronal cells and interneurons in the production of small Aβ peptides in both sporadic and familial AD.
Footnotes
Editor's Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
G.T.C. and E.W.B. are supported by a National Institutes on Aging Predoctoral training grant (5T32 AG000269, to Elliott J. Mufson). We thank Drs. Kalipada Pahan, Daniel A. Nicholson, Elliott J. Mufson, and Dustin R. Wakeman for their insightful discussions and helpful comments on this manuscript.
References
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J, Gabuzda DH, Matsudaira P, Yankner BA. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993;90:2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Meade RP, Davis LG. Immunocytochemical localization of the precursor protein for beta-amyloid in the rat central nervous system. Neuron. 1988;1:835–846. doi: 10.1016/0896-6273(88)90131-6. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP, Lansbury PT., Jr The C-terminus of the beta protein is critical in amyloidogenesis. Ann N Y Acad Sci. 1993;695:144–148. doi: 10.1111/j.1749-6632.1993.tb23043.x. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/S0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/S0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Veeraraghavalu K, Sisodia SS. Mutant presenilin 1 expression in excitatory neurons impairs enrichment-mediated phenotypes of adult hippocampal progenitor cells. Proc Natl Acad Sci U S A. 2013;110:9148–9153. doi: 10.1073/pnas.1302106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavalu K, Zhang C, Zhang X, Tanzi RE, Sisodia SS. Age-dependent, non-cell-autonomous deposition of amyloid from synthesis of beta-amyloid by cells other than excitatory neurons. J Neurosci. 2014;34:3668–3673. doi: 10.1523/JNEUROSCI.5079-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]