Abstract
For learning to occur through trial and error, the nervous system must effectively detect and encode performance errors. To examine this process, we designed a set of oculomotor learning tasks with more than one visual object providing potential error cues, as would occur in a natural visual scene. A task-relevant visual target and a task-irrelevant visual background both influenced vestibulo-ocular reflex learning in rhesus monkeys. Thus, motor learning does not identify a single error cue based on behavioral relevance, but can be simultaneously influenced by more than one cue. Moreover, the relative weighting of the different cues could vary. If the speed of the visual target's motion on the retina was low (≪1°/s), background motion dominated learning, but if target speed was high, the effects of the background were suppressed. The target and background motion had similar, nonlinear effects on the putative neural instructive signals carried by cerebellar climbing fibers, but with a stronger influence of the background on the climbing fibers than on learning. In contrast, putative neural instructive signals carried by the simple spikes of Purkinje cells were influenced solely by the motion of the visual target. Because they are influenced by different cues during training, joint control of learning by the climbing fibers and Purkinje cells may expand the learning capacity of the cerebellar circuit.
Keywords: cerebellum, climbing fiber, error signal, eye movement, motor learning, Purkinje cell
Introduction
Much learning occurs through trial and error. Performance errors on one trial guide the modification of the neural circuitry controlling a behavior, leading to improved accuracy on subsequent trials (Lee and Schmidt, 2005; Medina and Lisberger, 2008; Shadmehr et al., 2010). This form of learning relies on the ability of the nervous system to identify and encode the relevant errors amid an ongoing stream of sensory and motor signals.
We studied the encoding of performance errors during oculomotor learning. The function of smooth eye movements is to stabilize images on the retina, hence image motion on the retina, or “retinal slip,” can indicate a performance error, and the presence of retinal slip during eye movements can drive oculomotor learning. In the laboratory, oculomotor learning is usually studied using a single moving visual stimulus to create the retinal slip that drives learning. In a natural environment, however, there are typically multiple visual objects, whose motion on the retina can differ. For example, when a subject turns her head, images of objects at different distances move at different speeds and directions on the retina, depending on their position relative to the point of visual fixation. Moreover, primates can track a small visual target (T), which can create motion of earth-stationary objects on the retina. Thus, to understand learning in the real world, one must understand the encoding of multiple potential error cues and their effects on learning. Therefore, we studied vestibulo-ocular reflex (VOR) learning in the presence of more than one visual cue.
The VOR stabilizes images on the retina during head movements by generating compensatory smooth eye movements in the direction opposite that of head motion. The VOR presumably evolved to stabilize large-field, rather than foveal, images, because it is a phylogenetically primitive oculomotor behavior that functions in species without a fovea. Accordingly, VOR learning can be induced by pairing passive head motion with coherent, large-field image motion (Ito et al., 1974; Miles and Fuller, 1974; Gonshor and Jones, 1976; Robinson, 1976; Watanabe, 1984). In primates, however, the oculomotor system has evolved the ability to track a small object of interest, using smooth pursuit eye movements. In the natural world, it would rarely be possible to stabilize images of both a small T and large-field background (BG) simultaneously. Therefore, we evaluated whether VOR learning in primates functions to improve the stabilization of a T or BG. We assessed the influence of T and BG motion on the changes in the VOR induced by training. We also analyzed the encoding of T and BG motion by cerebellar climbing fibers and Purkinje cells, which are thought to carry error signals that induce learning (Marr, 1969; Albus, 1971; Ito et al., 1977; Miles and Lisberger, 1981; Ito and Kano, 1982; Ke et al., 2009; Nguyen-Vu et al., 2013; Kimpo et al., 2014).
Materials and Methods
General procedures.
Experiments were conducted on two male rhesus monkeys. The monkeys were trained to track a T to obtain liquid reinforcement. Surgery was performed to implant orthopedic plates for restraining the head, a coil of wire in one eye for measuring eye position (Robinson, 1963; CNC Engineering), and a recording cylinder, which was aimed at the cerebellar flocculus and ventral paraflocculus, using stereotaxic coordinates. During experiments, the head of the monkey was restrained by securing the implanted head holder to a primate chair. Vestibular stimuli were delivered using a servo-controlled turntable (Ideal Aerosmith) to rotate the animal about an earth-vertical axis. To induce learning, a vestibular stimulus was paired with motion of two visual stimuli: a small T, which the animal was rewarded for tracking, and a large visual BG. The T subtended 0.5° of visual angle. The BG was a 20 × 30° grid of 1.5 × 1.5° black and white squares. The T and BG visual stimuli were projected onto the back of a tangent screen 114 cm in front of the eyes, and could be moved independently using mirror galvanometers. All surgical and behavioral procedures conformed to guidelines established by the U.S. Department of Health and Human Services (National Institutes of Health) Guide for the Care and Use of Laboratory Animals, as approved by Stanford University. A subset of these data was published previously (Ke et al., 2009).
Behavioral experiments.
VOR learning was induced by presenting combined visual-vestibular stimuli for 2 h (Monkey L) or 1 h (Monkey E). The longer training period was used in Monkey L because he consistently tracked the T during the full 2 h of training, whereas Monkey E's tracking of the T fell off during the second hour of training. VOR performance was tested before and after training by delivering the vestibular stimulus (i.e., rotating the monkey) in total darkness to eliminate any visual influence on eye movements. The vestibular stimulus used to measure the VOR and induce learning was always 0.5 Hz sinusoidal head rotation about an earth-vertical axis, with peak speed of 10°/s.
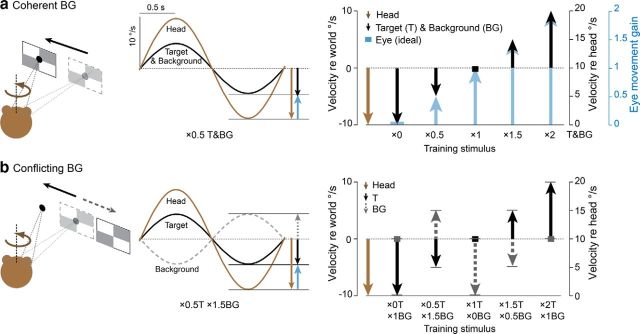
To induce learning, the vestibular stimulus was paired with different combinations of T and BG motion. The visual-vestibular training stimuli are described by a notation indicating the eye-movement gain (relative to head movement) required to stabilize the image of the T and BG on the retina. When the T moved exactly with the head, the eye velocity (relative to the head) and eye-movement gain required to stabilize the image of the T on the retina were zero, hence the training stimulus is described as “×0T” (Fig. 1a, ×0). When the T moved at the same speed as the head but 180° out of phase with the head, so that the eye movement required to stabilize the T on the retina had a peak speed of 20°/s, or 2× the speed of the head, the stimulus is described as “×2T” (Fig. 1a, ×2). During the ×0.5T training stimulus, the T moved in the same direction as the head, but at half the speed, so that the eye-movement speed required to stabilize the T was 5°/s, or 0.5× the speed of the head (Fig. 1a, ×0.5). During the ×1.5T training stimulus, the T moved in the opposite direction as the head, and at half the speed, so that the eye-movement speed required to stabilize the T was 15°/s, or 1.5× the speed of the head (Fig. 1a, ×1.5). During training stimuli with ×1T, the T was earth-stationary (Fig. 1a). Similar terminology was used to describe the motion of the BG stimulus during training (Fig. 1b, gray).
Figure 1.
Training stimuli. a, Coherent training stimuli. Head rotation (brown) was paired with motion of T and BG, and the T and BG moved exactly together (black trace). T and BG varied across the five training stimuli (black), and could be in the same direction (downward vectors) or opposite direction (upward vectors) from head motion. The motion of the T and BG determined the ideal eye-movement response (blue vectors) required to stabilize the images on the retina. Peak velocity of head, T, BG, and the ideal eye movement are indicated by the head of the vector in world coordinates (right, vertical axis on left) and relative to head motion (right, black vertical axis on right). The gain of the ideal eye movement (which equals the ratio of eye speed, relative to the head, to the head speed, relative to the world) is also indicated (right, blue vertical axis). b, Conflicting training stimuli. Head rotation was paired with motion of a T and BG that moved independently (see text for details). Black solid traces and vectors, motion of the T; gray dashed traces and vectors, motion of the BG. For all five conflicting stimuli, peak speed of the BG relative to the T was 10°/s (distance between heads of the gray and black vectors).
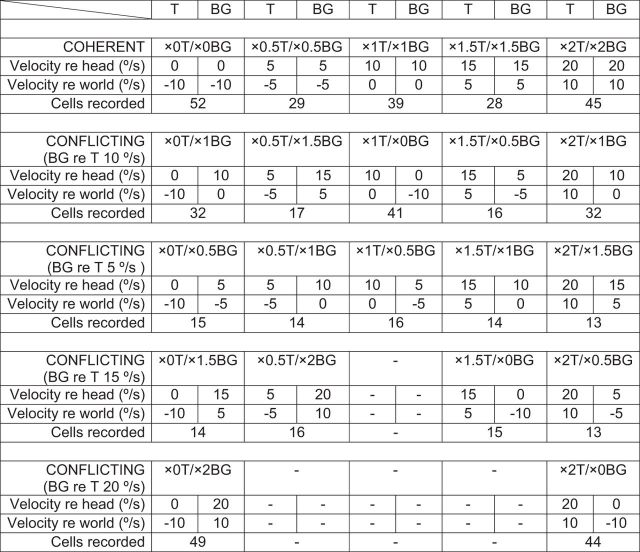
We used five pairs of visual-vestibular training stimuli (Table 1, top two rows). This set included five coherent training stimuli, in which the visual BG moved exactly with the T: ×0T/×0BG, ×0.5T/×0.5BG, ×1T/×1BG, ×1.5T/×1.5BG, and ×2T/×2BG. The five corresponding conflicting training stimuli were ×0T/×1BG, ×0.5T/×1.5BG, ×1T/×0BG, ×1.5T/×0.5BG, and ×2T/×0BG stimuli, where the visual BG moved independently from the T, with peak speed relative to the T of 10°/s. In Figure 1, the motion of the head, T, and BG, and the fully compensatory eye-movement responses for the coherent and conflicting training stimuli are represented in both world-centered and head-centered coordinates. For example, during the ×1.5 stimulus, the peak speed of the T and BG were 5°/s relative to the world (distance to head of black vector from zero on the vertical axis on left), but 15°/s relative to the head (distance to head of black vector from head of brown vector, which is what defines zero on the vertical axis on the right).
Table 1.
Summary of visual-vestibular training stimuli
In addition, we recorded climbing fibers when the T moved with the head, ×0T, and the BG moved at one of several different speeds (×0T/×0.5BG, ×0T/×1BG, ×0T/×1.5BG, ×0T/×2BG) or when the T moved in the direction opposite to the head, ×2T, and the BG moved at one of several different speeds (×2T/×0.5BG, ×2T/×1BG, ×2T/×1.5BG, ×2T/×2BG) (Table 1, bottom). We tested the ability of a subset of these additional stimuli (×0T/×0.5BG, ×0T/×2BG, ×2T/×0.5BG, and ×2T/×1.5BG) to induce VOR learning.
Experiments to induce learning were separated by ≥24 h to allow the gain of the VOR to recover to its normal value before the next experiment. No specific training was delivered to drive the VOR gain back to normal, aside from exposure to the normal visual-vestibular environment of the home cage. In each monkey, there were ≥3 replications of the behavioral experiments for each training stimulus.
Electrophysiology.
Tungsten electrodes (FHC, Microprobe) were used to make extracellular recordings from Purkinje cells in the floccular complex of the cerebellum, comprising the cerebellar flocculus and ventral paraflocculus. The floccular complex was identified based on stereotaxic coordinates and electrophysiological landmarks, such as the number of cerebellar layers penetrated by the electrode, the presence of eye-movement-related responses, and location relative to other structures, such as the dorsal cochlear nuclei and vestibular nerves, which have their own characteristic electrophysiological signatures. Purkinje cells were identified by their simple spike waveforms, mean firing rate, coefficient of variation of interspike interval, location within the layers of the cerebellar cortex (as determined by the BG electrophysiological activity), and, in most cases, by the presence of a complex spike. Once a Purkinje cell was isolated, its sensitivity to eye velocity and head velocity were measured by recording its simple spike responses during (1) smooth pursuit eye movements evoked by horizontal motion of the T with a sinusoidal velocity profile at a frequency of 0.5 Hz and a peak velocity of ≥10°/s and (2) as the monkey cancelled his VOR by tracking a T that moved exactly with sinusoidal head rotation about an earth-vertical axis at 0.5 Hz and at a peak velocity of ≥20°/s. Our analysis focused on horizontal gaze-velocity Purkinje cells (HGVPs), which have been implicated in VOR learning (Lisberger and Fuchs, 1978; Miles et al., 1980; Lisberger et al., 1994). Purkinje cells were classified as HGVPs if they met the following criteria: (1) during horizontal smooth pursuit eye movements, simple spike firing rate was modulated by ≥0.3 spikes/s per degree/s, and there was a phase difference of <45° between peak firing rate and peak ipsiversive eye velocity; and (2) during cancellation of the VOR, simple spike firing rate was modulated by ≥0.3 spikes/s per degree/s and the phase difference between peak firing rate and peak ipsiversive head velocity was <45° (Lisberger and Fuchs, 1978; Raymond and Lisberger, 1998).
The complex spikes recorded in a Purkinje cell provide a measure of activity in its climbing fiber input, since spikes in a climbing fiber trigger complex spikes in its Purkinje cell targets in a one-to-one manner (Eccles et al., 1967). Therefore we refer to the complex spike activity of a Purkinje cell as a climbing fiber response. Complex spikes were well isolated in 52 of 62 HGVPs recorded. In the other 10 HGVPs, complex spikes were sometimes detected, but were not well enough isolated to include in the analysis.
To compare neural responses across training stimuli, we recorded from the same Purkinje cell during the presentation of as many different visual-vestibular training stimuli as possible within a single recording session. The median number of training stimuli tested on each cell was 12. Each training stimulus was presented for 60–90 s. Training stimuli with the same T motion, combined with different BG motion, were delivered in one block; and the order of blocks was pseudorandomized. Data files were only included in the analysis if eye position was within 2° of T position for 90% of the entire 60–90 s presentation of a training stimulus. In separate behavioral experiments, we tested the ability of each training stimulus, presented continuously for 1–2 h, to induce VOR learning.
Statistical analysis.
Data analysis was performed in Matlab and Excel. The position and velocity of the eye, head, and visual stimulus were sampled at 500 Hz/channel. Eye-velocity records were edited to remove the rapid deflections caused by saccades. The smooth eye velocity data were then analyzed by aligning stimulus cycles on head velocity, and averaging. Most averages contained ≥10 cycles. Analysis of the VOR in the dark was limited to cycles for which gaze position was within 15° of straight-ahead gaze. A sinusoidal function with a frequency of 0.5 Hz was fit to the average eye and head velocity traces in Matlab, following previous procedures (Ke et al., 2009; Guo and Raymond, 2010). The peak eye velocity and peak head velocity derived from the fitted sinusoidal functions were used to calculate the gain of the VOR, as the ratio of peak eye velocity to peak head velocity. To calculate the retinal slip of the T and the BG, we computed the difference between the eye velocity and the T or BG visual stimulus velocity, respectively, at each time point. Sinusoidal functions were fit to these difference traces, and the amplitude of the sinusoidal function was used as the estimate of peak retinal slip.
Purkinje cell simple spikes were detected using a hardware window discriminator (Bak Electronics). Complex spikes were discriminated using off-line spike sorting with time and amplitude windows or template-matching algorithms (Spike2, Cambridge Electronic Design). We confirmed each sorted complex spike by examining the raw traces. The simple spike data were analyzed by aligning the records on head velocity. The amplitude and phase of the simple spike responses were determined from the fundamental components provided by Fourier analysis of the averages.
Because firing rate of the climbing fibers was close to zero in some cells during visual stimulus motion in the “off” (ipsiversive) direction, the climbing fiber responses were often not well fit by a sinusoid. Therefore, a vector analysis was used to quantify the response of each individual climbing fiber to each training stimulus, following previous methods (Ke et al., 2009). The stimulus cycle was divided into 1000 equal bins, with each bin corresponding to a particular phase relative to head velocity. A vector was assigned to each bin, with a length equal to the average firing rate of the climbing fiber in that bin (see Fig. 3a, for illustration purposes, 16 rather than 1000 bins are shown). The vector sum was then used to summarize the climbing fiber response of each cell to each stimulus. The phase of the vector sum (corresponding to the direction of the vectors in Figs. 3a, 4a) was used as our measure of the phase of the climbing fiber response. The amplitude of the climbing fiber response was measured as one half the amplitude of the vector sum (to correspond approximately to the amplitude that would be extracted using a Fourier analysis, which reflects half the trough-to-peak amplitude).
Figure 3.
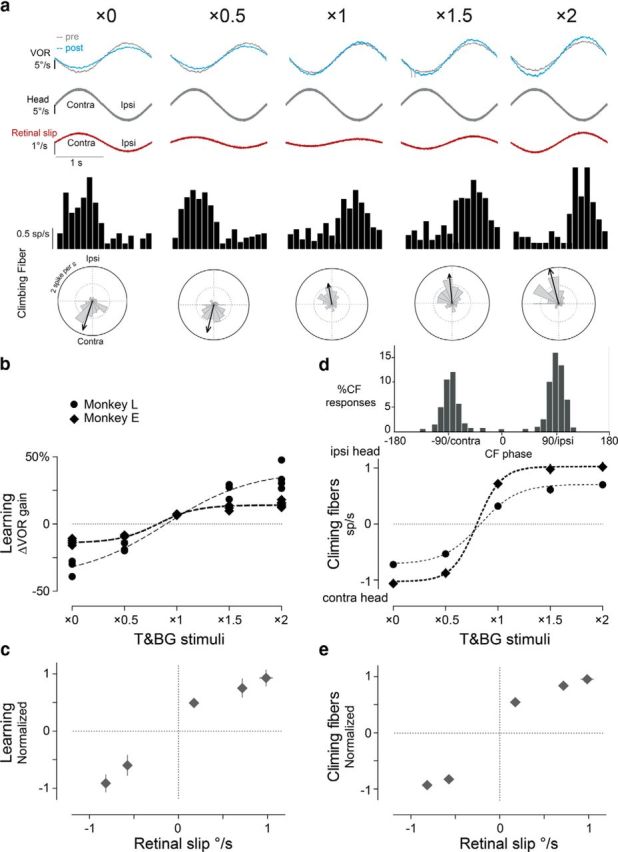
Learning and climbing fiber responses during the coherent training stimuli. a, Representative learning and climbing fiber responses. From top to bottom rows, Eye velocity traces showing VOR responses tested in the dark pretraining (gray) and post-training (blue); average head motion during training and VOR testing; retinal slip during training stimuli; spike frequency histograms and polar plots of the responses of a representative climbing fiber to the training stimuli. In the polar plots, the gray pie wedges represent firing rate at each phase of head motion, vectors quantify the amplitude and phase of firing rate modulation (see Materials and Methods). Clockwise, Phase lead. b, Learning was measured as the percentage change in VOR gain after training compared with pretraining. Each point represents the result from a single training session in Monkey L (circles) or Monkey E (diamonds). Dotted lines represent sigmoidal fits for each monkey. c, Normalized and averaged learning in two monkeys plotted against peak retinal slip of T and BG. d, Climbing fiber responses during training. Top, Histogram showing the distribution of phase of peak firing (relative to head velocity) for each climbing fiber to each training stimulus. Note clustering of values near 0 and 180°. Bottom, Average climbing fiber responses. Positive values indicate peak firing during ipsiversive head motion. Negative values indicate peak firing during contraversive head motion. Each point represents the mean response in the population of climbing fibers recorded in one monkey (in spikes/s). Dotted lines represent sigmoidal fits for each monkey. e, Normalized and averaged climbing fiber responses from two monkeys plotted against peak retinal slip of T and BG. In c–e, error bars signify SEM and are sometimes smaller than the symbol size.
Figure 4.
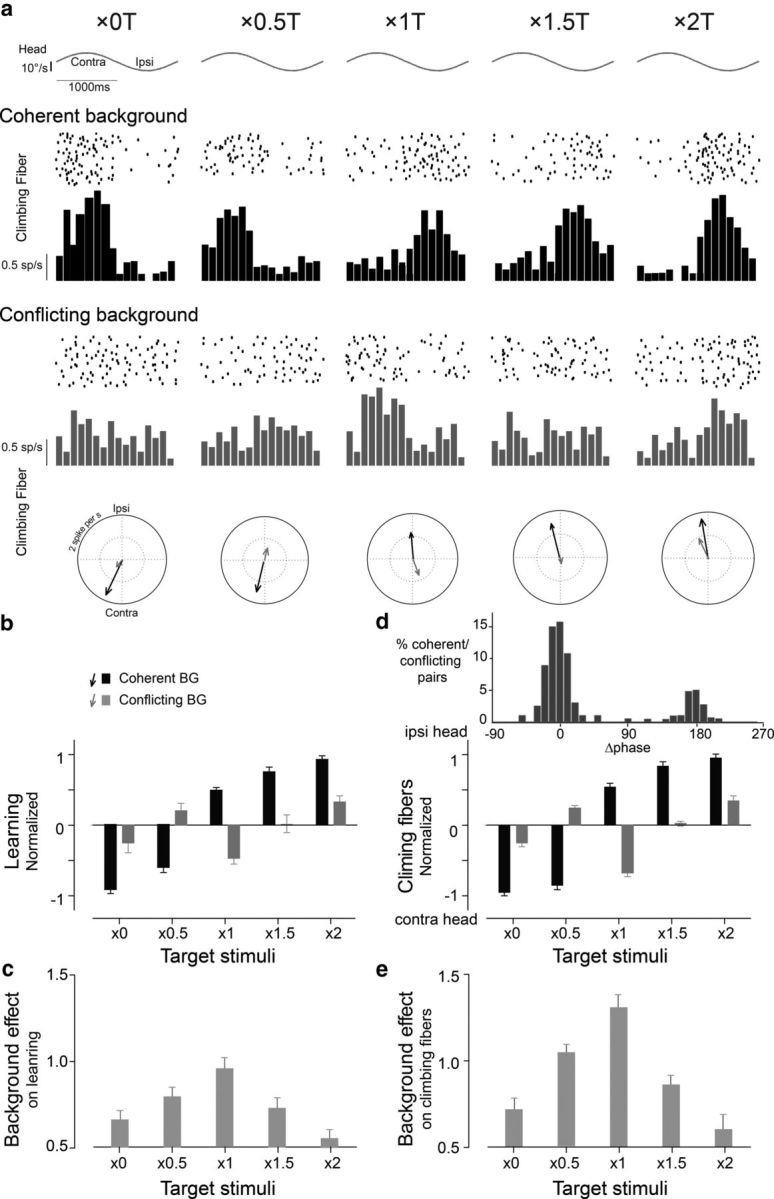
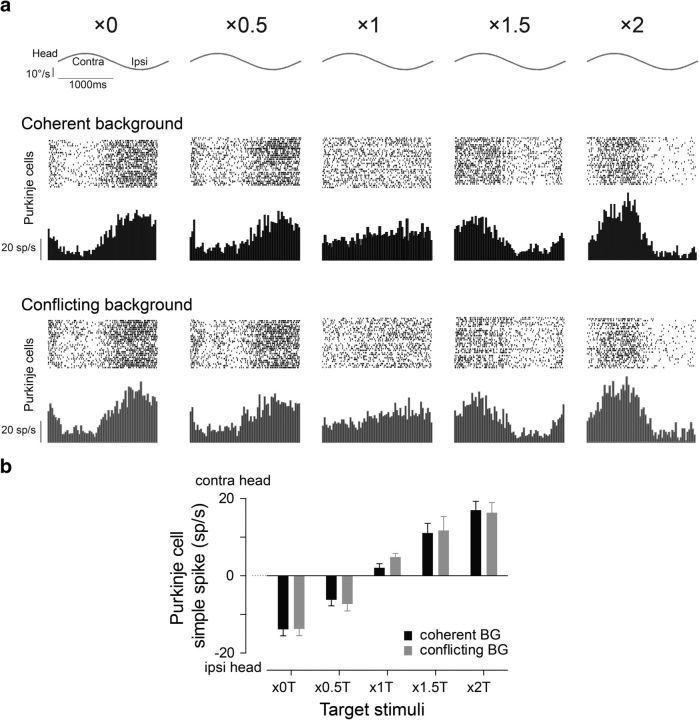
Learning and climbing fiber responses during the coherent (black) and conflicting (gray) training stimuli. a, Raster plots, spike frequency histograms, and polar plots showing the responses of a representative climbing fiber to the five pairs of coherent and conflicting training stimuli. b, Learned changes in VOR gain induced by training stimuli with different T motion (abscissa) and either coherent (black bars) or conflicting (gray bars) BG motion. c, The magnitude of the BG's effect on learning, calculated as the absolute difference between learning induced with coherent versus conflicting motion of the BG, for each T condition. d, Climbing fiber responses. Top, Histogram of differences in the phase of climbing fiber responses between pairs of coherent and conflicting training stimuli. Bottom, Average amplitude and direction of the climbing fiber responses to coherent training (black bars) and conflicting training stimuli (gray bars). Positive and negative values indicate peak firing during ipsiversive or contraversive head motion, respectively. e, The magnitude of the BG's effect on the climbing fibers, calculated as the absolute difference between the climbing fiber responses when there was coherent versus conflicting motion of the BG, for each T condition.
The data from each monkey were normalized to generate a combined dataset from the two monkeys. For each monkey, a sigmoidal curve was fit to the mean ΔVOR gain or mean climbing fiber responses measured for each of the different coherent training stimuli (Fig. 3b,d). The asymptotic values from the sigmoidal fits were used to normalize the individual data points from each monkey for each training stimulus, so that data from the two monkeys could be combined. Thus, the largest changes in VOR gain or climbing fiber responses in each monkey would have values close to 1.
A threshold of p < 0.05 was used to determine significance (ANOVA, post hoc Tukey's test, Pearson correlation analysis).
Results
We induced VOR learning in two rhesus monkeys by pairing a vestibular stimulus with two visual stimuli—a small visual T, which the animal tracked with his eyes for a reward, and a large visual BG that could move independently. In some cases, the T and BG moved together during training to provide consistent, unambiguous error cues (Fig. 1a). Five such coherent training stimuli were tested, denoted ×0, ×0.5, ×1, ×1.5, and ×2, where the notation ×a refers to a training stimulus that required a tracking eye-movement gain of a relative to the head movement to stabilize the visual stimuli on the retina (Fig. 1a; Table 1; see Materials and Methods for details). We further tested a set of five conflicting training stimuli, in which the T and BG did not move coherently during training, so that different eye movements would be required to stabilize the T versus the BG on the retina (Fig. 1b). The five conflicting training stimuli were as follows: ×0T/×1BG, ×0.5T/×1.5BG, ×1T/×0BG, ×1.5T/×0.5BG, and ×2T/×1BG, where the notation ×aT/×bBG indicates that a tracking eye-movement gain of a was required to stabilize the T on the retina, whereas a tracking eye-movement gain of b was required to stabilize the BG on the retina (Table 1). These conflicting stimuli were designed so that (1) the motion of the T on the retina should induce an increase in VOR gain, while the motion of the BG should induce a decrease in VOR gain, or vice versa, and (2) the difference between peak T and BG motion was always 10°/s (Fig. 1b, distance between black and gray vector tips).
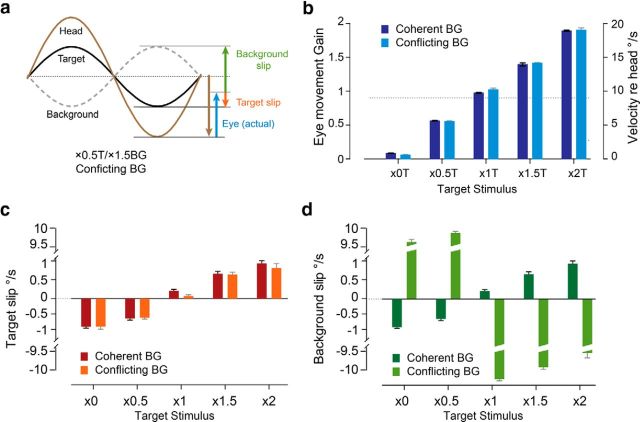
Monkeys were rewarded for and skilled at tracking the T. Therefore the actual eye velocity during training was close to that required to stabilize the T (compare actual eye movements in Fig. 2b, with ideal eye movements in Fig. 1a, blue bars). Moreover, eye velocity was not significantly influenced by the motion of the BG (Fig. 2b; coherent vs conflicting, p > 0.2, ANOVA). Therefore, the speed of T motion on the retina, or “T slip,” was not influenced by the BG (Fig. 2c; p > 0.2, ANOVA). Thus, by delivering the same T motion but different BG motion, we created pairs of coherent and conflicting training stimuli with similar vestibular input, eye movements (Fig. 2b), and T slip (Fig. 2c), but different BG slip on the retina (Fig. 2d; coherent vs conflicting, p < 0.0001, ANOVA). This design enabled us to assess the effect of BG motion on learning.
Figure 2.
Eye movements and retinal slip during training. a, Traces of head (brown), T (black), and BG (gray) motion during an example conflicting training stimulus (×0.5T/×1.5BG). Blue vector represents the peak velocity of the eye movement. Retinal slip is measured as the difference between movement of the visual stimuli and the eye. Red and green vectors represent the peak velocity of the retinal slip of T and BG, respectively. b, Eye movements (vertical axis on left, gain relative to head; vertical axis on right, peak velocity relative to head) measured during the coherent training stimuli (dark blue) were similar to those measured during the corresponding conflicting training stimuli (light blue) with the same T stimulus. During all training stimuli, the eye-movement gain was close to that required to stabilize the T on the retina (compare with Fig. 1a, blue arrows). Dotted line indicates the baseline gain of the VOR. c, T motion on the retina (slip) during coherent (dark red) and conflicting (light red) training stimuli. d, BG motion on the retina (slip) during coherent (dark green) and conflicting (light green) training stimuli. Broken scale bars are used to show both the subtle differences in the BG slip (or T slip) across different T stimuli as well as the large difference in BG slip for the conflicting versus coherent training stimuli.
Training with coherent T and BG motion
When the T and BG moved together during training, the direction of VOR learning was determined by the direction of motion of the visual stimuli relative to the head, as shown previously (Ito et al., 1974; Miles and Fuller, 1974; Gonshor and Jones, 1976; Robinson, 1976; Watanabe, 1984). The normal, baseline VOR gain, measured in darkness, was 0.87 ± 0.03 in Monkey L and 0.89 ± 0.04 in Monkey E. When a smaller eye-movement gain was required to track the visual stimuli, retinal slip was in the same direction as head motion (Fig. 3a, ×0, ×0.5, red traces; if the eye moves too much in the opposite direction from the head, image motion will be in the same direction as the head; Fig. 2a), and training reduced the VOR gain (Fig. 3a, blue traces; Fig. 3b; ×0, ×0.5 training stimuli). When a larger eye-movement gain was required to track the visual stimuli, retinal slip was in the opposite direction from head motion (Fig. 3a, ×1, ×1.5, ×2, red traces), and training increased the VOR gain (Fig. 3a, blue traces; Fig. 3b; ×1, ×1.5, ×2 training stimuli). The amount of learning varied with the speed of retinal slip during training (Fig. 3c).
The responses of climbing fibers during coherent training reflected the direction of the retinal slip, which is consistent with previous studies showing that the climbing fibers encode the error signals provided by retinal slip when oculomotor learning is induced using coherent, full-field image motion, or motion of a single visual stimulus against a dark BG (Simpson and Alley, 1974; Ghelarducci et al., 1975; Lisberger and Fuchs, 1978; Watanabe, 1984; Nagao, 1988; Stone and Lisberger, 1990). Climbing fiber activity increased during contraversive retinal slip (Waespe and Henn, 1981; Blanks and Precht, 1983; Graf et al., 1988; Kusunoki et al., 1990; Stone and Lisberger, 1990; Fushiki et al., 1994; Fig. 3a, spike frequency histograms), which coincided with contraversive head movement during the ×0 and ×0.5 training stimuli (Fig. 3a,d, negative values) and with ipsiversive head movement during the ×1, ×1.5, and ×2 training stimuli (Fig. 3a,d, positive values). The phases of the climbing fiber responses had a bimodal distribution, with phase values clustered near peak ipsiversive and peak contraversive head velocity (Fig. 3d, top). Therefore, a single scalar value was used to summarize the climbing fiber responses, with the absolute value indicating the amplitude of the firing rate modulation, and the sign indicating the phase (positive values to indicate peak firing near peak ipsiversive head velocity, and negative values to indicate peak firing near peak contraversive head velocity; Fig. 3d). The amplitude of the climbing fiber responses varied with the speed of retinal slip during the five coherent training stimuli tested (Fig. 3e), as also observed for learning (Fig. 3c).
The results from two monkeys were qualitatively similar, but differed quantitatively (Fig. 3b,d, diamonds and circles). On average, the climbing fiber responses were bigger in Monkey E than in Monkey L (Fig. 3d). However, the amount of learning was bigger in Monkey L than in Monkey E (Fig. 3b), at least in part because Monkey L reliably tracked the T for longer periods, making it possible to use longer training periods for the behavioral experiments (see Materials and Methods). Therefore, to compare the relative efficacy of the different coherent and conflicting training stimuli, we normalized both the behavioral and neural results for each monkey (see Materials and Methods for details) before combining the data from the two monkeys.
Conflicting T and BG motion: effects on learning
To determine how the small T and large visual BG each affect VOR learning, we compared the learned changes in the VOR induced by coherent and conflicting training stimuli (Fig. 4a–e). The same T stimuli induced different VOR learning when presented with conflicting (Fig. 4b, gray bars) versus consistent BG motion (Fig. 4b, black bars; p < 0.01, ANOVA; p < 0.001, post hoc Tukey's test). In some cases (×0 or ×2 T), the conflicting BG motion had a moderate effect on learning—it reduced the amount but did not change the direction of learning (Fig. 4b, gray vs black bars). However, in other cases, conflicting BG motion had a more dramatic effect—it eliminated learning (×1.5 T) or reversed the direction of learning (×0.5 or ×1 T; Fig. 4b, gray vs black bars). We evaluated which factor(s) determine the effect of conflicting BG motion on learning.
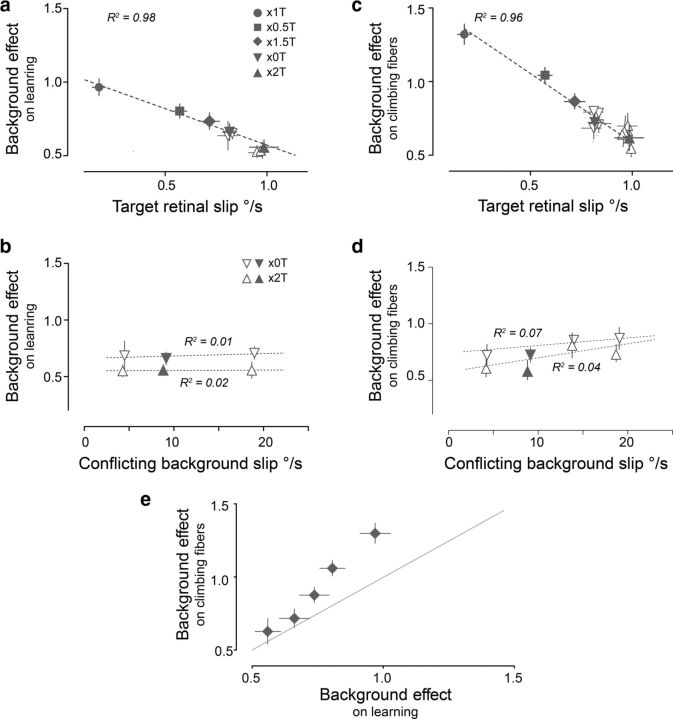
The effect of the BG on learning was quantified as the absolute difference between the learned changes induced when there was coherent versus conflicting BG motion for each T stimulus (Fig. 4c). The BG effect varied significantly across the five pairs of coherent and conflicting training stimuli (Fig. 4c; p < 0.001, ANOVA). These variations in BG effect were linearly correlated with the speed of T slip on the retina during training (Fig. 5a; R2 = 0.98, p < 0.001)—when T slip speed was the lowest (×1 T), the motion of the BG had the greatest effect on learning (Fig. 5a, circle), with progressively less effect of the BG for higher T slip speeds.
Figure 5.
a–d, Correlation between the magnitude of the conflicting BG's effects and retinal slip speed of the T (a, c) and BG (b, d). The effect of conflicting background motion on learning (a) and climbing fiber responses (c) was correlated with the peak speed of T slip on the retina, but did not vary with the peak speed of conflicting BG slip on the retina (b, d). Filled symbols represent data from the five pairs of coherent and conflicting training stimuli shown in Figure 3, with a peak BG speed of 10°/s relative to the T. Open symbols represent data from additional conflicting training stimuli with ×0 and ×2 T stimuli and peak BG speeds of 5–20°/s relative to the T. e, The effects of conflicting BG motion on learning plotted against the effects on climbing fiber responses. BG effects on learning and climbing fiber responses were the same as in Figure 4c and Figure 4e, respectively. Gray line: x = y.
Learning was relatively insensitive to the speed of the BG slip. The conflicting stimuli were designed to create similar BG motion on the retina (∼10°/s), yet there were small variations across the five conflicting training stimuli (from 9.1 to 9.9°/s; Fig. 2d, light green). To evaluate whether these small variations in the BG slip speed could account for its varying effects on learning (Fig. 4c), we designed additional stimuli to create varying conflicting BG motion (from 5 to 20°/s) with a given T, using either ×0 or ×2 T's (Fig. 5b, open symbols; see Materials and Methods for details). We found that the effect of the BG on learning was similar across the range of conflicting BG slip speeds tested (Fig. 5b; R2 < 0.05, p > 0.5). Thus, learning was fairly insensitive to the speed of the BG slip in the range from 5 to 20°/s, and therefore the substantial variations we observed in the amplitude of the BG's effect on learning across different training stimuli (Fig. 4c) could not be attributed to small variations in BG slip speed.
Together, the results indicate that the effect of a given BG stimulus does not simply sum with the effect of whatever T stimulus is present. Rather, the effect of the BG is diminished when T slip speed is high.
Conflicting T and BG motion: effects on climbing fiber responses
The effects of the conflicting BG motion on the responses of climbing fibers paralleled the effects of the BG on learning. The effect of the BG on the climbing fibers was quantified as the absolute difference between the climbing fiber responses during coherent versus conflicting motion of the BG, for each T condition. The putative error signals carried by the climbing fibers were significantly different when there was conflicting rather than coherent motion of the BG (Fig. 4a,d; p < 0.0001, ANOVA; p < 0.0001, post hoc Tukey's test). As observed for learning, the conflicting BG motion affected the climbing fiber signals to varying degrees across the five pairs of coherent and conflicting training stimuli (Fig. 4d,e). Conflicting BG motion could either reduce or reverse the phase of the climbing fiber responses, compared with the responses during coherent stimuli. Notably, changes in the phase of peak climbing fiber activity were bimodal—having either no effect, or flipping it by ∼180°, further supporting the use of a single, scalar value to summarize the climbing fiber responses (Fig. 4d, top).
The amplitude of the BG's effects on the climbing fibers was inversely correlated with the speed of T slip on the retina (Fig. 5c; R2 = 0.96, p < 0.001), as was also observed for its effects on learning. However, for a given T slip, the BG had a greater effect on the climbing fiber responses (Fig. 5c) than on learning (Fig. 5a,e), indicating that the climbing fiber responses were more sensitive to BG motion. Although sensitive to the direction of BG motion, climbing fiber responses were relatively insensitive to the speed of BG slip for peak speeds between 5 and 20°/s (Fig. 5d; R2 < 0.1, p > 0.3).
T and BG motion: effects on Purkinje cell simple spike responses
In addition to the climbing fiber responses, we measured the effects of BG motion on the Purkinje cell simple spike responses, which is another candidate neural instructive signal for cerebellum-dependent learning (Miles and Lisberger, 1981; Ke et al., 2009; Nguyen-Vu et al., 2013). As previously reported, Purkinje cell simple spike rate increased during ipsiversive head motion during training stimuli that induced a learned decrease in VOR gain (×0, ×0.5; Fig. 6a,b, black bars, negative values). In contrast, Purkinje cell simple spike rate increased during contraversive head motion during training stimuli that induced a learned increase in VOR gain (×1, ×1.5, ×2; Fig. 6a,b, black bars, positive values). The amplitude of the Purkinje cell simple spike responses was correlated with the T slip speed and the associated eye movements (compare Fig. 6b vs Fig. 2b,c; p < 0.001, Pearson correlation analysis). Conflicting BG motion had no significant effect on the simple spike responses of the Purkinje cells (Fig. 6b; coherent vs conflicting, ANOVA, p > 0.73). Thus, candidate instructive signals in the Purkinje cell simple spikes were influenced only by the motion of the T during training, and not by the motion of the BG visual stimulus. This contrasts with the candidate instructive signals in the climbing fibers, which were influenced by the motion of both the T and the BG.
Figure 6.
Purkinje cell simple spike responses during training stimuli. a, Raster plots and spike frequency histograms of neural responses of a representative Purkinje cell to the five pairs of coherent and conflicting training stimuli. b, Purkinje cell simple spike responses to coherent training (black bars) and conflicting training stimuli (gray bars). Positive values indicate peak firing during contraversive head motion, negative values indicate peak firing during ipsiversive head motion. Bars represent the averages from 62 HGVPs recorded in two monkeys. Error bars signify SEM.
Discussion
The VOR has been used extensively to study the mechanisms of cerebellum-dependent motor learning (du Lac et al., 1995; Raymond et al., 1996; Boyden et al., 2004; Schubert and Zee, 2010; Broussard et al., 2011). In the laboratory, coherent visual motion is usually used to study VOR learning. But in the natural world, image motion would be coherent on the retina only under rare circumstances, if ever. Hence the neural circuitry controlling VOR learning must extract an error signal from a visual scene composed of multiple visual objects. In our experiments with two visual objects, motor learning favored stabilization of a small T when there were large errors in T stabilization, but learning favored stabilization of the visual BG when errors in T stabilization were small. Here, we consider several factors that could determine the relative influence of the T and BG.
Previous studies have suggested that the behavioral relevance of a stimulus could gate its ability to influence climbing fiber responses. In animals making visually driven eye movements with the head stationary, motion of an irrelevant BG stimulus was reported to have little or no effect on climbing fiber responses in the floccular complex (Stone and Lisberger, 1990; Frens et al., 2001). Therefore, it was hypothesized that the decision to track a visual stimulus could gate the responses of the climbing fibers so that they would preferentially encode the motion of the visual object controlling the eye movements, with irrelevant information filtered out. Such a representation of the behaviorally relevant errors in eye-movement performance would seem to be an appropriate signal to guide learning. However, under all conditions we tested, the task-irrelevant BG motion had a substantial effect on the climbing fiber responses.
For all of the training stimuli, the T was more behaviorally relevant, in that it controlled the motor response (eye movements) and the delivery of rewards. In contrast, the BG had no significant effect on the ongoing eye-movement behavior (Fig. 2b). Despite the animals' decision to track the T, BG motion could reduce (Fig. 4a,b,d, ×0T, ×2T), eliminate (Fig. 4a,b,d, ×1.5T), or even dominate (Fig. 4a,b,d, ×0.5T, ×1T) the effects of the more behaviorally relevant T in driving climbing fiber responses and learning. Thus, the sensory cue with the highest task relevance does not necessarily control motor learning, nor do the climbing fibers simply encode the eye-movement performance error, as defined by the visual stimulus controlling the eye movements. Behavioral relevance may have some influence on the relative contribution of the T versus the BG, but it does not act as a binary gate. Rather, across a range of T and BG combinations, we found graded effects of the BG motion (Fig. 5a,c), which could not be explained by behavioral relevance.
Retinal slip speed is another factor that can influence climbing fiber responses. In such species as rabbit, cat, and rat, climbing fibers in the flocculus are tuned for low retinal slip speeds, and their responses fall off with speeds >1–2°/s (Simpson and Alley, 1974; Blanks and Precht, 1983; Kusunoki et al., 1990; Fushiki et al., 1994). Tuning for low speeds may explain the reduced sensitivity of climbing fibers to visual objects not controlling eye movements in nonprimate species (Frens et al., 2001), because those objects will tend to have higher retinal slip speeds, which are less effective for driving the climbing fibers. In monkeys, however, most climbing fibers exhibit a maximal response for retinal slip speeds of several degrees per second to >10°/s (Noda et al., 1987; Hoffmann and Distler, 1989). This sensitivity to high speeds may enable BG images to influence climbing fibers, and hence learning, in primates.
In our experiments, the effects of T and BG were not simply additive. Rather, T motion seems to suppress climbing fiber responses to BG motion in a manner that depends on the speed of T slip on the retina. Graded suppression of the BG's effects by the T could be directly related to T speed, or it could be mediated by other factors correlated with T speed, such as eye movements and attention. Previous studies have suggested that the visual signals carried by the climbing fibers might be gated by the presence of a tracking T at the fovea, the decision to track the T, or by the eye movement that results from that decision (Stone and Lisberger, 1990; Frens et al., 2001). Of these possibilities, our results are most consistent with the eye movements regulating the influence of the visual BG because the graded effects we observed are unlikely to result from a binary signal, such as the presence of a T or the decision to track it. Eye velocity was linearly correlated with T speed (Fig. 2b) and with Purkinje cell simple spike output (Fig. 6b, p < 0.0001). Purkinje cell output can influence the activity of climbing fibers (Bengtsson and Hesslow, 2006; Chaumont et al., 2013), and thus may account for the ability of T motion to gate the response of the climbing fibers to BG motion. Alternatively, attention could influence the relative effects of potential error cues provided by the two visual stimuli. If greater attention to the T is required to effectively track it at higher speeds, this could favor the neural representation of the T over the BG “distractor.” Currently, little is known about the effect of attention on climbing fiber responses.
Location on the retina could also influence visual responses in the climbing fibers. Visual receptive fields of climbing fibers in the flocculus are large (>10°; Simpson and Alley, 1974; Noda et al., 1987; Graf et al., 1988; Fushiki et al., 1994). Nevertheless, we found that the motion of a small T could, in some cases, dominate the effects of a large BG visual stimulus on climbing fiber responses. This may reflect privileged access of foveal image motion to the climbing fibers. Visual receptive fields of floccular climbing fibers always include the fovea or central retinal area, and in primates, a small foveal T is sufficient to elicit robust climbing fiber signals and to support VOR learning (Lisberger and Fuchs, 1978; Stone and Lisberger, 1990; Scudder and Fuchs, 1992; Lisberger, 1994; Shelhamer et al., 1994). However, it seems unlikely that the foveal location of the T can fully account for its powerful effect on climbing fiber responses, since the BG also occupied part of the fovea during training. The fovea represents the central 2° of visual space in rhesus monkeys, but the T used in the present experiment only subtended 0.5° (Rolls and Cowey, 1970).
Thus, the putative neural error signals carried by climbing fibers and motor learning do not identify a single error, but can be simultaneously influenced by more than one error cue, which can be differently weighted by such factors as their physical properties (e.g., speed or location on the retina), cognitive factors (such as attention), or the ongoing eye movements. There are several parallels between these new findings for oculomotor learning and previous findings regarding oculomotor performance in the presence of multiple T's. In particular, smooth pursuit eye movement performance, like VOR learning, can be simultaneously influenced by more than one visual cue, with the relative weighting of cues affected by several factors, including attention, the rewards associated with the different T's, their luminance, and their location on the retina (Ferrera and Lisberger, 1995, 1997; Niu and Lisberger, 2011; Gardner and Lisberger, 2001; Joshua and Lisberger, 2012).
The effects of the T and BG on the climbing fiber responses during training largely paralleled their effects on learning, which is consistent with the idea that the climbing fibers provide instructive signals that guide learning. However, previous work has suggested that learning is not completely determined by climbing fiber signals, but may also be influenced by additional neural instructive signals, such as the simple spike output of Purkinje cells (Miles and Lisberger, 1981; Ke et al., 2009; Nguyen-Vu et al., 2013). Accordingly, we found that the BG's effect on learning was smaller than its effect on the climbing fibers (Fig. 5e), as one would expect if the Purkinje cells, which are not affected by the BG, also contribute to learning. Because these neurons respond to different cues during training, joint control of learning by the climbing fibers and Purkinje cells could expand the capacity of the cerebellar circuit to flexibly draw on different cues to guide learning.
Footnotes
This work was supported by the U.S. National Institutes of Health (Grants R01 DC004154 and R01 NS072406 to J.L.R. and F31 DC008078 to M.C.K.), a Howard Hughes Medical Institute Fellowship for Medical Students and the Stanford Medical Scientist Training Program to M.C.K., and a Stanford Graduate Fellowship to C.C.G. We thank R. Levine and R. Hemmati for technical assistance.
The authors declare no competing financial interests.
References
- Albus J. A theory of cerebellar function. Math Biosci. 1971;10:25–61. doi: 10.1016/0025-5564(71)90051-4. [DOI] [Google Scholar]
- Bengtsson F, Hesslow G. Cerebellar control of the inferior olive. Cerebellum. 2006;5:7–14. doi: 10.1080/14734220500462757. [DOI] [PubMed] [Google Scholar]
- Blanks RH, Precht W. Responses of units in the rat cerebellar flocculus during optokinetic and vestibular stimulation. Exp Brain Res. 1983;53:1–15. doi: 10.1007/BF00239393. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Titley HK, Antflick J, Hampson DR. Motor learning in the VOR: the cerebellar component. Exp Brain Res. 2011;210:451–463. doi: 10.1007/s00221-011-2589-z. [DOI] [PubMed] [Google Scholar]
- Chaumont J, Guyon N, Valera AM, Dugué GP, Popa D, Marcaggi P, Gautheron V, Reibel-Foisset S, Dieudonné S, Stephan A, Barrot M, Cassel JC, Dupont JL, Doussau F, Poulain B, Selimi F, Léna C, Isope P. Clusters of cerebellar Purkinje cells control their afferent climbing fiber discharge. Proc Natl Acad Sci U S A. 2013;110:16223–16228. doi: 10.1073/pnas.1302310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Lac S, Raymond JL, Sejnowski TJ, Lisberger SG. Learning and memory in the vestibulo-ocular reflex. Annu Rev Neurosci. 1995;18:409–441. doi: 10.1146/annurev.ne.18.030195.002205. [DOI] [PubMed] [Google Scholar]
- Eccles SJC, Ito M, Szentagothai J. The cerebellum as a neuronal machine. New York: Springer; 1967. [Google Scholar]
- Ferrera V, Lisberger S. The effect of a moving distractor on the initiation of smooth-pursuit eye movements. Vis Neurosci. 1997;14:323–338. doi: 10.1017/s0952523800011457. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Lisberger SG. Attention and target selection for smooth pursuit eye movements. J Neurosci. 1995;15:7472–7484. doi: 10.1523/JNEUROSCI.15-11-07472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frens MA, Mathoera AL, van der Steen J. Floccular complex spike response to transparent retinal slip. Neuron. 2001;30:795–801. doi: 10.1016/S0896-6273(01)00321-X. [DOI] [PubMed] [Google Scholar]
- Fushiki H, Sato Y, Miura A, Kawasaki T. Climbing fiber responses of Purkinje cells to retinal image movement in cat cerebellar flocculus. J Neurophysiol. 1994;71:1336–1350. doi: 10.1152/jn.1994.71.4.1336. [DOI] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Linked target selection for saccadic and smooth pursuit eye movements. J Neurosci. 2001;21:2075–2084. doi: 10.1523/JNEUROSCI.21-06-02075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelarducci B, Ito M, Yagi N. Impulse discharges from flocculus Purkinje cells of alert rabbits during visual stimulation combined with horizontal head rotation. Brain Res. 1975;87:66–72. doi: 10.1016/0006-8993(75)90780-5. [DOI] [PubMed] [Google Scholar]
- Gonshor A, Jones GM. Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol. 1976;256:361–379. doi: 10.1113/jphysiol.1976.sp011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf W, Simpson JI, Leonard CS. Spatial organization of visual messages of the rabbit's cerebellar flocculus. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol. 1988;60:2091–2121. doi: 10.1152/jn.1988.60.6.2091. [DOI] [PubMed] [Google Scholar]
- Guo CC, Raymond JL. Motor learning reduces eye movement variability through reweighting of sensory inputs. J Neurosci. 2010;30:16241–16248. doi: 10.1523/JNEUROSCI.3569-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann KP, Distler C. Quantitative analysis of visual receptive fields of neurons in nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract in macaque monkey. J Neurophysiol. 1989;62:416–428. doi: 10.1152/jn.1989.62.2.416. [DOI] [PubMed] [Google Scholar]
- Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- Ito M, Shiida T, Yagi N, Yamamoto M. Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res. 1974;65:170–174. doi: 10.1016/0006-8993(74)90344-8. [DOI] [PubMed] [Google Scholar]
- Ito M, Nisimaru N, Yamamoto M. Specific patterns of neuronal connexions involved in the control of the rabbit's vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol. 1977;265:833–854. doi: 10.1113/jphysiol.1977.sp011747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Lisberger SG. Reward action in the initiation of smooth pursuit eye movements. J Neurosci. 2012;32:2856–2867. doi: 10.1523/JNEUROSCI.4676-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci. 2009;12:1171–1179. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpo RR, Rinaldi JM, Kim CK, Payne HL, Raymond JL. Gating of neural error signals during motor learning. Elife. 2014;3:e02076. doi: 10.7554/eLife.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunoki M, Kano M, Kano MS, Maekawa K. Nature of optokinetic response and zonal organization of climbing fiber afferents in the vestibulocerebellum of the pigmented rabbit. Exp Brain Res. 1990;80:225–237. doi: 10.1007/BF00228151. [DOI] [PubMed] [Google Scholar]
- Lee TD, Schmidt RA. Motor control and learning. 4th edition. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- Lisberger SG. Neural basis for motor learning in the vestibuloocular reflex of primates. III. Computational and behavioral analysis of the sites of learning. J Neurophysiol. 1994;72:974–998. doi: 10.1152/jn.1994.72.2.974. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978;41:733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Bronte-Stewart HM, Stone LS. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol. 1994;72:954–973. doi: 10.1152/jn.1994.72.2.954. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FA, Fuller JH. Adaptive plasticity in the vestibulo-ocular responses of the rhesus monkey. Brain Res. 1974;80:512–516. doi: 10.1016/0006-8993(74)91035-X. [DOI] [PubMed] [Google Scholar]
- Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- Miles FA, Fuller JH, Braitman DJ, Dow BM. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol. 1980;43:1437–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- Nagao S. Behavior of floccular Purkinje cells correlated with adaptation of horizontal optokinetic eye movement response in pigmented rabbits. Exp Brain Res. 1988;73:489–497. doi: 10.1007/BF00406606. [DOI] [PubMed] [Google Scholar]
- Nguyen-Vu TD, Kimpo RR, Rinaldi JM, Kohli A, Zeng H, Deisseroth K, Raymond JL. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci. 2013;16:1734–1736. doi: 10.1038/nn.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y-Q, Lisberger SG. Sensory versus motor loci for integration of multiple motion signals in smooth pursuit eye movements and human motion perception. J Neurophysiol. 2011;106:741–753. doi: 10.1152/jn.01025.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Warabi T, Ohno M. Response properties and visual receptive fields of climbing and mossy fibers terminating in the flocculus of the monkey. Exp Neurol. 1987;95:455–471. doi: 10.1016/0014-4886(87)90152-X. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci. 1998;18:9112–9129. doi: 10.1523/JNEUROSCI.18-21-09112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976;39:954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Cowey A. Topography of the retina and striate cortex and its relationship to visual acuity in rhesus monkeys and squirrel monkeys. Exp Brain Res. 1970;10:298–310. doi: 10.1007/BF00235053. [DOI] [PubMed] [Google Scholar]
- Schubert MC, Zee DS. Saccade and vestibular ocular motor adaptation. Restor Neurol Neurosci. 2010;28:9–18. doi: 10.3233/RNN-2010-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol. 1992;68:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Tiliket C, Roberts D, Kramer PD, Zee DS. Short-term vestibulo-ocular reflex adaptation in humans. Exp Brain Res. 1994;100:328–336. doi: 10.1007/BF00227202. [DOI] [PubMed] [Google Scholar]
- Simpson JI, Alley KE. Visual climbing fiber input to rabbit vestibulo-cerebellum: a source of direction-specific information. Brain Res. 1974;82:302–308. doi: 10.1016/0006-8993(74)90610-6. [DOI] [PubMed] [Google Scholar]
- Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. II. Complex spikes. J Neurophysiol. 1990;63:1262–1275. doi: 10.1152/jn.1990.63.5.1262. [DOI] [PubMed] [Google Scholar]
- Waespe W, Henn V. Visual-vestibular interaction in the flocculus of the alert monkey II. Purkinje cell activity. Exp Brain Res. 1981;43:349–360. doi: 10.1007/BF00238377. [DOI] [PubMed] [Google Scholar]
- Watanabe E. Neuronal events correlated with long-term adaptation of the horizontal vestibulo-ocular reflex in the primate flocculus. Brain Res. 1984;297:169–174. doi: 10.1016/0006-8993(84)90555-9. [DOI] [PubMed] [Google Scholar]