Abstract
Research has demonstrated the potential role of the brainstem in the pathobiology of autism. Previous studies have suggested reductions in brainstem volume and a relationship between this structure and sensory abnormalities. However, little is known regarding the developmental aspects of the brainstem across childhood and adolescence. The goal of this pilot study was to examine brainstem development via MRI volumetry using a longitudinal research design. Participants included 23 boys with autism and 23 matched controls (age range = 7–17 years), all without intellectual disability. Participants underwent structural MRI scans once at baseline and again at two-year follow-up. Brainstem volumetric measurements were performed using the BRAINS2 software package. There were no significant group differences in age, gender, handedness, and total brain volume; however, full-scale IQ was higher in controls. Autism and control groups showed different patterns of growth in brainstem volume. While whole brainstem volume remained stable in controls over the two-year period, the autism group showed increases with age reaching volumes comparable to controls by age 15 years. This increase of whole brainstem volume was primarily driven by bilateral increases in gray matter volume. Findings from this preliminary study are suggestive of developmental brainstem abnormalities in autism primarily involving gray matter structures. These findings are consistent with autism being conceptualized as a neurodevelopmental disorder with alterations in brain-growth trajectories. More longitudinal MRI studies are needed integrating longitudinal cognitive/behavioral data to confirm and elucidate the clinical significance of these atypical growth patterns.
Keywords: Autism, brainstem, development, gray matter, longitudinal, MRI
1. Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by impairments in reciprocal social interaction and verbal/non-verbal communication, and the presence of stereotyped behavior [1]. In addition to the core social-communication-behavioral symptoms that define ASD, there are a number of associated features widely ascribed to these disorders, including underreactivity and/or overreactivity to sensory stimuli and self-stimulation [2]. The prominence of these symptoms has fueled an early hypothesis suggesting that ASD is a disorder of impaired sensory modulation, resulting from developmental brainstem abnormalities [3]. However, only a limited number of studies have focused on this structure and its relationship to the pathophysiology of ASD.
Compared to the cerebrum, the brainstem is small in size; however, its development and neuroanatomy are quite complex [4]. Like the rest of the brain, the brainstem originates from the ectoderm; however, its structures mature early and precede the development of limbic and cortical structures. The brainstem consists of four main divisions: diencephalon, mesencephalon, pons, and medulla oblongata. Similar to the cerebrum, it contains both white and gray matter. The gray matter of the brainstem (i.e. neuronal cell bodies) is found in clumps and clusters throughout the entire structure to form the cranial nerve nuclei, the reticular formation, and pontine nuclei. The white matter consists of fiber tracts (i.e. axons of neurons) passing down from the cerebral cortex (important for voluntary motor function) and up from peripheral nerves and the spinal cord (where somatosensory pathways travel). Ultimately, it serves as the connection between the cerebral hemispheres with the medulla and the cerebellum and is responsible for many regulatory functions.
Brainstem abnormalities in ASD might cause disruptions in key neural networks leading to the expression of both core and associated symptoms. Some neurobiological models of emotion and behavior regulation adopt a vertical-integrative perspective [5, 6]. Effective regulation depends on the integration of a hierarchical system consisting of the brainstem, limbic system, and cerebral cortex. The brainstem serves as the foundation of this system via its role in regulation of basic vital functions, such as respiration, pulse, blood pressure, consciousness, sleep, etc. Recent research has culminated in a conceptual model for the development of behavior and emotion regulation that incorporates three integrated levels of processing: 1) brainstem-related physiological regulation of cyclic processes and sensory integration; 2) emotion and attention regulation capacities that draw on the integration of brainstem and limbic systems; and 3) higher-level outcomes that draw on the intactness of brainstem and limbic networks, including socio-emotional self-regulation, inhibitory control, and cognitive processing [7].
Over the past two decades, numerous cross-sectional studies have suggested the presence of brainstem abnormalities in ASD. With the advent of electrophysiological techniques for audiological and neurological assessment, several auditory response studies were reported in ASD suggesting brainstem involvement [2]. Moreover, structural magnetic resonance imaging (MRI) studies have examined the brainstem and reported various morphometric abnormalities. Early studies using area measurements from midsagittal images, reported reductions in brainstem size [8–10]; however, not all studies are in agreement [11–15]. Additionally, two cross-sectional volumetric MRI investigations observed no differences in the brainstem volume in ASD compared to controls [16, 17]. In contrast, a recent study of brainstem volumes reported reductions in whole brainstem volume and total brainstem gray matter in the group of mainly adult participants with autism [18]. While many studies have examined the brainstem for abnormalities in ASD, the findings remain both inconsistent and inconclusive.
There are many factors that may account for the inconsistent findings in these cross-sectional studies. First, there may be major differences in demographics such as age, gender, handedness, and ethnicity. While many groups report adequate group matching, the difference between studies from different groups can be substantial. Second, there is significant diagnostic heterogeneity across sites with some studies including only autism proper and others including the entire autism spectrum. Third, implementations of different neuroimaging methods create another layer of complexity. Sites vary in terms of scanning equipment, protocols and image processing/analysis techniques. Many of these issues can be eloquently addressed using a longitudinal research design.
To address these limitations, several longitudinal studies have recently been conducted over the last few years. These investigations have contributed to the description of the aberrant development of the brain in autism, reporting on measures of regional brain volumes [19], cortical thickness [20], surface area [21], and social brain modules [22]. However, no study to date has specifically examined the brainstem in a longitudinal design. In light of the mounting evidence implicating the brainstem in ASD pathophysiology, this pilot study was completed to examine brainstem development in children and adolescents with autism via MRI volumetry using a longitudinal research design.
2. Methods
2.1 Participants
The original sample consisted of 50 boys; however, four were excluded for issues related to follow-up and data quality. Therefore, quantitative volumetric analysis was performed on brain MRI scans obtained from 46 boys: 23 with ASD and 23 healthy controls recruited at the Western Psychiatric Institute and Clinic (Pittsburgh, PA). The study was confined to boys because the sample size was too small to accommodate for the structural variability associated with gender.
Subjects with ASD were referred to a research clinic from the community and met the following inclusion criteria: 1) diagnosis of ASD through expert clinical evaluation and two structured research diagnostic instruments, including the Autism Diagnostic Interview-Revised (ADI-R) [23] and the Autism Diagnostic Observation Schedule (ADOS, Module 3) [24], and 2) absence of other neurological disorders. Those with autistic disorder met both ADI-R and ADOS criteria for autism. Subjects with pervasive developmental disorder, not otherwise specified (PDD-NOS) had ADOS scores ranging from 7–10 while meeting ADI-R criteria for autism. Nine of the participants with ASD were taking psychotropic medications: most of them were either taking a psycho-stimulant or a selective serotonin reuptake inhibitor, with only one subject receiving an atypical antipsychotic.
Controls consisted of medically healthy individuals recruited from the community through advertisements in areas socioeconomically comparable to those of the families of origin of the participants with ASD. Control subjects had a full-scale IQ ≥ 70 and were screened by face-to-face interviews, questionnaires, telephone interviews, and observation during psychometric tests. Individuals with a family history of any neuropsychiatric disorder (such as ASD, learning disability, affective disorders, and schizophrenia) were not included. Potential subjects with a history of birth asphyxia, head injury, or a seizure disorder were also excluded. All control subjects had no present or lifetime history of psychiatric disorders and no learning disability as assessed by the Schedule for Affective Disorders and Schizophrenia for School-Age Children [25] and the Wide Range Achievement Test-Revised [26], respectively.
Evaluation of potential subjects also included obtaining a thorough medical history, as well as laboratory testing when indicated. The Wechsler Intelligence Scale for Children was administered to measure cognitive functioning [27]. The Hollingshead method was used to assess socioeconomic status (SES) of the family of origin of all participants [28]. After procedures were fully explained, all subjects or their legal guardians provided written informed consent. Verbal assent was obtained from all subjects. The University of Pittsburgh Institutional Review Board approved the methodology of the study.
2.2 Neuroimaging
Brain scans were obtained using the same acquisition protocol at baseline as well as at follow-up. The mean time difference between the two scans was two years. No difference in this interval period was observed between individuals with autism and controls. MRI scans were acquired using a 1.5-T General Electric Signa MRI scanner (GE, Milwaukee, WI, USA). Final images for each subject were generated by obtaining T1-, T2-, and proton density-weighted images from all participants. The T1-weighted spoiled gradient recalled acquisition in steady state sequence was acquired using the following parameters: slice thickness = 1.5 mm, slice number = 124, echo time = 5 ms, repetition time = 24 ms, flip angle = 40 degrees, number of excitations = 2, field of view = 260×260 mm2 and matrix = 256×192 mm2. Both proton density- and T2-weighted images were obtained with the following parameters: slice thickness = 5 mm, echo time = 96 ms for T2 and 36 ms for proton density, repetition time = 3000 ms, number of excitations = 1, field of view = 260×260 mm2 and matrix = 256×192 mm2 with an echo train length = 8. All images were obtained in the coronal plane. MRI data were identified by scan number alone to retain blindness of raters. All images were inspected by an experienced rater for motion and other artifacts. Images with major artifacts precluding complete data processing and analysis were discarded.
2.3 Processing and Analysis
Image processing was performed on a Silicon Graphics workstation (SGI, Mountain View, CA, USA) using the BRAINS2 software package [29]. Six brain-limiting points (anterior, posterior, superior, inferior, left, and right) were identified to normalize the image data to the standard Talairach stereotactic three-dimensional space [30] in which the anterior-posterior commissure line specifies the x-axis, a vertical line rising from the x-axis through the inter-hemispheric fissure specifies the y-axis, and a transverse orthogonal line with respect to x and y coordinates specifies the z-axis. After fitting the images to a standard three-dimensional space, the pixels representing gray matter, white matter, and cerebrospinal fluid were identified using a segmentation algorithm applied to T1-, T2-, and proton density-weighted image sequences as described elsewhere [31]. Raters were blinded and measurements were performed using the BRAINS2 masks as generated by a neural network and corrected by manual tracing (intraclass correlation coefficient > 0.9). Total brain volume (TBV) was defined as the cerebrum, cerebellum, and brainstem while excluding cerebrospinal fluid. The brainstem was defined as the infra-tentorial brain tissue volume superior to the foramen magnum and excluding the cerebellar volume [29, 32].
2.4 Analytic Plan
Mixed effects regression models estimated group differences in total brainstem growth. Separate models were computed for whole brainstem volume and left and right gray and white matter volumes. Diagnostic group (ASD vs. control) was a fixed-effects factor, baseline TBV was a time-invariant predictor, and age was a time-varying covariate in each model. Brainstem measurements at baseline and two-year follow-up were included as repeated measures. A significant age effect would specify brainstem growth with increasing age, regardless of diagnostic group. A significant diagnostic group effect would indicate differences in brainstem volume between individuals with ASD and controls, irrespective of age. A significant two-way interaction between age and diagnostic group would identify a unique pattern of brainstem growth in ASD. This interaction is a longitudinal comparison where each participant contributes two ages and two brainstem volumes to the analysis, equivalent to a time by diagnostic group interaction in a traditional repeated measures ANOVA model.
Mixed effects regression models are advantageous to repeated measures ANOVA in that they accommodate missing time points, explicitly evaluate the effects of age rather than the time of the measurement (baseline and follow-up), directly model the relationship between age and brain volumes, and utilize all available data. Analyses were computed with and without TBV adjustment, and where applicable, differing findings are presented. Analogous mixed effects regression models were used in recent longitudinal studies of the amygdala and corpus callosum in autism [22]. For each mixed effects regression model, F-tests for the main and interaction terms were converted to Cohen’s d to examine the magnitude of these effects. Conventions for interpretation of Cohen’s d were as follows: small (d = 0.20), medium (d = 0.50), and large (d = 0.80) [33]. The effect size for age gives the magnitude of growth in the brainstem regardless of diagnosis. The effect size for diagnostic group gives the magnitude of group differences in the brainstem regardless of age. The effect size for the interaction term of age and diagnostic group gives the magnitude of group differences in the slope of brainstem growth [34].
3. Results
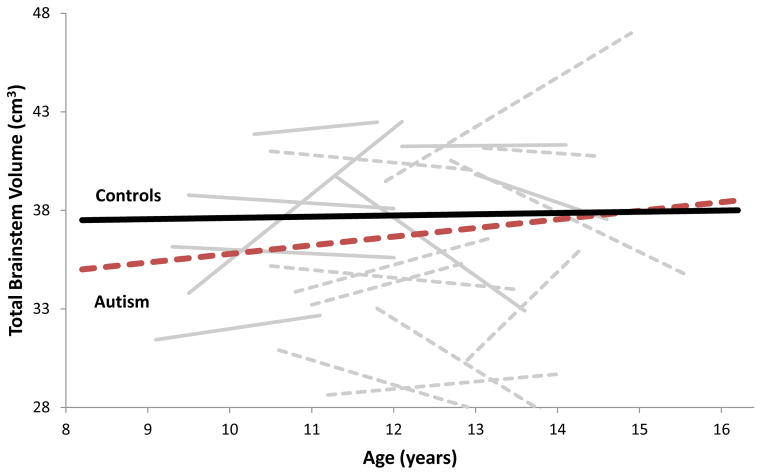
Sample characteristics are summarized in Table 1. There are no significant differences in age, SES, TBV, and handedness. While none of the study participants were intellectually disabled, full-scale IQ was significantly higher in the control group. Results for whole and subregion brainstem volumes are presented in Table 2. Figure 1 presents whole brainstem volume across ages 8–17 years for both ASD and control groups. ASD and control youth showed different patterns of growth in whole brainstem volumes. Whole brainstem volume remained relatively stable in healthy controls. In contrast, individuals with ASD showed increases with age [age X diagnosis interaction F(1,68) = 13.89, p < 0.001], reaching volumes comparable to controls by age 15 years. This effect was large (d = 0.90) and was even more pronounced in youth with ASD who had larger TBV [age X diagnosis X TBV interaction F(1,72) = 14.62, p < 0.001]. The increase in whole brainstem volume in ASD participants was primarily driven by whole brainstem gray matter volume (d = 0.85; p = 0.006) with only modest non-significant increases in whole brainstem white matter volume (d = 0.44, p = 0.167). Changes were similar across the left and right brainstem regions.
Table 1.
Subject characteristics.
| Autism | Controls | t-test | ||||
|---|---|---|---|---|---|---|
| N = 23 | N = 23 | df = 44 | ||||
| Mean | SD | Mean | SD | T | P | |
| Age | 10.6 | 1.4 | 10.5 | 1.3 | 0.091 | 0.928 |
| Full-scale IQ | 94.6 | 20.0 | 116.1 | 13.3 | −4.285 | < 0.001 |
| SES | 4.5 | 0.6 | 4.4 | 0.6 | 0.638 | 0.527 |
| TBV | 1349 | 119 | 1350 | 103 | −0.028 | 0.977 |
SES = socioeconomic status; TBV = total brain volume (cm3); age in years
Table 2.
Brainstem volumes (cm3) at baseline and 2-year follow-up.
| Baseline (T1) | 2-Year Follow-Up (T2) | T2-T1 | Mixed effects regression model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autism | Controls | Autism | Controls | Autism | Controls | Age | Diagnosis | Age X Diagnosis | |||||
| M | SE | M | SE | M | SE | M | SE | % Increase | % Decrease | Cohen’s d | Cohen’s d | Cohen’s d | |
| Whole Brainstem | 36.4 | 0.7 | 38.4 | 0.7 | 37.1 | 1.1 | 37.9 | 1.2 | 1.9 | 1.3 | .02 | .93* | .90* |
| Whole Gray Matter | 19.2 | 0.6 | 21.2 | 0.6 | 19.8 | 0.8 | 20.9 | 0.9 | 3.1 | 1.4 | .23 | .89^ | .85^ |
| Whole White Matter | 17.2 | 0.6 | 17.2 | 0.6 | 17.4 | 0.8 | 16.9 | 0.9 | 1.2 | 1.7 | .01 | .44 | .44 |
| Right Brainstem | 18.7 | 0.4 | 19.8 | 0.4 | 19.0 | 0.6 | 19.6 | 0.6 | 1.6 | 1.0 | .06 | .91^ | .91^ |
| Left Brainstem | 17.7 | 0.4 | 18.6 | 0.4 | 18.1 | 0.5 | 18.4 | 0.6 | 2.2 | 1.1 | .03 | 1.05* | 1.02* |
p<.05,
p<=.001. Positive effect sizes (d) indicate increasing volume with age (Age), lower volume in the autism group (Diagnosis), and larger increases in volume with age in the autism group relative to the controls (Age X Diagnosis).
Figure 1.
Age-related changes in total brainstem volume (cm3) in participants with autism (red and grey dotted lines) and healthy controls (black and grey solid lines).
4. Discussion
Characterizing abnormal brain growth trajectories in individuals with autism is an area of great interest and is best examined by studies implementing a longitudinal design. While several other brain regions have already been studied in this manner, no study to date has investigated whether the brainstem undergoes an abnormal growth trajectory in ASD. Therefore, the main goal of this pilot study was to assess whether there might be an abnormal pattern of brainstem growth in ASD. At baseline, brainstem volume in ASD youth is smaller compared to controls [18]. However, this study provides preliminary data supporting a gradual increase in volume in the ASD group, reaching volumes comparable to controls over a seven-year period. In contrast, brainstem volumes in control subjects remain relatively stable during the same time period. This apparent brainstem “growth” in ASD is largely attributed to increases in gray matter volume, providing an important clue to the underlying pathophysiology.
The gray matter of the brainstem represents neuronal cell bodies which are found in clumps and clusters throughout the structure to form the cranial nerve nuclei, reticular formation, and pontine nuclei. Gray matter can also represent the volume of synaptic density and connections. It follows that the apparent increase in gray volume may correspond to increases in total volume of neural cell bodies, increase in synaptic connections, and/or reduction in synaptic pruning. If there is indeed an increase in volume of neuronal cell bodies, what remains unclear is whether this is from an increase in the neural cell populations (i.e. proliferation), increase in size of existing cell bodies (i.e. hypertrophy), or some combination of these processes. The apparent stability of white matter volume does not support a process of increased myelination though increase in synaptic connectivity is consistent with the increase in gray matter volume. Finally, it also remains to be determined which brainstem regions or nuclei/neurons are involved in this volumetric increase. Additional studies looking at brainstem subregions are clearly needed to better characterize this abnormal pattern of growth.
Nevertheless, these preliminary findings are consistent with other studies reported in the research literature. As mentioned previously, our group has examined brainstem volume in ASD on a sample of adolescents and adults who had a mean age of 22 years and found no differences in whole brainstem volume between ASD and controls groups [16]. The lack of volumetric differences in a much older sample of ASD participants is congruent with the normalization of whole brainstem volume reported in this study. Remarkably, the findings of this study are consistent with reports of abnormal brain growth trajectories of youth with ASD reporting on measures of regional brain volumes [19], cortical thickness [20], surface area [21], amygdala and corpus callosum in autism [22]. Moreover, the most replicated finding is the increased brain size in early childhood followed by possible normalization later on in life [36–38]. However, it should be noted that abnormal brain growth has multiple dimensions. Brain volumes may increase, decrease, or stay the same depending on specific location and/or age range. For a particular age range, it is possible that two regions have different patterns of volumetric change. Overall, unusual growth patterns may represent a global or regional effect where different areas of the brain of individuals with autism grow at different stages of development dissimilar from the growth pattern seen in typically developing individuals.
Findings reported in this study must be interpreted in the context of several methodological limitations. The sample size is quite modest for a structural MRI study and consists of high-functioning participants with ASD. Small sample sizes amplify the effect of variability and may lead to erroneous results. Limiting the study to the higher-functioning ASD population limits its generalizability as the majority has some degree of intellectual disability. However, the implementation of longitudinal studies is very challenging with the need to minimize attrition and including subjects with a relatively narrow age range. Comprehensive longitudinal clinical characterization of participants is also lacking, precluding the examination of the relationships between behavioral/cognitive features and imaging measures. Moreover, this study did not separate the brainstem into individual components (i.e. midbrain, pons, and medulla) which limits specific regional localization of gray matter growth. Finally, no study-specific quality control was implemented over the two-year interval. Overtime, this may lead to systematic errors which may be reflected in 1% reduction in brainstem volume in controls of the current study. However, this would not be expected to bias the results given that the same effect applies equally to autism and control groups.
5. Conclusion
Although there are multiple cross-sectional studies examining brainstem size in ASD, results have been inconsistent and no studies to date specifically assessed brainstem development using a longitudinal design. While preliminary, findings from the present investigation are suggestive of abnormal brainstem growth patterns in ASD and are consistent with existing neuroimaging literature. The increase in gray matter, leading to brainstem volumes comparable to controls by age 15 years, supports previous observations of abnormal growth patterns also driven by gray matter. While these findings may represent small portion of a more global process, this does not preclude that the brainstem has an important role in the ultimate phenotypic expression of ASD given its potential role in emotion/behavior regulation and underreactivity and/or overreactivity to sensory stimuli. However, in light of the aforementioned observations, additional longitudinal studies are needed before any conclusions can be made. Multimodal, longitudinal morphometric and functional studies are warranted in larger samples of well-characterized individuals with ASD to examine the three sub-regions of the brainstem using well-validated analytic techniques.
Highlights.
Autism and control groups showed different patterns of brainstem growth
Whole brainstem volume remained stable in controls over the two-year period
Whole brainstem volume increased in the autism group over the two-year period
Normalization of brainstem volume was primarily driven by increases in gray matter
Autism is a neurodevelopmental disorder with alterations in brain-growth trajectories
Acknowledgments
This study was supported by an NIMH grant (MH 64027) to AH. Additional support to RJ received by: (1) The Hilibrand Autism Fellowship in Adolescence and Adulthood from the Hilibrand Foundation, (2) NARSAD, Brain & Behavior Research Fund Young Investigator Award (Foster Bam Investigator); and (3) ANA/Pfizer Fellowships in Clinical Practice from Pfizer’s Medical and Academic Partnership program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Roger J. Jou, Email: roger.jou@yale.edu.
Thomas W. Frazier, Email: fraziet2@ccf.org.
Matcheri S. Keshavan, Email: mkeshava@bidmc.harvard.edu.
Nancy J. Minshew, Email: minshewnj@upmc.edu.
Antonio Y. Hardan, Email: hardanay@stanford.edu.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision (DSM-IV-TR) [Google Scholar]
- 2.Klin A. Auditory brainstem responses in autism: brainstem dysfunction or peripheral hearing loss? J Autism Dev Disord. 1993;23:15–35. doi: 10.1007/BF01066416. [DOI] [PubMed] [Google Scholar]
- 3.Ornitz EM. The functional neuroanatomy of infantile autism. Int J Neurosci. 1983;19:85–124. doi: 10.3109/00207458309148648. [DOI] [PubMed] [Google Scholar]
- 4.Duvernoy HM. The human brainstem and cerebellum: surface, structure, vascularization, and three-dimensional anatomy with MRI. New York, NY: Springer-Verlag; 1995. [Google Scholar]
- 5.Panksepp J. Affective consciousness: core emotional feelings in animals and humans. Conscious Cogn. 2005;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Tucker DM, Luu P, Derryberry D. Love hurts: the evolution of empathic concern through the encephalization of nociceptive capacity. Dev Psychopathol. 2005;17:699–713. doi: 10.1017/S0954579405050339. [DOI] [PubMed] [Google Scholar]
- 7.Geva R, Feldman R. A neurobiological model for the effects of early brainstem functioning on the development of behavior and emotion regulation in infants: implications for prenatal and perinatal risk. J Child Psychol Psychiatry. 2008;49:1031–41. doi: 10.1111/j.1469-7610.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 8.Ciesielski KT, Harris RJ, Hart BL, Pabst HF. Cerebellar hypoplasia and frontal lobe cognitive deficits in disorders of early childhood. Neuropsychologia. 1997;35(5):643–55. doi: 10.1016/s0028-3932(96)00119-4. [DOI] [PubMed] [Google Scholar]
- 9.Gaffney GR, Kuperman S, Tsai LY, Minchin S. Morphological evidence for brainstem involvement in infantile autism. Biol Psychiatry. 1988;24:578–86. doi: 10.1016/0006-3223(88)90168-0. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Tayama M, Miyazaki M, Fujii E, Harada M, Miyoshi H, Tanouchi M, Kuroda Y. Developmental brain changes investigated with proton magnetic resonance spectroscopy. Dev Med Child Neurol. 1995;37:398–405. doi: 10.1111/j.1469-8749.1995.tb12023.x. [DOI] [PubMed] [Google Scholar]
- 11.Elia M, Ferri R, Musumeci SA, Panerai S, Bottitta M, Scuderi C. Clinical correlates of brain morphometric features of subjects with low-functioning autistic disorder. J Child Neurol. 2000;15:504–8. doi: 10.1177/088307380001500802. [DOI] [PubMed] [Google Scholar]
- 12.Garber HJ, Ritvo ER. Magnetic resonance imaging of the posterior fossa in autistic adults. Am J Psychiatry. 1992;149:245–7. doi: 10.1176/ajp.149.2.245. [DOI] [PubMed] [Google Scholar]
- 13.Hsu M, Yeung-Courchesne R, Courchesne E, Press GA. Absence of magnetic resonance imaging evidence of pontine abnormality in infantile autism. Arch Neurol. 1991;48:1160–3. doi: 10.1001/archneur.1991.00530230068024. [DOI] [PubMed] [Google Scholar]
- 14.Kleiman MD, Neff S, Rosman NP. The brain in infantile autism: are posterior fossa structures abnormal? Neurology. 1992;42:753–60. doi: 10.1212/wnl.42.4.753. [DOI] [PubMed] [Google Scholar]
- 15.Piven J, Nehme E, Simon J, Barta P, Pearlson G, Folstein SE. Magnetic resonance imaging in autism: measurement of the cerebellum, pons, and fourth ventricle. Biol Psychiatry. 1992;31:491–504. doi: 10.1016/0006-3223(92)90260-7. [DOI] [PubMed] [Google Scholar]
- 16.Hardan AY, Minshew NJ, Harenski K, Keshavan MS. Posterior fossa magnetic resonance imaging in autism. J Am Acad Child Adolesc Psychiatry. 2001;40:666–72. doi: 10.1097/00004583-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–92. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- 18.Jou RJ, Minshew NJ, Melhem NM, Keshavan MS, Hardan AY. Brainstem volumetric alterations in children with autism. Psychol Med. 2009;39:1347–54. doi: 10.1017/S0033291708004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Pierce K, Hagler D, Schork N, Lord C, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–27. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardan AY, Libove RA, Keshavan MS, Melhem NM, Minshew NJ. A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol Psychiatry. 2009;66:320–6. doi: 10.1016/j.biopsych.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, Vachet C, Piven J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68:467–76. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–16. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 24.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism Diagnostic Observation Schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Jastak S, Wilkinson JS. The Wide Range Achievement Test-Revised. Wilmington, DE: Jastak Associates; 1985. [Google Scholar]
- 27.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 28.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Department of Sociology; 1975. [Google Scholar]
- 29.Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 30.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: three-dimensional proportional system. New York, NY: Thieme Medical; 1988. [Google Scholar]
- 31.White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54:418–26. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 32.Pfaendner NH, Reuner G, Pietz J, Jost G, Rating D, Magnotta VA, Mohr A, Kress B, Sartor K, Hahnel S. MR imaging-based volumetry in patients with early-treated phenylketonuria. AJNR Am J Neuroradiol. 2005;26:1681–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1987. [Google Scholar]
- 34.Rosenthal R. Meta-analytic procedures for social research. Vol. 6. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- 35.Frazier TW, Keshavan MS, Minshew NJ, Hardan AY. A two-year longitudinal MRI study of the corpus callosum in autism. J Autism Dev Disord. 2012;42:2312–22. doi: 10.1007/s10803-012-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–83. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- 37.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–54. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 38.Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. J Child Neurol. 2001;16:421–24. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]