Abstract
Regulatory T cells (Tregs) play crucial roles in both fetal and tumor development. We recently showed that immunosurveillance by pre-existing CD44hiCD62Llow activated/memory Tregs (amTregs) specific for self-antigens protects emergent tumor cells in mice. This Treg response of a memory type is more rapid than and dominates the anti-tumor response of tumor-specific effector T cells. Here, we report striking similarities between the early Treg responses to embryo and tumor implantation. Tregs are (i) rapidly recruited to uterus-draining lymph nodes and activated in the first days after embryo implantation in both syngeneic and allogeneic matings; (ii) they express the markers of the amTreg subset; and (iii) are at least in part self-antigen-specific, as seen in tumor emergence. Unlike in the tumor emergence setting, however, for which pre-immunization against tumor antigens is sufficient for complete tumor eradication even in the presence of Tregs, Treg depletion is additionally required for high frequencies of fetus loss after pre-immunization against paternal tissue antigens. Thus, amTregs play a major role in protecting embryos in both naïve and pre-immune settings. This role and the ensuing therapeutic potential are further highlighted by showing that Treg stimulation, directly by low-dose interleukin-2 or indirectly by Fms-related tyrosine-kinase-3 ligand, lead to normal pregnancy rates in a spontaneous abortion-prone model.
Keywords: maternal-fetal tolerance, tolerance induction, conceptus, cancer, immunotherapy, IL-2, Flt3-L, cytokine
Introduction
Since Medawar’s classic 1953 paper (1), viviparity and especially placental pregnancy have been a riddle for immunologists. Survival of a semi-allogeneic conceptus appeared incompatible with self/non-self recognition being a fundamental function of the adaptive immune system. Systemic specific or non-specific immunosuppression cannot be invoked as an explanation of maternal-fetal tolerance since even during the first pregnancy the mother is perfectly capable of rejecting paternal strain allografts that are distant (2, 3) or even intra-uterine in close proximity to the implantation site (4). These data suggest there is active and specific protection that limits potentially detrimental immune responses while sparing useful ones (5–7).
The maternal immune system thus does sense the presence of the conceptus and mounts an active protective immune response. This task was attributed to suppressor T cells in the late 1970s (8, 9) and more recently to CD4+CD25+Foxp3+ Tregs (10). A Treg deficit severely hampers allopregnancy in nude mice reconstituted with Treg-depleted T lymphocytes (10) or in BALB/c mice treated with an anti-CD25 antibody (11). These and other data in mice (12–15) and humans (16, 17) emphasize the importance of Tregs in maintenance of successful allopregnancy.
This role of Tregs in protecting the foreign conceptus is reminiscent of the role of Tregs in protecting tumors, both the conceptus and tumors being proliferating cell masses that are partly self and partly non-self (i.e., possessing paternal or tumor antigens). We recently showed that the immune response at the first encounter between the immune system and emerging tumor cells dictates tumor outcome. Activated/memory Tregs (amTregs) (18) specific for self-antigen are recruited in the first days after tumor cell emergence in both transplanted and inducible murine cancer models (18). The response of amTregs precedes and preempts the slower response of naïve conventional T cells with effector potential (Teffs) that are specific for tumor neo-antigens, and rapidly establishes a dominant tolerant environment (18). We hypothesized that a similar phenomenon could be at work to protect the conceptus in the very early days of its implantation at the blastocyst stage. Thus, we studied the very early regulatory immune responses triggered by embryo implantation and examined the similarities of these responses to the one observed in the context of tumor cell implantation. Striking similarities are indeed observed, notably the early self-antigen-specific driven proliferation of activated memory Tregs, which have important therapeutic implications. We report that Tregs boosted by low-dose IL-2 or by Flt3-L can prevent fetal loss in a model of recurrent spontaneous abortion.
Materials and Methods
Mice
BALB/c, C57/Bl6, female CBA/J and male DBA/2J mice (6–8 weeks old at the initial time of experimentation) were from Charles River, Elevage JANVIER SAS or Jackson Laboratories. Thy1.1 BALB/c congenic mice, InsHA (18, 19), pgkHA (20) and SFE TCR-HA (21) mice (all in BALB/c background) were bred in our animal facility (ISO9001), in which the mice are kept under specific pathogen-free conditions (spf+). Flt3-L−/− mice (C57Bl/6 background) were a kind gift from Dr. Michel Nussenzweig and were tested in the immunocore of the Rockefeller University animal facility (New York, NY, USA). The rates of CBA X DBA fetal loss in the current study (15 to 45%) were similar to those in the majority of studies published in the field (22–30). All protocols and treatments were conducted either according to Rockefeller University Animal Care and Use Committee–approved protocols, or were approved by the Charles Darwin Animal Experimentation Ethics Committee of the CNRS. In order to compare experiments conducted in two different animal facilities in the US and France, we normalized the fetus rejection rates to the mean of the PBS-treated control groups, which was set at 100%.
Visual observation of mating and pregnancy outcome
The sighting of a vaginal plug was denoted as dpc 0.5. Female mice were sacrificed from dpi 8–12, and the numbers of viable fetuses (F) and resorbed fetuses (R) were recorded visually. Resorbed fetuses are smaller and usually hemorrhagic compared with viable ones. Resorption frequency was calculated as: Resorption% = R/(R+F)
Tumor experiments
5 × 105 B16 (melanoma, C57Bl/6 background), 4T1, 4T1-HA (breast carcinoma, BALB/c background), AB1, or AB1-HA (mesothelioma, BALB/c background) tumor cells were injected s.c. in the flank of the mice as described in (18) and (31). Tumor volume was determined by measuring perpendicular tumor diameters L and l using vernier calipers, calculated as (L × l2)/2, and expressed as mm3. The left inguinal LN was used as the dLN. The right inguinal and/or bilateral axillary LNs were used as ndLNs.
In vivo depletion of CD4+CD25+ T cells
One day before the mating or one to three days before tumor injections, female mice received 100 mg of anti-CD25 mAb (clone PC61; BD Biosciences) administered by i.p. injection. The anti-CD25 effect on Tregs lasted from 3 to 4 weeks at the dose used (11).
IL-2 and rhFLt-3 treatments
IL-2 treatment: Mice were injected i.p. daily with 25000 IU of recombinant human IL-2 (Proleukin, Novartis) for 10 consecutive days, starting 4 days before mating. rhFL treatment: Mice received 4 s.c. injections of 10 mg of Flt-3 Ligand (rhFL) (Amgen) 3 days apart starting 6 days before mating.
CFSE staining and adoptive transfer of cells
Experiments were performed essentially as described earlier (18, 21). Briefly, 5 – 10 × 106 Thy1.1+ CFSE-labeled unsorted cells from peripheral LNs and spleen from BALB/c mice (used in experiments in Figure 2), or from SFE TCR-HA transgenic mice (used in experiments in Figure 3A) were transferred i.v. on dpc 3. After adoptive transfer in wild-type hosts under our experimental conditions, donor Tregs represented 0.1% of splenocytes or LN cells (21). Therefore, 1–5 × 106 events were acquired for each analysis. The Treg division index based on CFSE decrease was calculated as: (division rate in dLNs – division rate in ndLNs)/division rate in ndLNs.
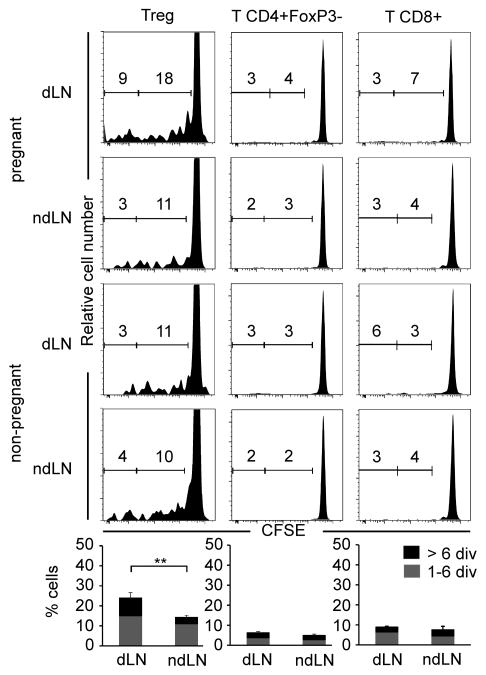
Figure 2. Treg division kinetics after embryo implantation.
Division profiles of CFSE-labeled Thy1.1+ donor cells in dLNs or ndLNs of embryo-bearing mice. Numbers in each panel represent the percentage of cells having undergone 1–6 divisions (right) or > 6 divisions (left) in B6-mated BALB/c at dpi 7 (upper panels) and unmanipulated female BALB/c mice (lower panels). Results are from one representative experiment out of 3 independent ones, with panels representing the results of pooled cells from 3 mice. Bar histogram statistics for Foxp3+ CD4+ Tregs in para-aortic vs. brachial LNs from pregnant mice are indicated below. ** P≤0.01.
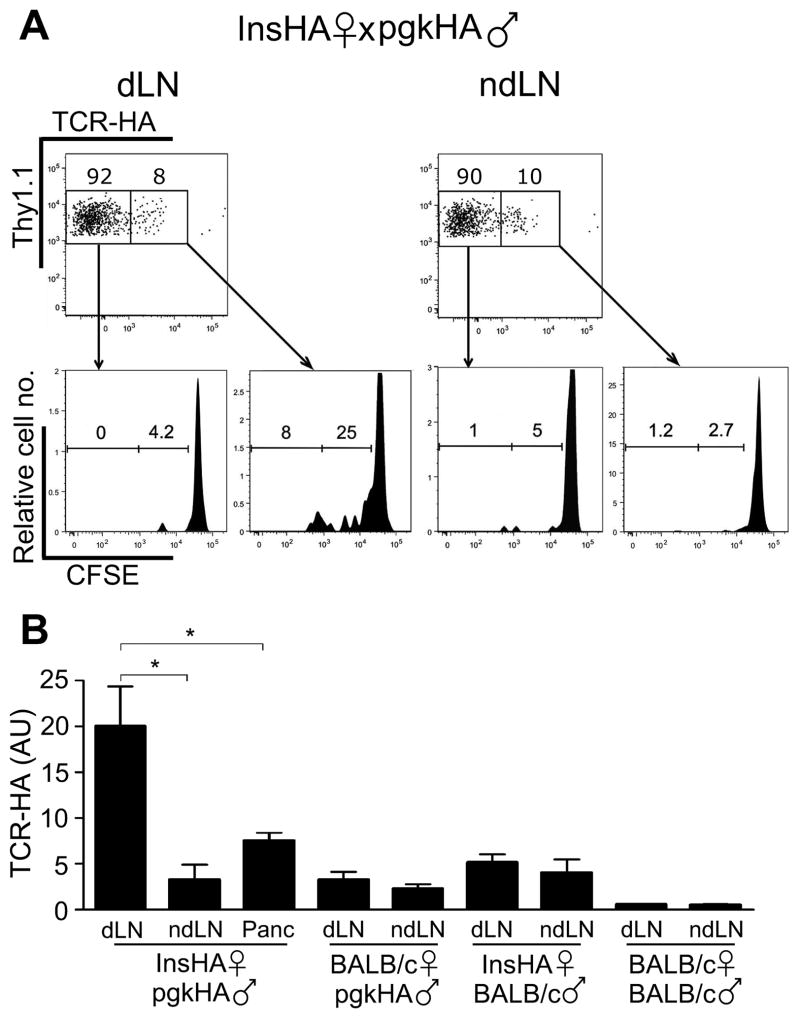
Figure 3. Treg proliferation after embryo implantation is self-antigen-driven.
(A) Division of TCR-HA+ and TCR-HA− donor Tregs in dLNs and ndLNs of pgkHA-mated InsHA females adoptively transferred with CFSE-labeled cells from Thy1.1+ SFE mice transgenic for the anti-HA 6.5 clonotypic TCR. Dot plots show gating strategy of the Thy1.1+/TCR-HA+ and the Thy1.1+/TCR-HA− among CD4+Foxp3+ cells in dLNs and ndLNs, and histograms below illustrate the level of division of these populations. Cells were transferred on dpc3 (dpi -1) and analyzed at dpi3 in pgkHA-mated insHA mice; one representative experiment out of 3. (B) Natural self-antigen specificity of the non-TCR-transgenic endogenous Tregs recruited in dLNs and ndLNs of the indicated pregnant mice was tested by measuring the presence of HA-specific TCR by qPCR (mean ± SEM). Means of 3 experiments, except for BALB/c x BALB/c combination (2 experiments). The Y-axis represents the arbitrary units of TCR-HA in the indicated LNs of the indicated mating combinations. Two-tailed Mann-Whitney test: * P<0.05.
Adoptive transfer of HA-specific naïve Teff and HA immunization
3 × 106 Naïve Teffs obtained by magnetically depleting CD25+ cells from SFE mice were transferred i.v. to each BALB/c mouse. The day after adoptive transfer, BALB/c mice were immunized s.c. at the base of the tail with the 126–138 peptide in CFA (Sigma-Aldrich, (32)). Two months later, these female mice were mated with pgkHA males either directly or after a single depletion of Tregs (as described above). Similarly, SFE mice were immunized, mated and Treg-depleted before mating as BALB/c mice. The mice used in the tumor experiments were similarly immunized and then challenged with tumor cells 2 months later.
Antibodies and flow cytometry analysis
Para-aortic and brachial LNs were mechanically dissociated in PBS (3% FBS) and stained with FITC- or V500-labeled anti-CD4, APC-labeled anti-CD8, PerCP-labeled anti-Thy1.1/CD90.1, biotin-labeled anti-CD62L (all from BD Biosciences), PE-Cy7-labeled anti-CD25, FITC-labeled anti-CD44, PE-labeled anti-PD-L1, APC-labeled anti-CD103 or PE-labeled anti-GITR, APC-labeled anti-CTLA-4 and PE-Cy5-labeled anti-ICOS mAbs (all from eBiosciences) The PE-labeled clone 6.5 anti-clonotypic mAb specific to TCR-HA was produced in rats immunized with the soluble TCR (a kind gift from Dr. H. von Boehmer, Dana-Farber Cancer Institute). Intracellular staining with PE- or eFluor 450-labeled anti-Foxp3, APC-labeled anti-CTLA-4 and FITC-labeled Ki67 antibodies (all from eBioscience) was done using a kit (FJK-16s, eBioscience). Lymphocytes were acquired with an LSR II cytometer (BD Biosciences) and analyzed with FlowJo (Tree Star, Inc.) software.
TCR-specific qPCR assays
On dpi 6, para-aortic and brachial LNs were harvested from pregnant or non-pregnant female mice, as were pancreatic LNs from InsHA mice and inguinal LNs from 5-day-old 4T1 tumor-bearing mice. Tregs were sorted on a FACSAria cytometer (BD Biosciences) based on the CD4 and CD25. mRNA was prepared using TRIzol reagent (Invitrogen) and phenol chloroform extraction. Reverse transcription PCR and quantification of TCR-HA cDNA were done as previously described (18): TCR-HA cDNA in each sample was quantified by real-time qPCR (Applied Biosystems). The primers for the first amplification of the TCR-HA gene were forward primer Vβ8.2, 5′-ACAAGGTGGCAG- TAACAGGA-3′, and reverse primer Jβ2.1, 5′-CCTCTAGGACGGT- GAGTCGTG-3′. For the nested qPCR, the forward primer Vβ8.2 was 5′-AGTTGGCTACCCCCTCTCAGA-3′, the reverse primer was 5′-GGCC- GGGGGAGTTATGC-3, and the probe labeled with fluorescent reporter was 5′-FAM-ATCAGTGTACTTCTGTGCCAGCGGTGG-TAMRA-3′. As an internal control, endogenous mouse HPRT was also amplified: forward, 5′-CACGTGGGCTCCAGCATT-3′; reverse, 5′-TCACCAGT-CATTTCTGCCTTT-3′; probe, 5′-FAM-CCAATGGTCGGGCACTGCT- CAA-TAMRA-3′. Primers and probes were designed with Primer Express software (version 1.5; Applied Biosystems). Real-time PCR was performed twice using TaqMan Universal PCR master mix (Applied Biosystems) with 200 ng of equivalent mRNA in the case of nested qPCR or 600 ng of equivalent mRNA in the case of classic direct qPCR. The average Ct of the triplicates was used to calculate the fold change relative to positive control cDNA of cells from TCR-HA SFE mice.
Statistics
Statistical significances were evaluated using GraphPad Prism software (GraphPad Software Inc.). Data are presented as mean ± SD unless otherwise indicated. A P value less than 0.05 was considered statistically significant. For some experimental groups with non-matching time points, unpaired t tests with Welch’s correction were used to compare groups with pooled time points.
Results
Embryo implantation triggers early recruitment of activated/memory Tregs in uterine-draining lymph nodes
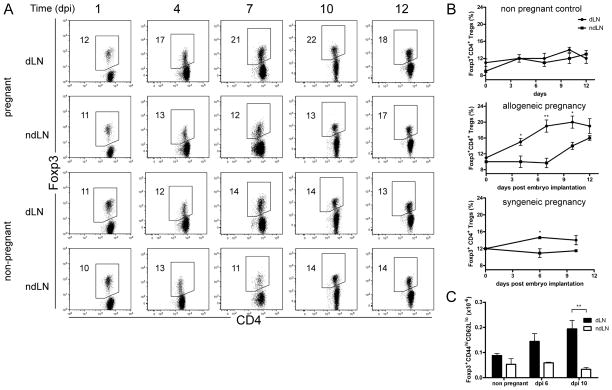
We investigated the recruitment of CD4+Foxp3+ Tregs in the lymph nodes of pregnant B6-mated BALB/c female mice. We analyzed Treg numbers and proportions in the uterine para-aortic draining lymph nodes (dLNs) and brachial non-draining lymph nodes (ndLNs), on days post blastocyst implantation (dpi) 1 to 12 (i.e., days post coitum (dpc) 5 to 16). By dpi 4, we observed a significantly augmented proportion of Tregs in the pregnant mouse dLNs compared with the ndLNs, or with the control LNs from non-pregnant mice (Figure 1A). This increase in Treg proportions in pregnant mice corresponded to more than a doubling of their absolute numbers (Supplemental Figure 1A). The proportion and numbers of Tregs continued to increase by dpi 7, 10, and 12 in the dLNs. By dpi 12 an increase of Tregs was also observed in the ndLNs, albeit smaller (Figure 1 A & B, Supplemental Figure 1A).
Figure 1. Early recruitment of activated, memory Tregs after embryo implantation.
(A) Dot plots illustrate the percentages of Foxp3+ CD4+ Tregs in para-aortic dLNs and brachial ndLNs harvested from pregnant B6-mated BALB/c mice on days post implantation (dpi) 1, 4, 7, 10, and 12, or from unmanipulated BALB/c female mice. One representative mouse per time point is depicted, n = 3 to 6 mice per group. Associated statistics are shown in (B). (B) Kinetics of Foxp3+ CD4+ Treg frequency in dLNs and ndLNs harvested at the indicated times from: Top panel: non-pregnant female BALB/c mice; Middle panel: B6-mated pregnant BALB/c mice; Bottom panel: BALB/c-mated pregnant BALB/c mice. (C) absolute numbers of CD4+ Foxp3+ CD44hi CD62Llo activated/memory Tregs in dLNs and ndLNs harvested from pregnant B6-mated or non-pregnant BALB/c mice at the indicated times. Results are shown in mean ± SEM. n = 3 to 5 mice per group. Two-tailed unpaired t-test significance: * P≤0.05, ** P≤0.01. Stats on graph compare each dLN with their respective ndLN. Mouse dLNs: Pregnant allogeneic vs. pregnant syngeneic (Welch’s t-test with P value = 0.1501); pregnant allogeneic vs. non pregnant (Welch’s t-test P value = 0.0458; Two-way Anova P values < 10−4 for both time and pregnant status).
We previously identified the CD44hiCD62Llo Treg subset as self-specific activated/memory Tregs (18, 21). In the uterus dLNs of allogeneically mated BALB/c females, compared with non-pregnant virgin controls, we observed a specific and continuous increase in both number and frequency of amTregs from dpi6 to dpi10 (Figure 1C and supplemental Figure 1B). There were no major changes in CD103, CTLA-4, ICOS, PD-L1, CD25 and GITR expression on the recruited Tregs (Supplemental Figure 1D).
In syngeneic pregnancy (BALB/c-mated BALB/c mice), we also observed an increase of Treg numbers in the dLNs compared with the ndLNs, although to a lesser extent than in allogeneic pregnancy (Figure 1B, Supplemental Figure 1C). This suggests that Tregs respond at least in part to self-antigen.
The rapid augmentation of Tregs in the uterus dLNs in pregnancy (B6-mated BALB/c), starting approximately 3 or 4 days post embryo implantation, was very similar to that observed in an emergent cancer model, 4T1 breast carcinoma cells implanted in BALB/c mice (Supplemental Figure 1A).
Embryo implantation triggers Treg expansion in uterine draining lymph nodes
Next, we studied the proliferation of the different T cell subsets in the dLNs and ndLNs of pregnant mice. Ex vivo CFSE-labeled congenic Thy1.1+ cells were adoptively transferred to B6-mated BALB/c mice on implantation day, and to non-mated BALB/c mice. On dpi 7, 27% of the transferred CD4+Foxp3+ Tregs had undergone at least 1 division, with 9% having divided more than 6 times (Figure 2). In contrast, the division rates were approximately half those in ndLNs, and identical to those observed at the steady state in nLNs from control non-pregnant mice, the latter reflecting the high turnover of Tregs in a physiological setting (21). The division rates of CD4+Foxp3− T and CD8+ Teffs in dLNs were low and not significantly different from those in ndLNs and control nLNs (Figure 2). Similar observations were made at dpi 4 and 10 (data not shown).
These data are strikingly similar to those observed in an emergent cancer model, 4T1 breast carcinoma cells implanted in BALB/c mice (Supplemental Figure 2A), although the magnitude of Treg division is higher in this case. The Treg division index ([division rate in dLNs – division rate in ndLNs]/division rate in ndLNs) increased faster and reached a higher level in the cancer than in the pregnancy setting (Supplemental Figure 2B).
Treg proliferation during early pregnancy is antigen-driven and self-specific
We next studied the importance of self-antigens in the recruitment/division of Tregs induced by the conceptus implantation using influenza hemagglutinin (HA) as a model antigen. In insHA mice, HA is expressed in pancreatic islet β-cells under the control of the insulin promoter (18, 19). We mated insHA female mice with homozygous pgkHA males, in which HA is expressed ubiquitously and from an early embryonic stage by the phosphoglycerate kinase (pgk) promoter. HA is thus a self-antigen in both insHA and pgkHA mice.
We first analyzed the recruitment and division of Tregs obtained from SFE mice that express an HA-specific transgenic TCR (18), adoptively transferred in pgkHA mated insHA female mice. At dpi 3, 33% of the CFSE-stained donor TCR-HA+ Tregs had already divided in the dLNs, vs. 3.9% in the ndLNs of these insHA mice (Figure 3A). In contrast, there was little proliferation of donor TCR-HA− Tregs (Figure 3A).
These data are strikingly similar to those obtained after the transfer of CFSE-stained donor TCR-HA+ Tregs in mice implanted with HA-expressing tumors, although in that case the recruitment and division of donor TCR-HA+ Tregs were even more pronounced (18).
Next, we wanted to confirm the recruitment of self-specific Tregs in a wild-type, non-TCR transgenic system. In insHA mice, HA is expressed in the thymus by an AIRE-dependent process, and HA-specific TCRs are expressed almost exclusively on Tregs (18). We assessed the recruitment of endogenous natural HA-specific Tregs by quantifying TCRs specific for an immunodominant epitope of HA by qPCR in insHA mice mated with pgkHA or BALB/c mice (18). Among the various mating combinations tested, we detected the presence of endogenous HA-specific Tregs (i.e., increased qPCR signal of the HA-specific TCR) only in the pancreas and para-aortic dLNs of the pgkHA-mated insHA females, which are the two main sites where HA antigens are drained in these mice (Figure 3B).
These results are strikingly similar to those obtained in the setting of cancer where endogenous HA-specific amTregs are recruited only in the HA-expressing tumor itself and in its dLNs (Supplemental Figure 2C and (18)).
Effects of Treg deficit or pre-immunization on the fate of embryos
We assessed the effect of Treg ablation in the classic immunological abortion-prone model of CBA/J (H2k) females mated with DBA/2J (H2d) males. Treg ablation was achieved by anti-CD25 mAb treatment administered one day prior to mating, which led to a Treg deficiency for approximately 3–4 weeks ((11) and Supplemental Figure 3A). Diphtheria toxin (DT)-mediated Treg elimination in mice which express DT in Tregs could not be used in our setting, because of the different genetic background and also because DT-induced Treg ablation is transient (≤4 days) and incomplete in DEREG mice (33), or leads to rapid and catastrophic lethal autoimmunity in Foxp3DTR mice (34).
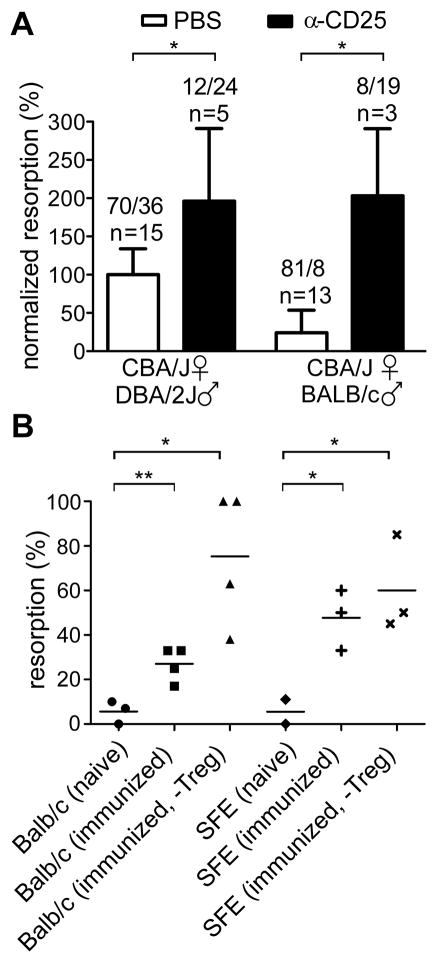
By dpi 9, when the animals were sacrificed, examination of the uteri showed similar numbers of implantation sites (whether with viable or resorbing conceptus) in control and Treg-depleted females, indicating that the antibody treatment did not affect fertility. The fetus resorption frequency was increased two-fold in the Treg-depleted group, compared with controls (Figure 4A). These rates were compared with those of BALB/c-mated CBA/J females, a normal pregnancy model, which underwent the same antibody-induced Treg ablation. Fetal resorption frequency in this case was increased eight-fold in the Treg-depleted group, compared with the control group (Figure 4A).
Figure 4. Treg ablation and fetal-antigen preimmunization impair fetal survival.
(A) Treg ablation was achieved by anti-CD25 mAb treatment administered one day prior to the mating of CBA/J mice. Histograms depict the % of fetal resorption in the indicated groups. The results are shown as normalized to the mean resorption frequency of the PBS-treated CBA x DBA control group, which was set at 100%. Numbers above bars indicate the cumulative numbers of resorbed/viable fetuses and the numbers of mice per experimental condition on dpi 8. Two-tailed Mann-Whitney test: * P < 0.05, ** P < 0.01. (B) Evaluation % of fetus resorption after fetal antigen preimmunization of the indicated mice with or without anti-CD25 mAb-induced Treg depletion at the time of the mating. BALB/cfemale first received anti-HA naïve CD4+CD25− Teffs from SFE donor mice (i.v.), and were then immunized by HA peptide (s.c., CFA condition). 2 months later, HA-immunized SFE-transferred BALB mice, similarly HA-immunized SFE mice and naïve SFE and BALB/c female were mated with pgkHA males directly or after Treg depletion by anti-CD25 treatment (- Treg condition). Viable fetuses or resorption sites were counted on dpi 8. (n = 2–4 mice per group). Two-tailed unpaired t-test: * P < 0.05, ** P < 0.01.
The quantitative differences in fetal resorption frequencies upon ablation of Tregs in different genetic backgrounds are reminiscent of similar variations observed in different tumor models (18, 35) (Supplemental Figure 3B).
Next, we investigated whether pre-immunization against a single paternal antigen influences pregnancy. We immunized (i) BALB/c mice transferred with CD4+CD25− T cells from SFE mice, or (ii) SFE mice directly. In these two settings, Teffs contain approximately 1% or 15–30% of anti-HA specific cells, respectively, and the HA peptide is not a self-antigen in this context. These females were then mated with pgkHA males, with or without prior Treg depletion by anti-CD25 mAb. Compared with the 5% basal spontaneous resorption frequency in unmanipulated BALB/c mice, HA-immunized BALB/c had an average of 27% fetal resorption, which increased dramatically to 75% when immunized mice were Treg-depleted before mating, and even to 100% in a few cases. A resorption frequency of 48% was observed in the group of HA immunized SFE females and this reached 60% when immunization was followed by Treg depletion (Figure 4B). Collectively, these results show that after HA-immunization, resorption of HA-expressing fetuses substantially increased in both BALB/c and SFE females as compared with the resorption level in the naïve controls (about 5% in both strains). Moreover, the resorption levels did not differ significantly between immunized BALB/c (27%) vs. immunized SFE females (48%), despite the difference in the initial number of anti-HA Teffs (1 and 15–30%, respectively).
In contrast, in the cancer setting, pre-immunization resulted in a 100% rejection of AB1-HA tumors in BALB/c mice, without requiring Treg ablation (Supplemental Figure 3C).
Treg expansion by Flt3-L or low-dose IL-2 treatments prevents recurrent spontaneous abortion in abortion-prone mice
As Treg depletion increases the frequencies of fetal loss, we investigated whether, on the contrary, Treg induction would reduce the spontaneous abortion rates in the abortion-prone CBA/J x DBA/2J mating model. We previously reported that Treg homeostasis is tightly correlated with the homeostasis of conventional dendritic cells (cDCs) (36). Mice deficient in Fms-related tyrosine kinase 3 ligand (Flt3-L) (C57Bl/6 background) are genetically deficient in cDCs and exhibit a 50% decrease in Tregs compared with syngeneic Flt3-L-sufficient mice ((36) and Supplemental Figure 4A). Compared with B6 x BALB/c matings, Flt3L−/− x BALB/c matings showed an increase in fetal loss (i.e. visible resorption sites), which did not reach statistical significance (Supplemental Figure 4B), but resulted in significantly smaller litters (Supplemental Figure 4C). This suggests a scenario of very early rejection of embryos without detectable resorption sites in this model.
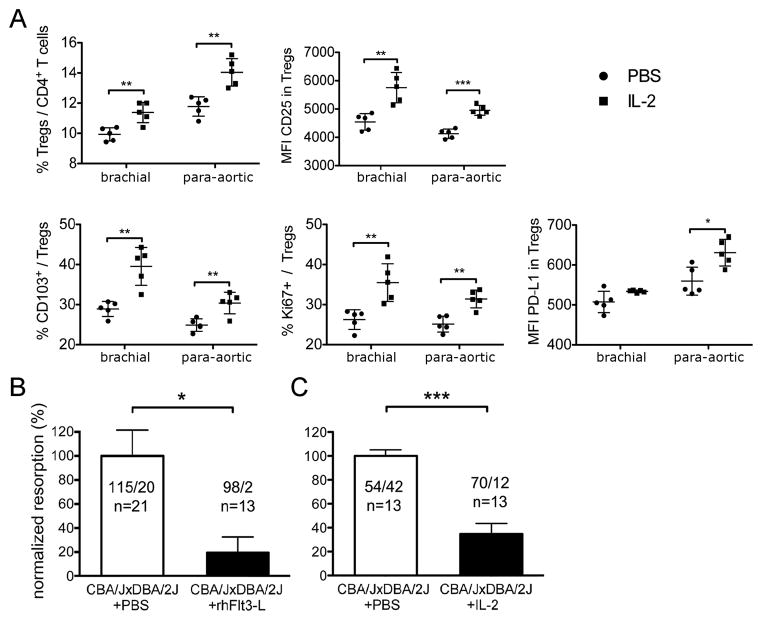
As Flt3-L treatment increases the proliferation of natural Tregs in a DC-dependent manner both at the steady state (36) and also during the pregnancy of DBA/2J-mated CBA/J females (Supplemental Figure 4D), we evaluated whether Flt3-L treatment could improve the impaired pregnancy in DBA/2J-mated CBA/J females. We observed that the proportion of fetus rejection in CBA/J x DBA/2J matings dropped by 80% after Flt3-L treatment, with 115 viable fetuses vs. 20 resorptions in the PBS-treated group and 98 viable fetuses vs. 2 resorptions in the Flt3L-treated group (Figure 5B).
Figure 5. Low-dose rhFlt3-L or IL-2 reverse pregnancy loss in a spontaneous abortion-prone model.
(A) Effects of IL-2 treatment on Treg population of female CBA/J mice. Percentages of CD103 and Ki67 expressing Tregs and CD25 and PD-L1 mean fluorescence index (MFI) of Tregs in para-aortic and brachial LNs after a 10-day treatment with IL-2 (n = 5) or PBS (n = 5). (B-C) Effect of rhFlt3-L and IL-2 treatment on the outcome of CBA/J x DBA/2J matings.
The results are shown as normalized to the mean resorption frequency of the PBS-treated control groups, which was set at 100%. (B) Viable fetuses and resorption sites were counted on dpi 11, post PBS or rhFlt3-L treatment. In the PBS-treated group: 115 viable fetuses and 20 resorptions (n = 21); in the Flt3L-treated group, 98 viable fetuses and 2 resorptions (n = 13); * P = 0.0123, two-tailed Mann-Whitney test. (C) Viable fetuses and resorption sites were counted on dpi 8 post IL-2 or PBS treatment. In the PBS-treated group: 54 viable fetuses and 42 resorptions (n = 13); in the IL-2-treated group, 70 viable fetuses and 12 resorptions (n = 13); *** P < 0.001, two-tailed Mann-Whitney test.
IL-2 is also known to modulate Treg homeostasis directly by promoting Treg survival, proliferation and function. For example, we have recently shown that IL-2 administration (i) can cure recent-onset diabetes in NOD mice (37) and (ii) significantly induces Tregs and improves clinical symptoms in human HCV-induced vasculitis (38). We therefore investigated whether low-dose IL-2 treatment could improve pregnancy outcome in CBA/J x DBA/2J matings. First, we checked the effect of low-dose IL-2 treatment on wild-type CBA/J animals. We observed a statistically significant increase of Tregs in para-aortic and brachial lymph nodes in IL-2-treated vs. control mice, which correlated with a higher proliferation (measured by the expression of Ki67+ cells among Tregs) and increased expression of CD25 and CD103. Tregs were thus activated and expanded systemically in those mice. Notably, we observed an increase of PDL-1 expression by Tregs only in the para-aortic LN of the CBA/J mice.
Next, we evaluated pregnancy outcome in the low-dose IL-2-treated animals. We observed that the proportion of fetus rejection in CBA/J x DBA/2J matings dropped to 65% after IL-2 treatment, with 54 viable fetuses vs. 42 resorptions in the PBS-treated group and 70 viable fetuses vs. 12 resorptions in the IL-2-treated group (Figure 5C). It is noteworthy that 4 out of 13 IL-2-treated CBA/J females had not a single observable resorption site and all the fetuses were alive. We thus conclude that pregnancy outcome in the abortion-prone CBA/J x DBA/2J mating is substantially improved by low-dose IL-2 treatment.
DISCUSSION
Understanding the mechanisms that protect the allogeneic fetus from attack by the maternal immune system during pregnancy is still a major challenge and has vast heuristic and therapeutic implications in autoimmune diseases and organ transplantation. A large body of findings has demonstrated that fetuses are protected from immune attack in various and redundant ways, with Tregs having a major role. However, relatively little is known about Treg modulation of immune responses at the precise time of embryo implantation, concomitant with the passage from an inflammatory environment (5, 6) to a locally tolerant one. We report here that rapid recruitment and activation of pre-existing self-antigen-specific memory Tregs enforces a local tolerogenic environment by outrunning the primary response of Teffs and appears to be a key to embryo survival.
The early requirement for Tregs during pregnancy is supported by the expansion of CD4+CD25+FOXP3+ Tregs in the late follicular phase of the menstrual cycle in mice, in preparation for a possible implantation event, followed by a decrease in Treg numbers in the metestrus-disestrus phase as fertility recedes after the window of implantation in a non-gravid cycle (39). Women who have experienced recurrent spontaneous abortions have low numbers of Tregs, comparable to numbers observed in postmenopausal women at both the follicular and luteal phases (17), and/or these cells are functionally deficient (40).
Nature of the antigens driving early Treg recruitment/activation
Recently, two groups reported the generation and pivotal role of maternal Tregs specific for paternal alloantigens in successful allopregnancies (14, 41). In particular, Way and colleagues demonstrated that these cells are recruited and actively proliferate starting from mid-gestation, and later persist as memory Tregs after delivery (14). They concluded that these cells are important for the success of both primary and secondary pregnancies with males expressing the same alloantigens. However, these data did not shed light on how allogeneic embryos are protected from immune attack at implantation (~ day 4) and early gestation, before the appearance of maternal Tregs with paternal specificity at mid-gestation (~ day 11).
Here we show that Tregs specific for self-antigens are mobilized very early (3–4 dpi), earlier than the reported mobilization of allospecific Tregs (7.5 days pi (14)), and are essential components of the Treg response to the conceptus. Our results in pgkHA-mated InsHA females unequivocally show that most of the amTreg response at very early gestational stages is self-antigen-triggered. This is also supported by our observations and those of others (14) that syngeneic mating triggers an expansion of Tregs in the dLNs. We thus suggest that the recruitment and proliferation of amTregs specific for self-antigens play a crucial role in establishing an early tolerogenic environment that protects the fetus before allospecific Tregs come into play. amTregs are engaged faster and dominate the allospecific Teffs.
Memory alloantigen-specific Tregs (14) are absent at embryo implantation and are thus not crucial in the early establishment of tolerance in primary pregnancies. They may, however, contribute to the tolerant immune response at a later stage as their number increases (14), and could have an important role in secondary pregnancies when the immune attack against the fetus could be more violent due to the presence of allospecific memory Teffs generated during the first pregnancy. Here, they could reinforce the self-specific amTreg responses for successful fetus protection (14). Tolerant maternal immune responses to the fetus thus appear to be a subtle intercourse of self-specific memory Tregs and allospecific Tregs, the role of which depends on the moment of pregnancy and its primary or secondary status.
Similarities and differences between embryo and tumor handling by Tregs
There are striking similarities in the early T cell response to tumor or embryo implantation: the response (i) is that of CD44hiCD62low amTregs; (ii) is driven by self-antigens; (iii) is detectable in the first 3–4 days after implantation, preceding the response of effector T cells; (iv) depends more on memory status than on relative numbers of the cells; and (v) Treg depletion at an early, but not a late, time point induces embryo or tumor rejection (13, 18). The self-antigen-driven response is the main property shared by the immune reactions to cancer and conceptus, as recently confirmed for the cancer setting (42).
There are also important differences between conceptus and tumor handling by Tregs: (i) After dpi 10–12, the frequencies and numbers of Tregs in uterine dLNs did not continue to increase, although they remained higher than those in control LNs. In contrast, Treg proliferation continues after dpi 10 in emergent tumor models. This difference could be due to a post-implantation transition from an inflammation-like environment to a Th2 dominant environment, which rises at later gestational stages (43). This latter state may involve other mechanisms for embryo/fetus protection, which act as additional and possibly by then predominant protectors of the conceptus. Hormonal changes at the end of pregnancy could also explain this phenomenon (40, 44). (ii) More importantly, pre-immunization against a paternal antigen – HA in our experiments – only increased the resorption rate, as has also been seen by others in different experimental models (45, 46), while in marked contrast, HA-pre-immunization resulted in 100% eradication of HA-tumors. This result is noticeable because fetus rejection rates have never been reported to reach 100% consistently, except in the somehow artificial setting of nude mice reconstituted with Treg-depleted T cells (10). In our hands, combining Treg depletion with pre-immunization resulted in a marked increase in the fetus resorption rate. Finally, (iii) local T cell tolerance to (semi)-allogeneic embryos faces the difficulty that allo-immune responses mobilize many more effector cells than antigen-specific responses do. To control these effectors, one could speculate that it would be necessary to engage a large enough number of suppressor cells and/or to build a resistant environment before Teffs could become active and deleterious. The rapid intervention of amTregs, which are quickly put into action because of their memory nature, fulfills these two requirements (number and speed) and appears to be a major component of the early protective responses to embryo implantation in mice. Altogether, our results point to the importance of Tregs in protecting the growing fetus, but also highlight the fact that other mechanisms come into play most likely to ensure at multiple levels survival of the embryo and perpetuation of the species.
Treg response to embryo and tumor in evolution
Viviparity and even placentation appear to have developed before antigen-specific adaptive cellular immunity. Placentae are already found in invertebrates such as onychophora (47) – whose representatives have existed since over 500 million years – as well as in jawed vertebrates (48). T lymphocytes expressing variable immune receptors that interact with antigens presented by major histocompatibility complex molecules were first found in jawed vertebrates (49). The development of the adaptive immune system in viviparous species thus called for concomitant selection of mechanisms for robust tolerance to embryo implantation and development. Functional Tregs are already found in Tetraodon (-400 million years), in which their depletion produced an enhanced mixed lymphocyte reaction in vitro and inflammation of the intestine in vivo (50). Also, Foxp3 from zebrafish is able to confer suppressive activity on murine T lymphocytes (51). Thus several lines of evidence suggest that Tregs have played a significant role in implementing maternal fetal tolerance during the development of adaptive immunity. Induced Tregs that emerged in evolution concomitantly with placentation (15) have also been found to play a significant role in maternal/fetal tolerance (41).
The similarities in embryo and tumor handling by the maternal/host system are not unexpected if one considers analogies between pregnancy and cancer (reviewed in (52)). First, embryo and tumor implantations correspond to the development of highly invasive cells that penetrate/migrate through normal structures to establish their own nutrient supplies. Second, semi-allogeneic fetal cells and cancer cells are characterized as being partly self to the mother/patient. That similar responses should be mounted to similar challenges seems logical, but one can then wonder how an immune system that protects deadly tumor cells survived evolution. We speculate that this type of response has been positively selected to protect allogeneic fetuses against immune rejection, in the case of placental or histotrophic viviparity, while developing an adaptive immune system, which appeared after such a reproductive strategy was first developed. It was not counter-selected because cancer development is usually a late-in-life process (53) which usually does not affect reproductive life span. Tregs were probably selected in part to protect allogeneic fetuses against immune rejection, but protection of cancer cells by Tregs became the price paid for an efficient protection of embryos.
Therapeutic implications
Tregs accumulate within the decidua during early human pregnancy (40). Circulating Tregs peak in the second trimester, and decrease to a pre-pregnancy level after delivery (54). Several studies have suggested a close correlation between lower numbers of Tregs and/or reduced expression of Foxp3 and pregnancy failure such as recurrent spontaneous abortions (17, 40, 55–57), in which Treg numbers in the decidua and peripheral blood decrease compared with normal pregnancy levels and reach non-pregnancy levels in the blood (57). In such patients, Tregs are also qualitatively hampered (40). Altogether, these data suggest that an increase in Treg number and/or function could be therapeutic in pregnancy-related diseases. Therapeutics that boost Treg number and function therefore hold promise.
There is much evidence showing that low-dose IL-2 administration induces Tregs. For example, 5 days of low-dose IL-2 cured recent-onset diabetes in ~40% of treated mice (37). We therefore investigated whether IL-2 could improve the poor fetal survival in the classic murine abortion model, the CBA x DBA/2 system (3). Boosting Treg number did indeed lower the high abortion frequency to a level that is comparable to what is seen in normal, non-abortion-prone mating combinations. However, these data conflict with reports showing that injection of IL-2 in pregnant mice is abortifacient (58, 59). We previously reported a high abortion rate after 2,000 IU in three repeated i.p. injections at days 6.5, 8.5, and 10.5 (60). There are several possible, not necessarily mutually exclusive, explanations, such as quantity of IL-2 used (higher doses may recruit CD25-low effector T cells), contamination by LPS of crude IL-2 preparations and/or different injection schedules. In addition, our data obtained with low-dose IL-2 are fully supported by the results obtained with Flt3-L treatment. Initially known as a DC inducer, Flt3-L also acts as a Treg inducer because DCs and Treg homeostasis are closely correlated, the number of Tregs increasing when the number of DCs increases and decreasing when DC number decreases (36). Flt3-L treatment also reduced the high abortion frequency to a level similar to what is seen in normal, non-abortion-prone mating combinations. Moreover, fully consistent results were obtained with Flt3-L−/− mice in which both the fetal loss rate and the survival of mice challenged with tumor cells were increased.
Our recent clinical trial showed that low-dose IL-2 quite specifically induces Tregs without inducing Teffs, and is very well tolerated in patients with autoimmunity (38). Thus, low-dose IL-2 warrants investigation in recurrent spontaneous abortions and infertility caused by implantation failure.
Supplementary Material
Acknowledgments
Grants: This work was supported by an Institut national du Cancer (INCA) grant to DK and by the authors’ institutions. GDJ was supported by a Human Frontier long-term fellowship (LT00291/2008), and JLS by an NIH research grant (AI053330).
We thank Michel Nussenzweig for the Flt3-L−/− mice and rhFlt3-L, Katrina Podsypanina for critical reading of the manuscript, Hang-Phuong Pham for statistical advice. Yenkel Grinberg-Bleyer for assisting with the immunization, and Claude Baillou for cell sorting.
Abbreviations used in this article
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- dpc
day(s) post coitum
- dpi
day(s) post implantation
- Flt3-L
Fms-related tyrosine kinase 3 ligand
- Foxp3
forkhead/winged-helix protein 3
- GITR
glucocorticoid-induced TNFR-related protein
- HA
hemagglutinin
- ICOS
inducible T-cell costimulator
- Ins
insulin
- LNs
lymph nodes
- dLNs
draining lymph nodes
- ndLNs
non-draining lymph nodes
- nLNs
unmanipulated mouse lymph nodes
- PD-L1
programmed cell death ligand 1
- pgk
phosphoglycerate kinase
- TDO
tryptophan 2,3-dioxygenase
- Teff
effector T cell
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–338. [Google Scholar]
- 2.Beer AE, Billingham RE. The embryo as a transplant. Sci Am. 1974;230:36–46. doi: 10.1038/scientificamerican0474-36. [DOI] [PubMed] [Google Scholar]
- 3.Chaouat G, Monnot P. Systemic active suppression is not necessary for successful allopregnancy. Am J Reprod Immunol. 1984;6:5–8. doi: 10.1111/j.1600-0897.1984.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 4.Beer AE, Billingham RE, Hoerr RA. Elicitation and expression of transplantation immunity in the uterus. Transplant Proc. 1971;3:609–611. [PubMed] [Google Scholar]
- 5.Sanford TR, De M, Wood GM. Expression of colony-stimulating factors and inflammatory cytokines in the uterus of CD1 mice during days 1 to days 3 of pregnancy. J Repro Fert. 1992;94:213–220. doi: 10.1530/jrf.0.0940213. [DOI] [PubMed] [Google Scholar]
- 6.McMaster MT, Newton RC, Dey SK, Andrews GK. Activation and distribution of inflammatory cells in the mouse uterus during the preimplantation period. J Immunol. 1992;148:1699–1705. [PubMed] [Google Scholar]
- 7.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaouat G, Voisin GA, Escalier D, Robert P. Facilitation reaction (enhancing antibodies and suppressor cells) and rejection reaction (sensitized cells) from the mother to the paternal antigens of the conceptus. Clin Exp Immunol. 1979;35:13–24. [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RN, Powell AE. The adoptive transfer of pregnancy-induced unresponsiveness to male skin grafts with thymus-dependent cells. J Exp Med. 1977;146:899–904. doi: 10.1084/jem.146.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolernace to the fetus. Nature Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 11.Darrasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol. 2008;77:14–22. doi: 10.1016/j.jri.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006;12:301–308. doi: 10.1093/molehr/gal032. [DOI] [PubMed] [Google Scholar]
- 18.Darrasse-Jeze G, Bergot AS, Durgeau A, Billiard F, Salomon BL, Cohen JL, Bellier B, Podsypanina K, Klatzmann D. Tumor emergence is sensed by self-specific CD44hi memory Tregs that create a dominant tolerogenic environment for tumors in mice. J Clin Invest. 2009;119:2648–2662. doi: 10.1172/JCI36628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo D, Freedman J, Hesse S, Palmiter RD, Brinster RL, Sherman LA. Peripheral tolerance to an islet cell-specific hemagglutinin transgene affects both CD4+ and CD8+ T cells. Eur J Immunol. 1992;22:1013–1022. doi: 10.1002/eji.1830220421. [DOI] [PubMed] [Google Scholar]
- 20.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behaviour in vitro. Proc Natl Acad Sci U S A. 2003;100:8886–8891. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisson S, Darrasse-Jèze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaouat G, Kiger N, Wegmann TG. Vaccination against spontaneous abortion in mice. J Reprod Immunol. 1983;5:389–392. doi: 10.1016/0165-0378(83)90248-6. [DOI] [PubMed] [Google Scholar]
- 23.Chaouat G, Kolb JP, Kiger N, Stanislawski M, Wegmann TG. Immunologic consequences of vaccination against abortion in mice. J Immunol. 1985;134:1594–1598. [PubMed] [Google Scholar]
- 24.Clark DA, Chaput A, Tutton D. Active suppression of host-vs-graft reaction in pregnant mice. VII. Spontaneous abortion of allogeneic CBA/J x DBA/2 fetuses in the uterus of CBA/J mice correlates with deficient non-T suppressor cell activity. J Immunol. 1986;136:1668–1675. [PubMed] [Google Scholar]
- 25.Hamilton MS, Hamilton BL. Environmental influences on immunologically associated spontaneous abortion in CBA/J mice. J Reprod Immunol. 1987;11:237–241. doi: 10.1016/0165-0378(87)90060-x. [DOI] [PubMed] [Google Scholar]
- 26.Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, Wegmann TG. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol. 1995;154:4261–4268. [PubMed] [Google Scholar]
- 27.Kiger N, Chaouat G, Kolb JP, Wegmann TG, Guenet JL. Immunogenetic studies of spontaneous abortion in mice. Preimmunization of females with allogeneic cells. J Immunol. 1985;134:2966–2970. [PubMed] [Google Scholar]
- 28.Zenclussen ML, Anegon I, Bertoja AZ, Chauveau C, Vogt K, Gerlof K, Sollwedel A, Volk HD, Ritter T, Zenclussen AC. Over-expression of heme oxygenase-1 by adenoviral gene transfer improves pregnancy outcome in a murine model of abortion. J Reprod Immunol. 2006;69:35–52. doi: 10.1016/j.jri.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Du MR, Dong L, Zhou WH, Yan FT, Li DJ. Cyclosporin a improves pregnancy outcome by promoting functions of trophoblasts and inducing maternal tolerance to the allogeneic fetus in abortion-prone matings in the mouse. Biol Reprod. 2007;76:906–914. doi: 10.1095/biolreprod.106.056648. [DOI] [PubMed] [Google Scholar]
- 30.Zhou WH, Dong L, Du MR, Zhu XY, Li DJ. Cyclosporin A improves murine pregnancy outcome in abortion-prone matings: involvement of CD80/86 and CD28/CTLA-4. Reproduction. 2008;135:385–395. doi: 10.1530/REP-07-0063. [DOI] [PubMed] [Google Scholar]
- 31.Bergot AS, Durgeau A, Levacher B, Colombo BM, Cohen JL, Klatzmann D. Antigen quality determines the efficiency of antitumor immune responses generated in the absence of regulatory T cells. Cancer Gene Ther. 2010;17:645–654. doi: 10.1038/cgt.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billiard F, Litvinova E, Saadoun D, Djelti F, Klatzmann D, Cohen JL, Marodon G, Salomon BL. Regulatory and effector T cell activation levels are prime determinants of in vivo immune regulation. J Immunol. 2006;177:2167–2174. doi: 10.4049/jimmunol.177.4.2167. [DOI] [PubMed] [Google Scholar]
- 33.Lahl K, Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol Biol. 2011;707:157–172. doi: 10.1007/978-1-61737-979-6_10. [DOI] [PubMed] [Google Scholar]
- 34.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 35.Chaput N, Darrasse-Jeze G, Bergot AS, Cordier C, Ngo-Abdalla S, Klatzmann D, Azogui O. Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J Immunol. 2007;179:4969–4978. doi: 10.4049/jimmunol.179.8.4969. [DOI] [PubMed] [Google Scholar]
- 36.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 39.Kallikourdis M, Betz AG. Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PLoS One. 2007;2:e382. doi: 10.1371/journal.pone.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 41.Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, Neph S, Sabo P, Kim JM, Liao W, Li MO, Leslie C, Stamatoyannopoulos JA, Rudensky AY. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, Savage PA. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63:624–636. doi: 10.1111/j.1600-0897.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 44.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 45.Moldenhauer M, Hayball JD, Robertson SA. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J Reprod Immunol. 2010;87:1–13. doi: 10.1016/j.jri.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Drake BL, Head JR. Murine trophoblast can be killed by lymphokine-activated killer cells. J Immunol. 1989;143:9–14. [PubMed] [Google Scholar]
- 47.Morera-Brenes B, Monge-Najera J. A new giant species of placented worm and the mechanism by which onychophorans weave their nets (Onychophora: Peripatidae) Rev Biol Trop. 2010;58:1127–1142. doi: 10.15517/rbt.v58i4.5398. [DOI] [PubMed] [Google Scholar]
- 48.Haines AN, Flajnik MF, Wourms JP. Histology and immunology of the placenta in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Placenta. 2006;27:1114–1123. doi: 10.1016/j.placenta.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 50.Wen Y, Fang W, Xiang LX, Pan RL, Shao JZ. Identification of Treg-like cells in Tetraodon: insight into the origin of regulatory T subsets during early vertebrate evolution. Cell Mol Life Sci. 2011;68:2615–2626. doi: 10.1007/s00018-010-0574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quintana FJ, Iglesias AH, Farez MF, Caccamo M, Burns EJ, Kassam N, Oukka M, Weiner HL. Adaptive autoimmunity and Foxp3-based immunoregulation in zebrafish. PLoS One. 2010;5:e9478. doi: 10.1371/journal.pone.0009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holtan SG, Creedon DJ, Haluska P, Markovic SN. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc. 2009;84:985–1000. doi: 10.1016/S0025-6196(11)60669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 54.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Jin LP, Chen QY, Zhang T, Guo PF, Li DJ. The CD4+CD25 bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin Immunol. 2009;133:402–410. doi: 10.1016/j.clim.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Tezabwala BU, Johnson PM, Rees RC. Inhibition of pregnancy viability in mice following IL-2 administration. Immunology. 1989;67:115–119. [PMC free article] [PubMed] [Google Scholar]
- 59.Lala PK. Interruption of murine pregnancy by activation of antigen-non-specific killer cells in the endometrium with indomethacin, high dose IL-2 or a combination. Res Immunol. 1990;141:159–164. doi: 10.1016/0923-2494(90)90136-m. [DOI] [PubMed] [Google Scholar]
- 60.Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J Reprod Fertil. 1990;89:447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.