Abstract
Evidence suggests that inflammation plays a central role in the pathogenesis of atherosclerosis (Libby, Nature 420:868–874, 2002). Inflammation is a physiologic process with highly regulated and often redundant mechanisms to balance pro-inflammatory and anti-inflammatory responses. The complexity of these networks has made it challenging to identify those specific pathways or key enzymes that contribute directly to atherogenesis and could act as a valuable therapeutic target. Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a member of the phospholipase A2 family of enzymes and is believed to contribute to atherosclerotic plaque progression and instability by promoting inflammation. A large number of epidemiologic studies have demonstrated that elevated levels of Lp-PLA2 are associated with an increased risk of cardiovascular events across diverse patient populations, independent of established risk factors including low-density lipoprotein cholesterol. Further, a growing number of preclinical and genetic studies support a causal role for Lp-PLA2 in atherosclerosis. The development of a novel therapeutic agent that directly inhibits the Lp-PLA2 enzyme has provided a unique opportunity to directly test the hypothesis that inhibition of this inflammatory enzyme will translate into improved clinical outcomes. In this article, we will review the evidence to support the notion that Lp-PLA2 is causally implicated in the pathobiology of atherogenesis and discuss the potential utility of inhibiting this enzyme as a therapeutic target.
Keywords: Cardiovascular events, Darapladib, Lp-PLA2 inhibitors
Lp-PLA2: Biologic Mechanisms
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a calcium-independent enzyme that circulates in plasma in its constitutively active form [1, 2]. It is secreted by the inflammatory cells, including monocyte-derived macrophages, T cells and mast cells, and circulates primarily bound to low-density lipoprotein (LDL) cholesterol. It is a member of the phospholipase A2 superfamily of enzymes that are characterized by their ability to hydrolyze the sn-2 ester bond of phospholipid substrates. The catalytic activity of Lp-PLA2 is believed to be lipoprotein-dependent with more of its activity concentrated on small, dense LDL cholesterol, including lipoprotein(a) particles, that are presumed to be the most atherogenic. Discovered because of its ability to catalyze the hydrolysis of platelet-activating factor (PAF), Lp-PLA2 was originally referred to as PAF-acetylhydrolase before adopting its current name. Aside from PAF, the enzyme has specificity for a wide variety of polar phospholipids, including oxidized and short-chain phospholipids. Through this action, Lp-PLA2 is believed to play a key role in the hydrolysis and depletion of oxidized phospholipids (oxPL) associated with lipoproteins [1, 2].
The biologic role of Lp-PLA2 in the pathogenesis of atherosclerosis continues to be debated. Initial reports suggested a possible cardioprotective role for Lp-PLA2 through degradation of PAF and thereby indirect inhibition of platelet activation. An anti-atherogenic role for Lp-PLA2 was also hypothesized due to its ability to hydrolyze oxPLs on LDL cholesterol, therefore theoretically reducing the pathogenicity of oxidized LDL particles. However, the latter was not supported by more recent evidence that suggested that oxPL may in fact play an anti-inflammatory role and the hydrolysis of these lipids might, therefore, only contribute to further inflammation [3].
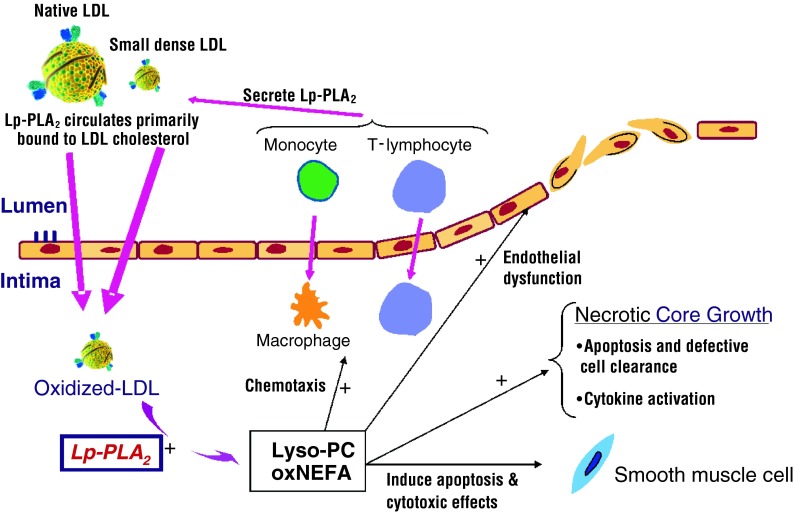
Overall, the weight of the evidence now favors a pro-atherogenic role for Lp-PLA2. Within the atherosclerotic plaque, Lp-PLA2 hydrolyzes oxidized LDL particles leading to the formation of lysophosphatidylcholine (lyso-PC) and oxidized nonesterified fatty acids which are believed to be potent pro-inflammatory mediators [2]. The production of these by-products is believed to contribute to atherogenesis and plaque destabilization through propagation of the inflammatory cascade and contributing to endothelial dysfunction, necrolysis, and apoptosis (Fig. 1). The culmination of which may lead to the production of more thin-cap fibroatheromas (TCFAs), an unstable plaque type that is more vulnerable to rupture [4]. These assertions are supported through histological staining that has shown that the Lp-PLA2 protein appears to be more concentrated in TCFAs than in smaller and more stable plaques [5].
Fig. 1.
The proposed effects of Lp-PLA2 on the progression of atherosclerosis. Lp-PLA2 circulates primarily bound to low-density lipoprotein (LDL) cholesterol and is most concentrated in small dense LDL particles. In the atherosclerotic plaque, it is believed that Lp-PLA2 hydrolyzes modified phospholipids on oxidized LDL particles to generate lysophosphatidylcholine (lysoPC) and oxidized non-esterized free fatty acids (NEFA). In vitro effects of these compounds include endothelial dysfunction, chemotaxis, cytokine activation, and cytotoxic effects. These pro-inflammatory mediators are believed to contribute to atherosclerotic plaque inflammation and instability, thereby leading to further disease progression and plaque instability. Adapted with permission from Motiwala and O’Donoghue [30]. Copyright © Saunders, an imprint of Elsevier Inc. (2011)
In addition, both biologic and animal data support a pro-atherogenic role for the Lp-PLA2 enzyme. Lp-PLA2 mRNA and protein have been identified in macrophages in both human and rabbit atherosclerotic lesions [6]. Hypercholesterolemic pigs have demonstrated an association between higher levels of Lp-PLA2 enzyme activity, higher levels of oxidized lipids, and accelerated progression of atherosclerosis. As well, direct inhibition of Lp-PLA2 activity was shown to inhibit progression of coronary atherosclerosis [7]. In humans, Lp-PLA2 expression is upregulated in unstable and ruptured carotid artery plaques along with increased concentrations of lysoPC [8]. Moreover, Lp-PLA2 gene expression in retrieved carotid plaques post endarterectomy has been shown to be independently associated with an increased risk of future cardiovascular (CV) events [9]. In TCFAs, Lp-PLA2 expression is strongly expressed in macrophages and deposition of the Lp-PLA2 protein preferentially co-localizes with apoptotic macrophages near the fibrous cap and in the necrotic core, a region that is abundant in lipids and oxidation products [5]. In contrast, minimal Lp-PLA2 activity has been identified in thicker capped or more stable fibroatheromas.
Lp-PLA2: Epidemiology and Risk Stratification
Since Lp-PLA2 is an enzyme, it can be quantified either through assessment of its mass or activity. Lp-PLA2 mass is typically assessed through an immunoassay that quantifies the concentration of Lp-PLA2 in serum or plasma. The commercially available PLAC™ test (diaDexus, Inc.; San Francisco, CA, USA) is an enzyme-linked immunosorbent (ELISA) assay that has been approved by the United States Food & Drug Administration for the quantitative determination of Lp-PLA2 and to be used as an aid for the assessment of risk of coronary heart disease or ischemic stroke. Since Lp-PLA2 is highly selective for phospholipids with very short acyl groups at the sn-2 position, Lp-PLA2 activity can be measured through assays that quantify the rate of formation of the reaction by-product through radiometric or calorimetric methods. Although early evidence suggested a high correlation between these two measures, more recent studies have shown only a modest correlation between Lp-PLA2 activity and mass [10]. It is plausible that the two measures provide complementary information, since the Lp-PLA2 mass assay quantifies Lp-PLA2 that is primarily accessible on the lipoprotein surface, whereas the activity assay may assess complete Lp-PLA2 activity under denaturing conditions.
To date, several studies have examined the prognostic utility of Lp-PLA2 activity and mass for predicting the risk of CV events in primary and secondary prevention patient populations.
The West of Scotland Coronary Prevention Study (WOSCOPS) was the first large-scale analysis to demonstrate an association between Lp-PLA2 mass concentration and the risk of subsequent CV events in hyperlipidemic men [11]. Importantly, in this study, Lp-PLA2 provided incremental information for risk stratification that was independent of established cardiac risk factors, LDL cholesterol and other markers of risk including C-reactive protein, fibrinogen, and white blood cell count.
Subsequent to the publication of the WOSCOPS results, several studies have since examined the prognostic utility of Lp-PLA2 activity or mass in healthy individuals and in those with established disease. Although several studies in primary prevention validated the previously observed results in WOSCOPS, other studies did not show an association between Lp-PLA2 and CV outcomes after multivariable adjustment [12–14]. In the Atherosclerosis Risk in Communities (ARIC) study, an association between Lp-PLA2 and the risk of future coronary disease was only demonstrated in those subjects with an LDL cholesterol <130 mg/dl [15].
The prognostic utility of Lp-PLA2 has also been examined in individuals with established coronary disease. Lp-PLA2 activity or mass do not appear to be useful for risk stratification in the acute phase of an acute coronary syndrome (ACS) [1, 10, 16], but Lp-PLA2 activity levels are associated with an increased risk of CV events once stabilized a few weeks after the event [10]. It remains incompletely understood why Lp-PLA2 mass or activity is not associated with the risk of recurrent CV events when measured early after ACS. In contrast, a large study that included 3,766 patients with stable coronary artery disease (CAD) demonstrated that higher levels of Lp-PLA2 mass were independently associated with an increased risk of CV events [17]. In both stable CAD and in those at least 1 month from an ACS, Lp-PLA2 is only minimally correlated with C-reactive protein (CRP) and adds incremental prognostic utility for prediction of CV events. The Lp-PLA2 Studies Collaboration combined patient-level data for 32,453 individuals with established individuals with stable CV disease and found that Lp-PLA2 was independently associated with an increased risk of coronary heart disease (CHD), as well as vascular and non-vascular death [18].
In all, the Lp-PLA2 Studies Collaboration report combined data for more than 79,000 subjects across 32 prospective studies in primary and secondary prevention and demonstrated that Lp-PLA2 activity and mass both have a continuous association with the risk of CHD and vascular death that is similar in magnitude to non-HDL cholesterol and systolic blood pressure and is independent of conventional risk factors [18]. When data were combined across studies, there existed a strong correlation between Lp-PLA2 activity and LDL surrogates, including non-HDL cholesterol (r = 0.49), apolipoprotein B (r = 0.45), and directly measured LDL cholesterol (r = 0.48). Lp-PLA2 activity was correlated with log triglyceride concentration (r = 0.22) and inversely correlated with HDL cholesterol (r = 0.24). Lp-PLA2 activity was higher in men than in women; however, only a weak or non-significant association was observed between Lp-PLA2 activity and age, systolic blood pressure, body-mass index, smoking, and CRP. Overall, similar correlations were observed for Lp-PLA2 mass and baseline covariates. A slightly weaker association was observed between Lp-PLA2 mass and the lipid parameters, whereas a stronger correlation was observed between Lp-PLA2 mass and smoking [18].
Lp-PLA2: Genetic Polymorphisms and Coronary Risk
Although several studies have shown that higher levels of Lp-PLA2 are associated with an increased risk of CV events, such studies cannot demonstrate causality. Genetic variants that lead to natural alterations in Lp-PLA2 activity provide a unique opportunity to begin to assess whether the enzyme may play a causal role in the development of CV disease. The gene encoding the Lp-PLA2 protein (PLA2G7) has 12 exons and is located on chromosome 6p21.2-12 [19]. A common loss-of-function (LOF) mutation (V279F allele) in the Lp-PLA2-encoding gene (PLA2G7) has been identified in individuals of Japanese, Chinese, and Korean descent and leads to natural deficiency or absence of Lp-PLA2 activity. Those with two LOF alleles (homozygotes) completely lack Lp-PLA2 activity, whereas those with one LOF allele (heterozygotes) have approximately a 50% reduction in Lp-PLA2 activity, as compared with those without this variant (wild-type).
Despite initial conflicting reports from smaller studies, a larger scale study of the V279F loss-of-function polymorphism supports a pro-atherogenic role for the Lp-PLA2 enzyme [19]. The study consisted of two large case–control populations in Korean men and demonstrated that genetic deficiency in Lp-PLA2 activity due to carriage of the V279F null allele was associated with reduced odds of coronary heart disease. There tended to be a gene-dose effect such that carriage of a single copy of the V279F allele was associated with a 21% reduction in the odds of CAD, whereas two copies were associated with a 31% reduction in risk of disease. In turn, the magnitude of this reduction in risk was consistent with what one would predict based on the epidemiologic data collected in the Lp-PLA2 Studies Collaboration [18].
Darapladib: Preclinical Studies
Since growing evidence supports a pro-atherogenic role for Lp-PLA2, ongoing research is investigating its utility as a therapeutic target. Since Lp-PLA2 circulates primarily bound to LDL cholesterol, drugs that influence lipoprotein concentration have been shown to influence Lp-PLA2 levels, including statins [20, 21], niacin [22], fenofibrate [23], and gemfibrozil [24]. The cholesteryl ester transfer protein (CETP) inhibitor dalcetrapib (no longer in development) was shown in phase II testing to increase Lp-PLA2 mass by approximately 17% as compared with placebo [25]. Since Lp-PLA2 is partly bound to HDL cholesterol, this effect may be perhaps explained by the marked rise in HDL cholesterol that is observed with CETP inhibitors. However, it remains unknown whether this effect on Lp-PLA2 is a class effect or if it is specific to dalcetrapib.
Unlike these lipid-modifying agents, darapladib is an orally active and reversible direct inhibitor of Lp-PLA2 enzyme activity. Although other direct inhibitors of Lp-PLA2 are in development, darapladib is the only direct Lp-PLA2 inhibitor in phase III testing. In pre-clinical studies in diabetic and hypercholesterolemic pigs, darapladib reduced the necrotic core area and medial destruction, resulting in fewer lesions with an unstable phenotype [7]. Importantly, darapladib inhibited Lp-PLA2 activity both in plasma and directly within atherosclerotic plaques, including a corresponding reduction in intra-plaque lysoPC. Darapladib also led to a downregulation of inflammatory gene expression, including 24 genes associated with T-lymphocyte and macrophage functioning. Expression of monocyte chemoattractant protein-1 (MCP-1) chemokine receptor CCR2, a marker of a subset of pro-inflammatory macrophages (M1 subtype) that is known to accumulate in atherosclerotic lesions, was also reduced. As expected from these gene expression findings, plaque macrophage content was reduced with darapladib. Darapladib did not modify plasma lipid levels, providing evidence that inhibition of inflammation without an effect on cholesterol concentration could diminish inflammation and reduce development of unstable atherosclerotic lesions.
Daraplapib: Clinical Studies
In a dose-ranging phase II study of patients with stable CHD on a background of atorvastatin (20 or 80 mg daily), darapladib 160 mg daily led to sustained inhibition of Lp-PLA2 activity by an average of 66% during 12 weeks of treatment, regardless of baseline lipid levels [26]. In this study, darapladib reduced interleukin-6, a marker of inflammation by 12.3%, but did not influence C-reactive protein or lipoprotein concentrations. Although Lp-PLA2 was originally named based on its ability to hydrolyze platelet-activating factor, inhibition of the Lp-PLA2 enzyme has not been shown to have any effect on platelet function.
The Integrated Biomarker and Imaging Study-2 (IBIS-2) trial was a randomized, double-blind, placebo-controlled phase II trial of darapladib 160 mg daily in high-risk patients with CHD [27]. All patients were to undergo intravascular ultrasound with additional assessment by virtual histology (IVUS-VH) at baseline and after 12 months. As part of the study design, subjects were treated with a background of intensive statin therapy. Consistent with the results from the dose-ranging study, darapladib reduced Lp-PLA2 activity by an average of 59% and did not reduce C-reactive protein concentration. At the end of 12 months, darapladib halted expansion of the plaque’s necrotic core, whereas the necrotic core had expanded in placebo-treated patients despite the background of statin therapy. Darapladib did not reduce the primary endpoint of total atheroma volume when compared with placebo [27]. In phase II testing, darapladib was well tolerated except for a higher incidence of diarrhea, dysgeusia (distortion of taste sensation), and malodor of feces and urine [26, 27]. Together, these pre-clinical and clinical studies support the concept of Lp-PLA2 inhibition as a therapeutic target for patients with atherosclerosis.
Currently, the efficacy and safety of darapladib are being evaluated in two large-scale, multicenter, double-blind, placebo-controlled randomized phase III clinical trials in subjects with stable and unstable coronary disease (Table 1). The STABILITY (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY, ClinicalTrials.gov identifier: NCT00799903) trial is evaluating the efficacy and safety of darapladib (160 mg daily) in more than 15,800 subjects with stable coronary disease on a background of evidence-based therapy [28]. The Stabilization Of pLaques usIng Darapladib-Thrombolysis in Myocardial Infarction 52 (SOLID-TIMI 52, ClinicalTrials.gov identifier: NCT 01000727) study is evaluating the efficacy and safety of darapladib (160 mg daily) in more than 13,000 subjects who were enrolled within 30 days of hospitalization for an acute coronary syndrome, including unstable angina, non-ST-elevation myocardial infarction (MI) and ST-elevation MI [29]. Both trials are event-driven with anticipated median treatment duration between 2 and 3 years. Both trials have completed enrollment and the topline trial results are anticipated in 2014. Together, these trials will directly test the hypothesis of whether Lp-PLA2 plays a causal role in atherogenesis and is, therefore, a valuable therapeutic target in patients with stable and unstable atherosclerotic disease.
Table 1.
Ongoing phase III trials of darapladib
| Name | STABILITY | SOLID-TIMI 52 |
|---|---|---|
| Compound and dose | Darapladib 160 mg daily | Darapladib 160 mg daily |
| Subjects randomized | ~15,828 | ~13,027 |
| Trial design | Randomized, placebo-controlled, double-blind, parallel group, event-driven trial | Randomized, placebo-controlled, double-blind, parallel group, event-driven trial |
| Population |
Stable coronary disease: (1) Prior MI >1 month prior to randomization and/or (2) Prior coronary revascularization (PCI >1 month and CABG >3 month) and/or (3) Documented multivessel CAD; |
Early Post ACS: ≤30 days post-ACS following hospitalization with confirmed UA, NSTEMI, or STEMI; |
| AND | ||
| and at least one additional high-risk predictor | ||
| AND | ||
| and at least one additional high-risk predictor | ||
| Background therapy | Optimized background therapy | Optimized background therapy |
| Primary endpoint | CV death, non-fatal MI, or non-fatal stroke | CV death, non-fatal MI, or non-fatal stroke |
| Target number of primary endpoint events (n) | 1,500 | 1,500 |
| Median treatment duration | 2–3 years | 2–3 years |
| Results expected | 2014 | 2014 |
ACS acute coronary syndrome, CAD coronary artery disease, CABG coronary artery bypass graft surgery, CV cardiovascular, MI myocardial infarction, NSTEMI non ST-elevation myocardial infarction, STEMI ST-elevation myocardial infarction, UA unstable angina
Conclusion
Inflammation plays a central role in the development of atherosclerosis and the Lp-PLA2 enzyme is hypothesized to play a causal role in its pathogenesis. Epidemiological and genetic data now support the concept that Lp-PLA2 may indeed be a risk factor for the progression of atherosclerotic disease. Darapladib is a selective inhibitor of the Lp-PLA2 enzyme that is now being evaluated in two large-scale phase III clinical trials. The results of these trials will test whether direct inhibition of Lp-PLA2 is useful for halting the progression of atherosclerosis and will provide valuable insights into the underlying pathobiology of atherothrombotic events and plaque rupture, in addition to evaluating the therapeutic utility of Lp-PLA2 inhibition.
Acknowledgments
Dr. O’Donoghue is the guarantor for this paper and takes responsibility for the integrity of the work as a whole. No funding was received for the publication of this article.
Conflict of interest
Dr. O’Donoghue has received grant funding from GlaxoSmithKline, Genzyme and AstraZeneca. She has received consulting fees from Aegerion. Dr. Steen reports no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Mallat Z, Lambeau G, Tedgui A. Lipoprotein-associated and secreted phospholipases A(2) in cardiovascular disease: roles as biological effectors and biomarkers. Circulation. 2010;122:2183–2200. doi: 10.1161/CIRCULATIONAHA.110.936393. [DOI] [PubMed] [Google Scholar]
- 2.Rosenson RS, Stafforini DM. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J Lipid Res. 2012;53:1767–1782. doi: 10.1194/jlr.R024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW. A prospective natural-history study of coronary atherosclerosis. NEJM. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 5.Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, Virmani R. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 6.Hakkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, Rice SQ, Tew DG, Karkola K, Yla-Herttuala S. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–2917. doi: 10.1161/01.ATV.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 7.Wilensky RL, Shi Y, Mohler ER, 3rd, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, Pelchovitz DJ, Yang J, Mirabile RC, Webb CL, Zhang L, Zhang P, Gelb MH, Walker MC, Zalewski A, Macphee CH. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008;14:1059–1066. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannheim D, Herrmann J, Versari D, Gossl M, Meyer FB, McConnell JP, Lerman LO, Lerman A. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke. 2008;39:1448–1455. doi: 10.1161/STROKEAHA.107.503193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrmann J, Mannheim D, Wohlert C, Versari D, Meyer FB, McConnell JP, Gossl M, Lerman LO, Lerman A. Expression of lipoprotein-associated phospholipase A(2) in carotid artery plaques predicts long-term cardiac outcome. Eur Heart J. 2009;30:2930–2938. doi: 10.1093/eurheartj/ehp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donoghue M, Morrow DA, Sabatine MS, Murphy SA, McCabe CH, Cannon CP, Braunwald E. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation. 2006;113:1745–1752. doi: 10.1161/CIRCULATIONAHA.105.612630. [DOI] [PubMed] [Google Scholar]
- 11.Packard CJ, O’Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. NEJM. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 12.Persson M, Berglund G, Nelson JJ, Hedblad B. Lp-PLA2 activity and mass are associated with increased incidence of ischemic stroke: a population-based cohort study from Malmo, Sweden. Atherosclerosis. 2008;200:191–198. doi: 10.1016/j.atherosclerosis.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol. 2001;38:1302–1306. doi: 10.1016/S0735-1097(01)01554-6. [DOI] [PubMed] [Google Scholar]
- 14.Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis. 2000;150:413–419. doi: 10.1016/S0021-9150(99)00406-2. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 16.Oldgren J, James SK, Siegbahn A, Wallentin L. Lipoprotein-associated phospholipase A2 does not predict mortality or new ischaemic events in acute coronary syndrome patients. Eur Heart J. 2007;28:699–704. doi: 10.1093/eurheartj/ehl565. [DOI] [PubMed] [Google Scholar]
- 17.Sabatine MS, Morrow DA, O’Donoghue M, Jablonksi KA, Rice MM, Solomon S, Rosenberg Y, Domanski MJ, Hsia J. Prognostic utility of lipoprotein-associated phospholipase A2 for cardiovascular outcomes in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2007;27:2463–2469. doi: 10.1161/ATVBAHA.107.151670. [DOI] [PubMed] [Google Scholar]
- 18.Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, Ballantyne C, Cannon CP, Criqui M, Cushman M, Hofman A, Packard C, Thompson SG, Collins R, Danesh J. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang Y, Waterworth D, Lee J-E, Song K, Kim S, Kim H-S, Park KW, Cho K-J, Oh I-Y, Park JE, Lee B-S, Ku HJ, Shin D-J, Lee JH, Jee SH, Han B-G, Jang H-Y, Cho E-Y, Vallance P, Whittaker J, Cardon L, Mooser V. Carriage of the V279F null allele within the gene encoding Lp-PLA2 is protective from coronary artery disease in South Korean males. PLoS ONE. 2011;6:e18208. doi: 10.1371/journal.pone.0018208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, MacFadyen JG, Wolfert RL, Koenig W. Relationship of lipoprotein-associated phospholipase A(2) mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin Chem. 2012;58:877–886. doi: 10.1373/clinchem.2011.180281. [DOI] [PubMed] [Google Scholar]
- 21.Albert MA, Glynn RJ, Wolfert RL, Ridker PM. The effect of statin therapy on lipoprotein associated phospholipase A2 levels. Atherosclerosis. 2005;182:193–198. doi: 10.1016/j.atherosclerosis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Kuvin JT, Dave DM, Sliney KA, Mooney P, Patel AR, Kimmelstiel CD, Karas RH. Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am J Cardiol. 2006;98:743–745. doi: 10.1016/j.amjcard.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Filippatos TD, Gazi IF, Liberopoulos EN, Athyros VG, Elisaf MS, Tselepis AD, Kiortsis DN. The effect of orlistat and fenofibrate, alone or in combination, on small dense LDL and lipoprotein-associated phospholipase A2 in obese patients with metabolic syndrome. Atherosclerosis. 2007;193:428–437. doi: 10.1016/j.atherosclerosis.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Robins SJ, Collins D, Nelson JJ, Bloomfield HE, Asztalos BF. Cardiovascular events with increased lipoprotein-associated phospholipase A(2) and low high-density lipoprotein-cholesterol: the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol. 2008;28:1172–1178. doi: 10.1161/ATVBAHA.107.160739. [DOI] [PubMed] [Google Scholar]
- 25.Luscher TF, Taddei S, Kaski JC, Jukema JW, Kallend D, Munzel T, Kastelein JJ, Deanfield JE. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J. 2012;33:857–865. doi: 10.1093/eurheartj/ehs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohler ER, 3rd, Ballantyne CM, Davidson MH, Hanefeld M, Ruilope LM, Johnson JL, Zalewski A. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008;51:1632–1641. doi: 10.1016/j.jacc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 27.Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Botker HE, von Birgelen C, D’Amico D, Hutchinson T, Zambanini A, Mastik F, van Es GA, van der Steen AF, Vince DG, Ganz P, Hamm CW, Wijns W, Zalewski A. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 28.White H, Held C, Stewart R, Watson D, Harrington R, Budaj A, Steg PG, Cannon CP, Krug-Gourley S, Wittes J, Trivedi T, Tarka E, Wallentin L. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with coronary heart disease. Am Heart J. 2010;160:655–661. doi: 10.1016/j.ahj.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 29.O’Donoghue ML, Braunwald E, White HD, Serruys P, Steg PG, Hochman J, Maggioni AP, Bode C, Weaver D, Johnson JL, Cicconetti G, Lukas MA, Tarka E, Cannon CP. Study design and rationale for the Stabilization of pLaques usIng Darapladib-Thrombolysis in Myocardial Infarction (SOLID-TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J. 2011;162(613–9):e1. doi: 10.1016/j.ahj.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Motiwala S, O’Donoghue ML. Emergence of phospholipase A2 enzymes as a therapeutic target. In: Bonow RO, et al. Braunwald’s heart disease (2011, online edition).