An association between hypertension and immune system activation has long been recognized as a potential mechanism of hypertensive end-organ damage. Indeed, recent accumulating evidence using mouse models of hypertension induced by angiotensin II (Ang II) supports the roles of T lymphocytes and macrophages in the pathogenesis of hypertension and its complications 1. Curiously, while neutrophil infiltration appears to be the earliest immune and inflammatory response observable with Ang II infusion, limited findings are available regarding the pathophysiological significance of neutrophil accumulation and its signaling mechanism in cardiovascular diseases. Neutrophil-derived myeloperoxidase was shown to be critical for atrial fibrosis and subsequent atrial fibrillation induced by Ang II, which involves HOCl-induced tyrosine chlorination and activation of matrix metalloprotease-9 2. Likewise neutrophil generated matrix metalloprotease-9 is required for aortic dissection induced by co-treatment with Ang II and a lysyl oxidase inhibitor. Thus, depletion of neutrophils with Gr-1 antibody prevented the dissection 3. However, whether neutrophils and their signal transduction play a role in Ang II-induced hypertensive organ damage had not been investigated.
In this issue of Hypertension, Wu et al 4 provide the first compelling evidence supporting that neutrophil-generated S100a8/S100a9 proteins are the key molecules to initiate Ang II-induced cardiac inflammation and fibrosis independently from high blood pressure response. Consistent with the immediate and transient nature of neutrophil activation and infiltration, S100a8/S100a9 mRNAs and proteins demonstrated a sharp rise and fall in CD11b+Gr1+ neutrophils infiltrating the heart upon Ang II infusion. In vitro analysis also showed acute and dominant induction of S100a8 and S100a9 mRNA expression by Ang II in bone marrow-derived monocytes, but was relatively lower in cardiac myocytes and cardiac fibroblasts. S100a8 (calgranulin A or migration inhibitory factor-related protein 8; MRP-8) and its binding partner S100a9 (calgranulin B, or MRP-14) are members of the S100 calcium-binding family of proteins primarily expressed in myeloid cells such as neutrophils and monocytes. These proteins show increased levels in a number of inflammatory states and have been characterized as significant immune regulatory multifunctional molecules through their intracellular and extracellular actions 5. They are also recognized as a potential biomarker for the pathophysiological condition including various types of cardiovascular diseases 5. The intracellular functions of S100a8 include scavenging reactive oxygen species generated by activated neutrophils whereas the S100a8/S100a9 complex contributes to NADPH oxidase activation in phagocytes 6. The extracellular functions appear to be mediated through membrane receptors including Toll-like receptor 4 (TLR4) and the receptor for advanced glycation end products (RAGE) 5, 6. The authors’ findings further suggest that RAGE, which activates nuclear factor-kB in cardiac fibroblasts, is the critical receptor for neutrophil-derived S100a8/S100a9 in mediating cardiac inflammation and subsequent fibrosis upon Ang II infusion.
The involvement of S100a8/S100a9 in mediating cardiovascular inflammation is supported by several past studies (reviewed in 5). For example, S100a9 gene deficiency in mice, which also depresses S100a8 expression, has reduced leukocyte accumulation, vascular inflammatory responses and neointima formation in femoral artery upon wire injury. Deletion of S100a9 also protected apolipoprotein E −/− mice from atherosclerosis, which was associated with less accumulation of macrophages in the plaques. Cardiac overexpression of S100a8 and S100a9 led to RAGE-dependent suppression of calcium flux and decreased ejection fraction in mice, whereas S100a9 knockdown attenuated lipopolysaccharide-induced cardiac dysfunction. The crucial role of RAGE in cardiovascular inflammation was also demonstrated by a recent study in which formation of atherosclerosis and associated vascular inflammation in apolipoprotein E−/− mice were attenuated by RAGE-deficiency or dominant-negative RAGE expression 7.
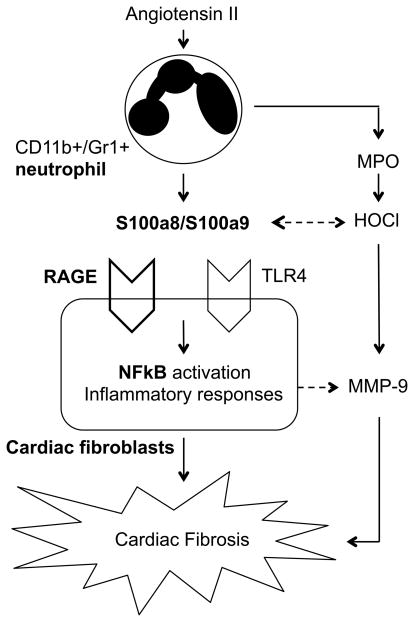
Interestingly, utilizing the same mouse model with Ang II infusion, the authors’ group has also demonstrated the crucial role of granulocyte colony stimulating factor as a key mediator for Ang II-induced recruitment of neutrophils into the heart and cardiac fibrosis 8. However, the cell type responsible for granulocyte colony stimulating factor production as well as its relation to the neutrophil S100a8/S100a9 system has not been addressed. While accumulating evidence strongly implicates participation of TLR4 in inflammatory responses associated with atherosclerosis as well as ischemic heart diseases 9, potential S100a8/S100a9 signaling via TLR4 in cardiac fibrosis remains obscure. S100a8/S100a9 synergistically stimulate HOCl production with myeloperoxidase. HOCl modified albumin is a high affinity agonist for RAGE 10. HOCl-modified S100a8/S100a9 proteins are also implicated in atherogenesis. Schematic diagram of the potential signal transduction stimulated by Ang II in neutrophil and cardiac fibroblast is illustrated in Figure 1.
Figure 1.
Schematic diagram of neutrophil derived S100a8/S100a9 protein potentially contributing hypertensive cardiac fibrosis induced by Ang II. Induction and secretion of S100a8/a9 from activated neutrophils plays a central role in triggering NFkB pathway and subsequent inflammatory responses in cardiac fibroblasts via RAGE. Blockade of S100a8/S100a9 function by anti-S100a9 antibody attenuates the inflammatory response and cardiac fibrosis. MPO indicates myeloperoxidase; RAGE, receptor for advanced glycation end products; TLR-4, Toll-like receptor 4; NFkB, nuclear factor-kB; and MMP-9, matrix metalloprotease-9.
Taken together, the above information and the study by Wu et al. provide new insight into the neutrophil specific mechanism leading to hypertensive end-organ damage such as that occurring in the heart independently from high blood pressure regulation. However, occasional anti-inflammatory roles of the S100a8/S100a9 oppose the use of anti-S100a8/S100a9 therapy against cardiovascular diseases in general. Clinical studies to look for correlations of S100a8/S100a9 with hypertension, the risk factors, incidence of complication and mortality with or without the usage of Ang II blockers seem to be critical. Limitations of the study include the lack of exploration into the intracellular mechanism by which Ang II enhances S100a8/S100a9 gene expression as well as the molecular link between secretion of S100a8/S100a9 by neutrophils and the ability of S100a8/S100a9 to act as autocrine factors for neutrophil infiltration. Therefore, further research is desired to look for the detailed molecular mechanisms by which neutrophil-derived S100a8/S100a9 participates in cardiac inflammation and fibrosis.
Acknowledgments
Sources of Funding: This work is supported in part by the National Institutes of Health (HL076770 to S.E. and DK096521 to R.S.) and the American Heart Association (13GRNT17060036 to S.E.).
Footnotes
Disclosures: None.
References
- 1.Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension. 2013;62:226–230. doi: 10.1161/HYPERTENSIONAHA.113.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph V, Andrie RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, Schwoerer AP, Lau D, Fu X, Klingel K, Sydow K, Didie M, Seniuk A, von Leitner EC, Szoecs K, Schrickel JW, Treede H, Wenzel U, Lewalter T, Nickenig G, Zimmermann WH, Meinertz T, Boger RH, Reichenspurner H, Freeman BA, Eschenhagen T, Ehmke H, Hazen SL, Willems S, Baldus S. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010;16:470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, Itoh H, Hori S, Aikawa N, Okada Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126:3070–3080. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 4.Wu YLY, Zhang CAX, Wang Y, Cui W, Li H, Du J. S100A8/A9 released by CD11B+GR1+ neutrophils activates cardiac fibroblasts to initiate angiotensin ii-induced cardiac inflammation and injury. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.113.02843. [DOI] [PubMed] [Google Scholar]
- 5.Averill MM, Kerkhoff C, Bornfeldt KE. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol. 2012;32:223–229. doi: 10.1161/ATVBAHA.111.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geczy CL, Chung YM, Hiroshima Y. Calgranulins may contribute vascular protection in atherogenesis. Circ J. 2014;78:271–280. doi: 10.1253/circj.cj-13-1505. [DOI] [PubMed] [Google Scholar]
- 7.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang HM, Wang HX, Yang H, Zeng XJ, Tang CS, Du J, Li HH. Role for granulocyte colony stimulating factor in angiotensin II-induced neutrophil recruitment and cardiac fibrosis in mice. Am J Hypertens. 2013;26:1224–1233. doi: 10.1093/ajh/hpt095. [DOI] [PubMed] [Google Scholar]
- 9.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsche G, Semlitsch M, Hammer A, Frank S, Weigle B, Demling N, Schmidt K, Windischhofer W, Waeg G, Sattler W, Malle E. Hypochlorite-modified albumin colocalizes with RAGE in the artery wall and promotes MCP-1 expression via the RAGE-Erk1/2 MAP-kinase pathway. FASEB J. 2007;21:1145–1152. doi: 10.1096/fj.06-7439com. [DOI] [PMC free article] [PubMed] [Google Scholar]