Abstract
We investigated the distribution of gamma aminobutyric acid, tyrosine hydroxylase and nitric oxide-producing elements in a cherry salmon Oncorhynchus masou brain at various stages of postnatal ontogenesis by immunohistochemical staining and histochemical staining. The periventricular region cells exhibited the morphology of neurons and glia including radial glia-like cells and contained several neurochemical substances. Heterogeneous populations of tyrosine hydroxylase-, gamma aminobutyric acid-immunoreactive, as well as nicotinamide adenine dinucleotide phosphate diaphorase-positive cells were observed in proliferating cell nuclear antigen-immunoreactive proliferative zones in periventricular area of diencephalon, central grey layer of dorsomedial tegmentum, medulla and spinal cord. Immunolocalization of Pax6 in the cherry salmon brain revealed a neuromeric construction of the brain at various stages of postnatal ontogenesis, and this was confirmed by tyrosine hydroxylase and gamma aminobutyric acid labeling.
Keywords: neural regeneration, neurogenesis, teleostei, adult neurogenesis, neurotransmitter signaling, migration, tyrosine hydroxylase, gamma aminobutyric acid, development, Pax6, NADPH-diaphorase, proliferation, grant-supported paper, neuroregeneration
Research Highlights
(1) Distribution features of nitric oxide and classical neuromediators γ-aminobutyric acid and catecholamines are closely associated with the ability of the fish brain to grow during the entire lifespan.
(2) Nitric oxide and classical neuromediators γ-aminobutyric acid and catecholamines not only regulate the functional activity of neurons and modulate the synaptic transmission in mature neural networks, but also are regarded as the inductors of fish brain development (morphogenetic factors) in postembryonic ontogenesis.
Abbreviations
RG, radial glial cells; GFAP, glial fibrillary acidic protein; TH, tyrosine hydroxylase; GABA, gamma-aminobutyric acid; NADPH-d, nicotinamide adenine dinucleotide phosphate diaphorase; PVZ, periventricular zone; PCNA, proliferating cell nuclear antigen; SVZ, subventricular zone
INTRODUCTION
The presence of proliferating periventricular regions has been demonstrated in various fish species, including a neurogenetic model Danio rerio[1]. A recent study conducted in the zebra fish Danio rerio[2], has shown that the newly generated cells migrate from the ventricle to the deep brain where they differentiate into neurons.
It is interesting to investigate this process in a fish species, taking into account the widely known fact that a lot of radial glial cells (RG) persist in a fish brain during adulthood, which ensures the capacity for brain development throughout the whole life[3]. In spite of a certain data present in the literature, the role of RG in a neurogenesis process in adult animal remains poorly understood. This is possibly because of absent suitable markers of RG in lower vertebrates.
Immunohistochemical markers of astrocytic glial cells in mammals, such as glial fibrillary acidic protein (GFAP), vimentin (V) or protein S-100, label merely the fish RG, and the staining is limited to the cell body or its long processes[4]. Our previous study has shown that tyrosine hydroxylase (TH)-immunoreactive cells with long radial outgrowths localize along the 3rd ventricular lumen and in the caudal region of the brain of cherry salmon, which is different from other fish species[5]. We also acquired similar results from gamma-aminobutyric acid (GABA) immunolabeling and nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) histochemical labeling[5,6]. The aim of this study is to investigate the distribution of several neurochemical markers and their changes during adult neurogenesis.
RESULTS
Feature of distribution of GABA, TH, NADPH-d and Pax6 labeling in 3-month-old, 6-month-old and 1-year-old cherry salmon brain
Detection of GABA, TH, NADPH-d and Pax6 in the cherry salmon brain has revealed the cells in the periventricular zone (PVZ) of different areas of the brain. The results of the brain investigations in various age groups of cherry salmon have shown that GABA, TH and Pax6 selectively label the cells and radial fibers in the diencephalon, tectum, cerebellum, medulla oblongata and spinal cord. The distribution patterns of these immunomarkers correspond to a neuromeric organization of the salmon brain. On the borders of the first three prosomers, including pretectum (P1), dorsal thalamus (P2), ventral thalamus (P3) and medullar rhombomers, the GABA, TH and Pax6 labeling was not detected.
TH served as an immunohistochemical marker of RG-like cells in the spinal cord of 1-year-old young cherry salmon (Figures 1A–C); it labels some cells near the central channel and radially oriented fibers that formed the so-called “end feet” on the periphery of the spinal cord, which is a characteristic of this cell type (Figures 1A–C).
Figure 1.
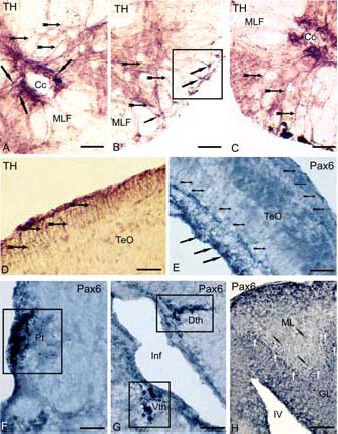
Expression of tyrosine hydroxylase (TH) and transcription factor (Pax6) in the brain of cherry salmon Oncorhynchus masou (immunoperoxidase staining, light microscopy) at different years of age.
(A) Immunolocalization of TH in a spinal cord of a 1-year-old cherry salmon, the arrows show radial glia (RG) cell bodies, localized near a central channel, RG fibers are shown by small arrows on B and C, square marks the sites of fibers with “end feet”, signed by black arrows on B.
(D) TH-immunoreactive RG cell bodies in the superficial layers of the tectum in a 3-month-old cherry salmon (black arrows), and the fibers are shown by small arrows.
(E) Pax6 in tectum of a 6-month-old cherry salmon, the bodies of immunoreactive cells of periventricular layer are shown (solid arrows), the migrated cell bodies (arrows with a cut), the RG fibers (arrow shapes); Pax6- immunoreactive elements in the pretectum on F, thalamus on G and in the cerebellum on H of a 1-year-old cherry salmon, square marks the accumulations of immunoreactive cells, corresponding to pretectal (Pt), dorsal (Dth) and ventral (Vth) thalamic (P1–P3) prosomers; the bodies of Pax6-immunoreactive cells are shown by white arrows, the bodies of migrated cells are shown by black arrows with a cut.
Scale bars: 50 μm for A–D, F; 100 μm for E, G, H. Cc: Central channel, MLF: medial longitudinal fascicle, TeO: optical tectum, Pt: pretectum, Inf: infundibulum; Dth: dorsal thalamus, Vth: ventral thalamus, ML: molecular layer, GL: granular layer, IV: the fourth ventricle.
Immunohistochemical labeling of transcription factors Pax6 and TH has shown the presence of Pax6- and TH-immunoreactive cells with radial glial characteristics in the superficial layer of the tectum in 3-month-old and 6-month-old young fish as well as in the periventricular and central fibrous layers (Figures 1D, E). The Pax6 labeling in 1-year-old cherry salmon has allowed us to identify P1–P3 prosomers (Figures 1F, G). In the cerebellum of a 1-year-old cherry salmon, Pax6- immunoreactive radial fibers were present in the Purkinje cell layer and molecular layer, with a majority of Pax6-immunoreactive cells in the Purkinje cell layer (Figure 1H). The highest density of GABA-immunoreactive fibers and cell bodies appeared in the diencephalon of the 6-month-old cherry salmon.
Immunolocalization of TH
In the 3-year-old cherry salmon specimen, a TH-labeled population of cells with radially oriented processes was revealed in zona limitans – the border, separating the pallial part of the telencephalon and the subpallial part (Figures 2A, B). In the periventricular areas of the diencephalon, TH-labeled cells with radial glial characteristics were localized along the infundibulum; and in the dorsal region of the diencephalon, TH-labeled similar cells were localized in the territory of the pretectum, dorsal and ventromedial thalamic nuclei (Figure 2C), the anterior part of the paraventricular organ (Figure 2D), and in the ventral region, such TH-labeled cells were found in the parvocellular part of the preoptic nucleus (Figure 2E) and in the anterior tuberal nucleus. In the diencephalon, TH labeling revealed the borders of prosomers (Figure 2F). In the cherry salmon brain medulla, TH-labeled cells with their long processes around the medial longitudinal fascicle (Figure 2G) and in the ventromedial medullar segment (Figure 2H) were observed. The largest population of the TH-labeled cells with long radial processes in the medulla of cherry salmon was present in the interfascicular area (Figure 2G). In the caudal part, TH-labeled cells with a similar morphology were detected in the subventricular area in the territory of the nucleui of IX–X nerves (Figure 2G). Immunoreactive cells with radial glial characteristics were present in the medial and lateral regions of the area postrema (Figure 2I) and in the interfascicular area of the spinal cord.
Figure 2.
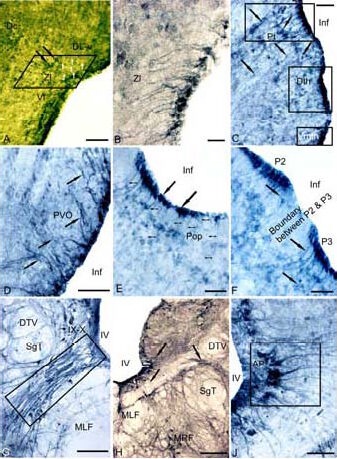
Expression of tyrosine hydroxylase (TH) in the 3-year-old cherry salmon Oncorhynchus masou brain (immunoperoxidase staining, light microscopy).
(A) Immunoreactive cells and fibers (white arrows) of radial glia (RG) in zona limitans (delineated by parallelepiped). (B) RG under large magnification. (C) Periventricular region of dorsal thalamus (rectangles) delineates the pretectal region (Pt), dorsal thalamic nuclei (Dth) and ventromedial thalamic nuclei (Vmth), the RG fibers are shown by black arrows, the migrating cells are shown by arrows with a cut.
(D) Paraventricular organ (PVO), (E) parvocellular preoptic area (Pop), thin arrows show the RG fibers, black solid arrows indicate periventricular bodies of TH- immunoreactive cells. (F) A border between dorsal forebrain neuromers P2 and P3. (G) Interfascicular segment of the medulla (delineated by rectangle). (H) Ventromedial medullar segment. (I) RG in ventro-medial region of area postrema (delineated by the rectangle).
Scale bars: 100 μm for A, D, G; 20 μm for B; 50 μm for C, E, F, I; 200 μm for H. DL-V: Ventral segment of dorso-lateral region; Zl: zona limitans; Vl: ventro-lateral region; Inf: infundibulum; DTV: descending pathway of N. trigeminus; SgT: secondary gustatory tract; IX–X: nuclei of glossopharyngeal nerve and vagus; MLF: medial longitudinal fascicle; MRF: medial reticular formation; IV: the fourth ventricle; AP: area postrema.
Immunolocalization of GABA
Immunolocalization of GABA in the area of the diencephalon in 3-year-old salmon is similar to distribution of TH. The GABA-immunoreactive cells with radial glial characteristics were present along the infundibular lumen, in the area of magnocellular and parvocellular parts of the preoptic nucleus (Figure 3A), dorsal thalamic nuclei (Figure 3B), paraventricular organ and anterior tuberal nucleus (Figure 3C), dorsal and ventral thalamus and periventricular pretectum (Figure 3D).
Figure 3.
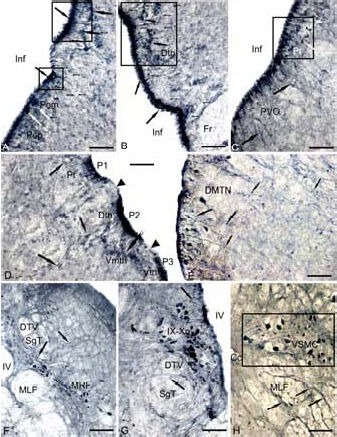
Expression of gamma amino butyric acid (GABA) in the 3-year-old cherry salmon Oncorhynchus masou brain (immunoperoxidase staining, light microscopy).
(A) Clusters of immunoreactive radial glia (RG) (delineated by squares) in preoptical region, the neurons of a magnocellular nucleus (Pom) are shown by white arrows, the periventricular cells bodies by black arrows, RG fibers by a figure like arrows, and the migrated cells by arrows with a cut.
(B) RG in a region of dorsal thalamus. (C) Paraventricular organ, the cells of anterior tuberal nucleus (delineated by a square) are indicated by white arrows. (D) RG in the content of forebrain prosomers P1, P2 and P3 without GABA immunolabeling on the border (black edges of black arrows). RG fibers (black arrows) and migrated cells in the content of pretectum (Pt), dorsal thalamus (Dth) and ventro-medial nuclei of thalamus (Vmth) are shown by arrows with a cut, (E) RG in the content of dorsomedial tegmentum by black arrows, and white arrows for large neurons of DMTN. (F) RG in the interfascicular area (black arrows) and immunoreactive cells MRF (white arrows). (G) RG on the territory of nuclei of IX–X craniocerebral nerves. (H) RG on the territory of the spinal cord (neuronal bodies are shown by white arrows).
Scale bars: 100 μm for A–D, G, H; 200 μm for E, F. Fr: Fasciclus retroflexus; Inf: infundibulum; PVO: paraventricular organ; DMTN: dorsomedial tegmental nuclei; Vth: ventral thalamu; DTV: descending pathway of N. trigeminus, SgT: secondary gustatory tract, MLF: medial longitudinal fascicle; MRF: medial reticular formation; IV: the fourth ventricle; IX–Xn: nuclei of glossopharyngeal nerve and vagus; Cc: central channel; VSMC: ventral spinal motor column.
Immunolocalization of GABA in the periventricular area of the diencephalon had permitted to verify the borders of telencephalic prosomers (Figure 3D). In the mesencephalon, GABA-immunoreactive cells were revealed in the region of the dorsomedial tegmentum (Figure 3E) and torus semicircularis. In medulla, the GABA-immunoreactive elements with radial processes were present in the interfascicular area of reticular formation (Figure 3F), in a territory of the nuclei of nerves IX–X (Figure 3G), and in the dorsal part of the area postrema, among neurons of the ventral spinal motor column (Figure 3H).
Localization of NADPH-d
While histochemical labeling for NADPH-d in the brain of a 3-year-old cherry salmon, cells with a high activity of NADPH-d were present in the periventricular diencephalon, the central grey layer of the medulla, tectum, basal mesencephalon, and valvula cerebelli (Figure 4). A rostrally localized population of NADPH-d-positive cells was present in a parvocellular preoptic nucleus, composed of small neurons in the periventricular diencephalon (Figure 4A). A mesencephalic population of cells was detected in the area of basal tegmentum and superficial layers of the tectum (Figure 4B). In the cerebellum, NADPH-d-positive radial fibers were localized in the medial part of the valvula (Figure 4C). More caudally, NADPH-d-positive populations of cherry salmon glial cells were present in the subventricular region on a territory of the nuclei of V, VII, IX–X nerves (Figure 4D).
Figure 4.
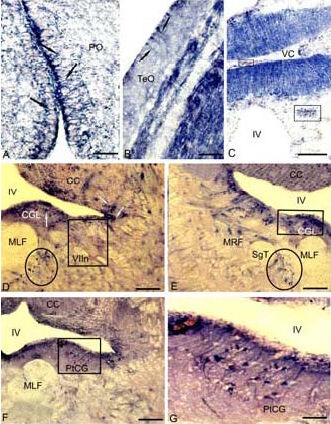
Localization of nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) in the 3-year-old cherry salmon Oncorhynchus masou brain (histochemical staining; light microscopy).
(A) Radial glia (RG) in a parvocellular preoptical region (black arrows), in superficial layers of tectum on B, in medial part of valvula cerebelli (VC) on C, complexes of vessels and perivascular glia are delineated by the rectangle.
(D) RG (white arrows) in a central grey layer (CGL) of subventricular region in the territory of nucleus of facial nerve (VIIn) (NADPH-d-negative nucleus VIIn is delineated by a rectangle), accumulation of RG adjacent to medial longitudinal fascicle (in an oval).
(E) RG clusters CGL in the territory of reticular formation, a cluster of NADPH-d-positive cells of RG (in a square). (F) RG in the territory of posttrigeminal group of a central grey layer (in a square). (G) Fragment inside a square in F on a large magnification.
Scale bars: 50 μm for A, G; 20 μm for B; 200 μm for C–F. PO: Preoptical region; CC: corpus cerebellum; MLF: medial longitudinal fascicle; MRF: medial reticular formation; IV: the fourth ventricle; SgT: secondary gustatory tract; PtCG: posttrigeminal group of central grey layer; TeO: tectum opticum.
In contrast to TH- and GABA-immunoreactive cells in these regions, NADPH-d-positive cells had processes of various lengths and some cells did not have any processes at all. Other population of NADPH-d-positive glial cells was present around a medial longitudinal fascicle (Figures 4D, E), in the territory of a medial reticular formation (Figure 4E) and a posttrigeminal cellular group of the central grey layer (Figures 4F, G).
Immunolocalization of Pax6
Labeling of transcription factor Pax6 in the 3-year-old cherry salmon brain differed essentially from immunolocalization of this marker in the 6-month-old specimens (Figure 5). Populations of Pax6-immunoreactive cells were present in the diencephalon and medulla of 6-month-old specimens; their distribution corresponded to that of dorsal telencephalic P2–P3 prosomers and rhombomers (Figures 5A, B). In adult cherry salmon, Pax6-immunoreactive cells were detected in the periventricular diencephalon in the region of dorsal and ventromedial thalamic nuclei and the anterior thalamic nucleus; and the radial fibers were present in the sites between dorsal and ventral parts of the thalamus (Figure 5C). More caudally, the similar fibers and Pax6-immunoreactive cells were present along the infundibular lumen within paraventricular organ and anterior tuberal nucleus (Figure 5D). On the border of P2 and P3 neuromers, there were Pax6-immunoreactive cells and long fibers with a small weakly marked Pax6 cells, distributed along them (Figure 5E). In the mesencephalon in the area of the dorsal tegmentum and mesencephalic central grey layer, we have observed a lot of small Pax6-immunoreactive cells and radially oriented thick fibers organized in bunches (Figure 5F), along which a multiple small Pax6-immunoreactive cells were scattered (Figure 5G). A majority of mesencephalic bunches of Pax6-immunoreactive fibers were directed towards a reticular formation region.
Figure 5.
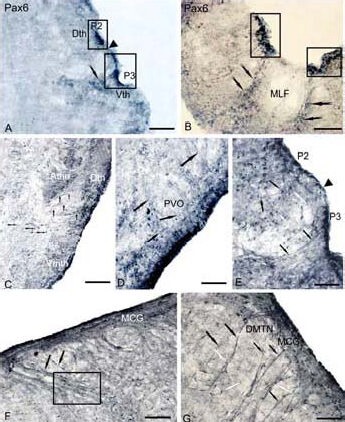
Expression of the transcription factor Pax6 in the brain of 6-month-old young (A, B) and 3-year-old (C–G) cherry salmon Oncorhynchus masou (immunoperoxidase staining, light microscopy).
Accumulations of immunoreactive cells in the diencephalon (A) and medulla (B). Part of the brain (in rectangles) labels its neuromer structure, the sites without immunolabeling constitute the borders of forebrain P2 and P3 prosomers (black edges of arrows), arrows with a cut show accumulations of migrating cells.
(C) Labeling of Pax6 in periventricular nuclei of thalamus, cells, with the absent processes (small figure like arrows), radial glia (RG) fibers (black arrows). (D) Immunoreactive cells (white arrows) and fibers of RG (black arrows) in the paraventricular organ. (E) Pax6-immunoreactive cells and RG fibers on the border of forebrain neuromers P2 and P3, the site without RG fibers is marked by a black arrow edge.
(F) Pax6-immunoreactive complexes in the region of mesencephalic central grey layer of dorsal tegmentum, fascicles of centripetal fibers of RG (black arrows) along which small Pax6-immunoreactive cells are scattered (delineated by a rectangle). (G) Pax6-immunoreactive complexes in the region of dorsomedial tegmental nuclei (DMTN), fascicles of RG fibers are shown by black arrows, the cells migrating along them are shown by white arrows.
Scale bars: 100 μm for A, D, E, G; 200 μm for B, C, F. MCG: Mesencephalic central grey layer, Athn: anterior thalamic nucleus; Dth: dorsal thalamic nuclei; Vmth: ventromedial thalamic nuclei; PVO: paraventricular organ.
Immunolocalization of proliferating cell nuclear antigen (PCNA)
The PCNA labeling in periventricular regions of the diencephalon and medulla oblongata of 1-year-old and adult specimens of cherry salmon revealed a vast population of proliferating cells (Figures 6A, C–E). On the level of the diencephalon and rhombencephalon, TH-immunoreactive cell distribution in 1-year-old specimens corresponded to the brain neuromeric construction, and this localization was confirmed also by the PCNA labeling of proliferative zones (Figures 6B, G). In the diencephalons, the proliferative PCNA-immunogenic zones, the localization corresponded to the prosomeric construction of the forebrain (Figure 6C). On the border between the dorsal prosomers P1–P3, the immunoreactive labeling of TH and PCNA was not expressed (Figures 6B–C). In the medulla of 1-year-old and adult cherry salmon, the PCNA-immunoreactive cells were localized in the bottom of fossa rhomboidea rostrally, on the level of the V–VII nerve pairs (Figures 6D, E). A similar TH immunolocalization was revealed in the cherry salmon specimens of the same age groups (Figures 6F–G).
Figure 6.
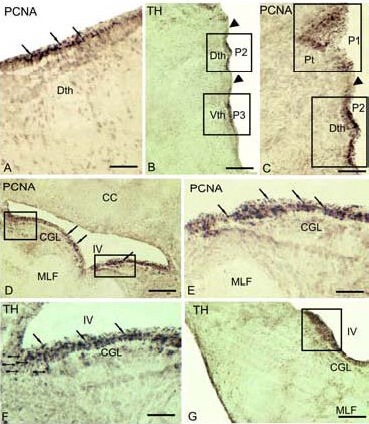
Expression of proliferating cell nuclear antigen (PCNA) and tyrosine hydroxylase (TH) in the brain cherry salmon Oncorhynchus masou of different age groups (immunoperoxidase staining; light microscopy).
(A) PCNA-immunoreactive cells (black arrows) in dorsal thalamus of 3-year-old cherry salmon. (B) Clusters of TH-immunoreactive cells of 1-year-old cherry salmon in the content of dorsal and ventral thalamic (Dth and Vth) neuromers (P2 and P3) divided by immunonegative zone (black edge of an arrow) (delineated by a rectangle).
(C) PCNA-immunoreactive pretectal (P1) and Dth neuromers (P2) of adult cherry salmon (delineated by a rectangle). (D) PCNA-immunoreactive proliferative zone (delineated by a rectangle) in the territory of central grey layer of medulla in 1-year-old cherry salmon.
(E) PCNA-immunoreactive cells in medulla of adult cherry salmon (shown by arrows). (F) TH-immunoreactive cells and fibers of RG (figure-like arrows) in the content of CGL of 1-year-old cherry salmon. (G) Accumulation of TH-ir cells (delineated by a square) in the caudal part of the medulla in 1-year-old cherry salmon.
Scale bars: 100 μm for A, C, E, F; 200 μm for B, D, G. CC: Corpus cerebellum; MLF: medial longitudinal fascicle; IV: the fourth ventricle; CGL: central grey layer; ir: immunoreactive.
DISCUSSION
Results from this study have demonstrated that the periventricular zone of the diencephalic, mesencephalic, medullar and spinal regions of the cherry salmon brain contains a cell population that are heterogenous in morphological and neurochemical respects; some of the cells have processes and are able to produce TH, GABA and NADPH-d.
The periventricular proliferative zones of fish brain were previously reported[1–2]. The transcription factor Pax6 was used as a marker of progenitor cells[7] and neurogenic niches in a cherry salmon (foci of postnatal neurogenesis in mammals[8-9]). We used a proliferative nuclear antigen as a marker of proliferating cells[10]. Newly generated cells migrated from the ventricle to the deep brain to differentiate into neurons and glia[4,11]. Results of this study have shown the presence of proliferative zones in the cherry salmon in different age brackets. In these zones situated in the diencephalon and the medulla oblongata, we observed the cells expressing TH, GABA and NADPH-d.
It has been demonstrated that neurotransmitter molecules exert a significant effect on the cell development during embryogenesis[12] and adult neurogenesis of the dentate gyrus subgranular zone in the medial and lower surfaces of the cerebral hemisphere[13–14]. Recently, there are the data that neurotransmitters regulate postnatal neurogenesis in the subventricular zone of the brain in various vertebrate animals[14-15]. Based on our results, it is supposed that TH, GABA and NADPH-d play a role of signaling molecules (or transducers) in the diencephalon and the medulla of the cherry salmon brain to regulate the process of adult neurogenesis. The subventricular zone (SVZ) in mammals develops from different cell types, including atrocyte-like cells[15]. During embryogenesis in mammals, GABA signaling provides a reciprocal relationship between astrocyte-like cells and neuroblasts[15]. It has been shown that GABA regulates the processes of postembryonic neurogenesis in the brain of adult mammals[14]. As to fishes, GABA participation in embryonic and early postembryonic development of the brain has been already reported[16], while its participation in the process of adult neurogenesis has not yet been described. In early ontogenesis, GABA depolarizes the newborn cells in the hippocampal dentate gyrus and moves the precursor cells because it has a high intracellular concentration of chloride ions[17]. An external GABA-induced depolarization and inflow of Ca2+ is necessary for the growth of dendrites and their elongation in postnatally generated olfactory interneurons[18]. From the genetic point of view, the switch of GABAergic signaling from depolarizing to hyperpolarizing by knockout of the chloride importer NKCC1 in predecessors of the hippocampal subgranular zone decreases dendritic arborization and arrests synapse formation[19].
It remains unknown whether GABAergic cells evolve in fish during postembryonic development. The development of GABAergic neurons during early ontogenesis has been earlier investigated in stickleback Gasterosteus aculeatus[16]. During late embryonic stages and early larval development, a number of GABAergic neurons in the stickleback brain inevitably arise to fill merely all the telencephalic nuclei. It has been shown in 1-month-old stickleback that GABA immunolocalization shifts from neuronal cell bodies and tracts towards the so-called “neuropyl staining”[16]. Our studies on 6-month-old and 1-year-old cherry salmon have revealed quite similar changes in GABA immunolocalization. There are a large number of GABAergic cells in the preoptic-hypothalamic periventricular region, thalamic and pretectal nuclei of the gold fish[20] and eel[21], like in the adult cherry salmon. It is interesting that in this species, like in the cherry salmon, the highest density of GABAergic cell distribution is revealed in the medial region, adjacent to the ventricle lumen. A previous study[16] reported that all GABAergic clusters revealed in the telencephalon of the stickleback after 101-hour development are also present in adult gold fish and eel. In the young and adult cherry salmons, GABA immunolocalization is revealed in cells of the periventricular zone and in radial fibers of the diencephalon, mesencephalic tegmentum, periventricular grey layer of the medulla, interfascicular area, nuclei of the IX–X craniocerebral nerve pairs, the area postrema and the ventral spinal cord column. Our results suggest that the regions of GABA immunolocalization may take part in the process of adult morphogenesis.
TH-labeled RG cells were detected in the diencephalon, medullar region, spinal cord and the tectum of 3-month, 6-month and 1-year-old cherry salmon fish. In the adult cherry salmon individuals, TH-labeled periventricular RG-like cells with radially oriented processes were detected in the diencephalon and medullar regions. TH labeling has revealed a neuromeric organization of the cherry salmon brain in all the age groups.
In adult cherry salmon, TH labeling was detected on the border which separates a pallial part of the telencephalon and the subpallial part. Our results regarding the expression of catecholamine synthesis enzymes in the RG cells corresponds to the data obtained on Danio rerio, where TH-immunoreactive RG was found in the telencephalon[22]. The TH- immunoreactive elements, such as radial glial cells, were localized in the rostral spinal cord, where the main neuromorphological signs of such a cell type, according to Rakic classification[23], are represented most distinctly. It has been shown that dopamine neuron precursors within the developing human mesencephalon show radial glial characteristics[24]. TH plays a role in catecholamine biosynthesis and morphogenesis in certain periods of ontogenesis, exerting a long-lasting effect on the differentiation of noncatecholaminic neurons and the expression of their specific phenotypes[25]. A possible mechanism underlying such an influence of TH is the presence of dopaminergic D1–D3 receptors in the cells-targets[26]. Using the methods of autoradiography and hybridization in situ, Diaz et al[27] have detected a high level of D3 receptors in the SVZ of embryos and adult animals. D2 receptors are revealed in transit-amplifying cells, while D1 and D2 receptors are found in neuroblasts[28]. Our present knowledge regarding distribution of dopamine receptor in the fish brain is limited to the data on D1 receptors and only for a single species[29]. The immunolocalization of TH in the telencephalic forebrain region of the fish brain differs substantially in mammals[30–32]. Therefore, we concluded that high TH content in periventricular cells with radial processes in the cherry salmon may be an alternative adaptation mechanism of the present unknown functional role. However, the presence of D1 receptors in paraventricular areas of eel[29], in our opinion, indicates that in these brain areas, containing proliferating cells, are regulated by dopamine. We suggest that catecholamines, which are excreted by TH- immunoreactive cells in the periventricular area, may indicate the presence of adult development of the cherry salmon brain.
Pax6 is considered to be a highly conservative transcription factor, playing an essential role in the process of CNS development in mammals, including formation of the neural tube, migration of neurons and creation of neuronal nets[9]. Neural stem cells express Pax6 at the initial stage of the CNS development as well as during the entire life period in the “neurogenetic niches”, areas of adult neurogenesis[9,33]. The Pax6 gene family is expressed mainly in cells of the mitotic ventricular zone of the cortex[34], where these genes execute a control function on the RG development and reproduction by asymmetric mitoses[33]. A detailed investigation and depiction of the patterns of Pax6 expression in embryogenesis of the zebrafish Danio rerio[31] have shown that a majority of telencephalic domains, expressing Pax6, correspond to the migration flow, depicted in the area of the pallial-subpallial border of amniotes. Nevertheless, the Pax6 expression in proliferating RG cells, localized periventricularly in the mouse pallium, including the isocortex[33], has not been detected in the developing pallium of the zebrafish[31].
Recently, it has been established that a Pax6 factor is expressed in the postnatal period by neurons of various regions of the brain, including the olfactory bulb, amygdala, thalamus and cerebellum[9]. A moderate expression of Pax6 has been revealed in the hippocampal subgranular zone[8] and in the ependymal layer of a subventricular zone of lateral ventricle[35], the mammalian brain regions, where neurogenesis persists during the entire life period. In the mammalian SVZ, the Pax6 factor is expressed in the neuronal stem/early progenitor cells, which have RG morphology and are specifically labeled by GFAP and nestin[8–9].
The results of Pax6 labeling of the cherry salmon brain have shown that this marker is expressed in early youngsters at the age of 3 and 6 months old, as well as in 1-year-old and adult animals. In 1-year-old cherry salmon, we have observed a specific labeling of periventricularly localized cells, creating clusters and domains. Distribution of forebrain Pax6-immunoreactive domains corresponds to localization of forebrain P1–P3 prosomers according to the accepted classification[35]. Our investigation of the late-age stages of the cherry salmon has revealed a specific accumulation of cells, with long radially oriented processes and the cells bodies localized near the brain ventricle lumen or along the immunoreactive fibers. The Pax6-immunoreactive complexes in the cherry salmon brain consisting of immunoreactive cells localized along the fibers and weakly labeled Pax6-cells belong, by our opinion, to the population of migrating neuroblasts. Thus, Pax6 labeling in the adult cherry salmon brain proves the presence of complexes, including periventricularly localized cells with long processes in subventricular regions of the diencephalon and mesencephalon; postmitotic immunonegative neuroblasts migrating along immunoreactive radial fibers; and Pax6-immunoreactive cells without processes.
Another neurochemical marker of the periventricular zone cells in the fish brain is NADPH-d, a histochemical marker of nitric oxide synthase, described primarily in the periventricular zone of the sunfish brain[36]. The description of NADPH-d-positive populations of periventricular cells in the adult cherry salmon brain confirms the participation of nitric oxide as a factor regulating postembryonic morphogenesis[14]. Nitric oxide regulates the processes of directed development of axons and dendrites, as well as migration of differentiating neurons[37]. Distribution of NADPH-d-positive periventricular cells in the cherry salmon brain is similar to localization of TH- and GABA-immunoreactive domains, but population of NADPH-d-positive cells is morphologically more heterogeneous.
Morphogenetic analysis and bromdesoxyuridine labeling have shown the presence of the individual neurons-predecessor cells among RG cells in the adult brain of the zebrafish[38]. So far, in the olfactory bulbs of adult representatives of Danio rerio, some cells were observed. They were generated by neuronal predecessors migrating from the telencephalic ventricular zone into the olfactory bulb within a rostral migration wave[22]. The migration of unripe neurons was depicted in turtle and various species of vertebrates, including primates[38].
The PCNA labeling in 1-year-old and adult cherry salmon fish has shown the presence of a vast population of proliferating cells in periventricular areas of the diencephalon and central grey layer of the medulla. Moreover, on the level of the telencephalon, distribution of TH-, GABA-, and Pax6-immunoreactive cells has labeled a neuromeric construction of the brain; this is confirmed by PCNA labeling of proliferative zones. On the border between the dorsal prosomers P2–P3, we have not detected the immunoreactive labeling with TH, GABA, Pax6 and PCNA. In the diencephalon, the localization of proliferating PCNA-immunogenic zones corresponds to the prosomeric construction of the forebrain. Thus, we have revealed several active PCNA-immunoreactive zones of proliferation, which include the TH, GABA and NADPH-d expressing cells. We suggest that these cells constitute newly born neurons, and this fact agrees well with the data obtained on Danio rerio[22]. This differs dramatically from the situation, which is observed in mammals, in whom, with aging, the zone of postnatal proliferation persists only in the ependymal layer of SVZ of the cerebral ventricles of the telecephalon and subgranular zone of the hyppocampus[23,39].
Thus, our study on the cherry salmon has demonstrated in fish for the first time the presence of heterogenous populations with radially oriented processes in the periventricular area of the diencephalon, in the central grey layer of the dorsomedian tegmentum, in the medulla oblongata and the spinal cord. TH-, GABA- immunoreactive and NADPH-d-positive cells are located in the PCNA-immunogenic proliferative zones. It remains unclear what kind of population (neurons, glia or radial glia) the TH-, GABA- and NADPH-d-positive cell groups used in this study in 3-year-old cherry salmon belong to. For this reason, a more detailed analysis is needed. However, the fact that these cells are localized in the PCNA-immunoreactive areas of the brain, and, together with Pax6, are immunolabeling a neuromeric structure in the brain of the cherry salmon of various age groups may signify that these cells are participating in the process of postembryonal neurogenesis.
MATERIALS AND METHODS
Design
A controlled, observational animal experiment.
Time and setting
This study was performed at Laboratory of Cytophysiology, A.V. Zhirmunskii Institute of Marine Biology, Far Eastern Branch, Russian Academy of Sciences, Russia, from February 2009 to May 2011.
Materials
The cherry salmon Oncorhynchus masou was obtained from Ryazanka experimental fish hatchery (Peter the Great Bay, Sea of Japan); 20 specimens were taken from each age bracket group: 3-month-old, 6-month-old, 1-year-old and 3-year-old mature fish. The fish were kept in aquaria with aerated fresh water at 17–18°C and anesthetized with a stock solution of 0.1% (w/v) tricaine methane sulphonate (MS-222; Sigma, St. Louis, MO, USA) for 10–15 minutes. The intracranial cavity of the immobilized fish was perfused with 0.1 M PBS (pH 7.2) containing 4% paraformaldehyde solution via a syringe. After prefixation, the brain was removed from the cranial cavity and fixed at 4°C for 2 hours in the same solution. Then, the brain was washed five times in 30% sucrose solution at 4°C during 24 hours. Serial frontal transverse brain sections (50 μm thick) of the cherry salmon were sliced using a freezing microtome.
Methods
Histochemical staining
The NADPH-d activity in the teleost fish brain was investigated according to a modification of conventional histochemical methods[40–41]. Free-floating sections were incubated in the medium, made up of 1mM β-NADPH, 0.8 mM nitro blue tetrazolium, and 0.06% Triton X-100 in 0.1 M PBS (pH 7.2), at 37°C for 2 hours. All chemicals were purchased from Sigma-Aldrich Chemie GmbH (USA). After incubation, the sections were rinsed in PBS, mounted on a gelatin-coated glass slide, and air-dried overnight. The next day, they were dehydrated according to the standard technique and embedded in balsam. In order to determine the specificity of histochemical reaction, the following controls were performed: incubation without the substrate β-NADPH and incubation without chromogen nitro blue tetrazolium in order to detect possible nonspecific formation of the reaction product. In all experiments, no residual reaction was observed. Control preparations were incubated in the media with addition of nitric oxide synthase inhibitor and 10 mM N-monomethyl-L-arginine. The sections were visualized under an Axiovert 200M fluorescent microscope (Carl Zeiss MicroImaging, Munich, Germany).
Immunohistochemical staining of proliferating cell nuclear antigen (PCNA)
Avidin-biotin-peroxidase complex (ABC) immunohistochemical staining of PCNA was performed to investigate the proliferative activity of cerebral cells. The fish brain was fixed using the above described technique and then divided into several blocks. The obtained samples were embedded in paraffin according to the conventional technique[10]. Serial frontal sections (25 μm thick) were prepared using a microtome, mounted on polylysine-covered glass slides and then deparaffinized. The obtained preparations were subjected to thermal treatment for 45 minutes at 95°C with Target Retrieval Solution (code No. S 3308; Dako Cytomation), citrate buffer (10 mM; pH 6.0) or Tris buffer (10 mM), and ethylenediamine tetraacetic acid (1 mM; pH 9.0; DAKO, Denmark) to increase membrane permeability. After cooling down to room temperature, the glass slides were rinsed in distilled water. To remove nonspecific peroxidase activity, the slices were incubated in 1% hydrogen peroxide solution for 5 minutes at 37°C and washed three times in 0.1 M PBS (pH 7.2). The immunocytochemical detection of PCNA was performed with a commercially available mouse MoAb against PCNA (PC 10, lgG, DAKO, Denmark) (1:400) for 20 minutes at room temperature and washed three times in 0.1 M PBS. The slices were incubated with secondary biotin-conjugated mouse anti-rabbit IgG (1:100 in PBS + NHS 1% LSAB 2 System, HRP; DAKO, Denmark) for 20 minutes at 37°C and then washed three times in 0.1 M PBS (pH 7.2). Subsequently, the sections were incubated with streptavidin-biotin visualization systems (LSAB 2 System, HRP; DAKO, Denmark) under the same condition (20 minutes, 37°C) and washed in 0.1 M PBS. The reaction products were detected with diaminobenzidine dissolved (to 0.5 mg/mL) in PBS; 1- or 2-mL aliquots were prepared. Before application, hydrogen peroxide, based on 0.1 M PBS, was added to aliquots to a final concentration of 0.03%. The obtained preparations were kept for 5–7 minutes in a thermostat at 37°C to reach a clear visualization of the used marker (process was controlled under a microscope). Glass slides were washed in distilled water, stained with hematoxylin (Lilly-Mayer, BioVitrum, Russia) for 30 seconds, washed with running water for 15–20 minutes, dehydrated according to the standard technique, and embedded in balsam.
Immunohistochemical staining for TH, GABA and Pax6
TH, GABA and transcription factor Pax6 were immunohistochemically identified using the standard avidin-biotin-peroxidase labeling on free-floating sections. The sections were incubated with mouse anti-GABA IgG1 (clone: 5A9; MP Biomedicals, Santa Ana, CA, USA; 1:5 000), mouse anti-TH IgG2a (clone: 1B5; Vector Laboratories, Burlingame, CA, USA; 1:10 000), mouse anti-Pax6 monoclonal antibody IgG1 (clone: AD2.38; Chemicon, Billerica, MA, USA; 1:4 000) at 4°C for 2 days. For visualization of immunohistochemical labeling, a Vectastain Elite ABC kit (Vector Laboratories) was applied. For identification of the reaction products, the substrates of blue (GABA, Pax6) and red (TH) colors (VIP Substrate Kit, Vector Labs, Burlingame, USA) were used. The staining process was controlled under an Axiovert 200M fluorescent microscope (Carl Zeiss MicroImaging). The sections were rinsed in water, mounted on slides, dehydrated according to the standard method, and embedded in balsam. Negative control was used to evaluate the specificity of immunohistochemical reaction. Brain sections were incubated for 1 day with 1% nonimmune horse serum rather than primary antibodies and then stained as described above. In all control experiments, no immunoreactive reactions occur.
Footnotes
Evgeniya V. Pushchina, Ph.D., D.Sc. Leader Researcher.
Funding: This work was supported by a grant from Far Eastern Branch of Russian Academy of Sciences, No. 12-III-A-06-095.
Conflicts of interest: None declared.
Ethical approval: The experiments were approved by the Animal Ethics Committee, A.V. Zhirmunskii Institute of Marine Biology, Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russia.
(Edited by Song LP)
REFERENCES
- [1].Zupanc GK, Horschke I. Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study. J Comp Neurol. 1995;353(2):213–233. doi: 10.1002/cne.903530205. [DOI] [PubMed] [Google Scholar]
- [2].Grandel H, Kaslin J, Ganz J, et al. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295(1):263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- [3].Zupanc GK. Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192(6):649–670. doi: 10.1007/s00359-006-0104-y. [DOI] [PubMed] [Google Scholar]
- [4].Pellegrini E, Mouriec K, Anglade I, et al. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J Comp Neurol. 2007;501(1):150–167. doi: 10.1002/cne.21222. [DOI] [PubMed] [Google Scholar]
- [5].Pushchina EV, Obukhov DK, Varaksin AA. Neurochemical markers of cells of the periventricular brain area in the masu salmon Oncorhynchus masou (Salmonidae) Ontogenez. 2012;43(1):39–53. [PubMed] [Google Scholar]
- [6].Pushchina EV, Obukhov DK. Loseva E, Loginova N, Sinelnikova V. Neuroscience for Medicine and Psychology. Moscow: MAKS Press, Russia; 2010. The participation of radial glia cells into histogenesis of nervous system; pp. 242–243. [Google Scholar]
- [7].von Bohlen und Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011;345(1):1–19. doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- [8].Maekawa M, Takashima N, Arai Y. Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neurogenesis. Genes Cells. 2005;10(10):1001–1014. doi: 10.1111/j.1365-2443.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- [9].Osumi N, Shinohara H, Numayama-Tsuruta K, et al. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells. 2008;26(7):1663–1672. doi: 10.1634/stemcells.2007-0884. [DOI] [PubMed] [Google Scholar]
- [10].Pushchina EV, Fleishman MY, Timoshin SS. Proliferative zones in the brain of the Amur sturgeon fry interactions with neuromeres and migration of secondary matrix zones. Rus J Dev Biol. 2007;38(5):286–293. [PubMed] [Google Scholar]
- [11].Extröm P, Johnsson CM, Ohlin LM. Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones. J Comp Neurol. 2001;436(1):92–110. [PubMed] [Google Scholar]
- [12].Platel JC, Laca B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38(4):602–610. doi: 10.1007/s10735-007-9153-y. [DOI] [PubMed] [Google Scholar]
- [13].Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15(6):4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aniol VA, Stepanichev MY. Nitric oxide and gamma-aminobutyric acid as regulators of neurogenesis in the brain of adult mammals: models of seizure activity. Neurochem J. 2007;1(4):265–274. [Google Scholar]
- [15].Platel JC, Stamboulian S, Nguyen I, et al. Neurotransmitter signaling in postnatal neurogenesis: the first leg. Brain Res Rev. 2010;63(1-2):60–71. doi: 10.1016/j.brainresrev.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Extröm P, Ohlin L. Ontogeny of GABA-immunoreactive neurons in the central nervous system in a teleost Gasterosteus aculeatus L. J Chem Neuroanat. 1995;9(4):271–288. doi: 10.1016/0891-0618(95)00093-3. [DOI] [PubMed] [Google Scholar]
- [17].Overstreet Wadiche L, Bromberg DA, Bensen AL, et al. GABA-ergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94(6):4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- [18].Gascon E, Dayer AG, Sauvain MO, et al. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26(50):12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ge S, Goh EL, Sailor KA, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Martinoli MG, Dubourg P, Geffard M, et al. Distribution of GABA immunoreactive neurons in the forebrain of the goldfish Carassius auratus. Cell Tissue Res. 1990;260(1):77–84. doi: 10.1007/BF00297492. [DOI] [PubMed] [Google Scholar]
- [21].Medina M, Reperant J, Dufour S, et al. The distribution of GABA-immunoreactive neurons in the brain of the silver eel (Anguilla anguilla L.) Anal Embryol. 1994;189(1):25–39. doi: 10.1007/BF00193127. [DOI] [PubMed] [Google Scholar]
- [22].Adolf B, Chapouton P, Lam CS, et al. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;295(1):278–293. doi: 10.1016/j.ydbio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- [23].Rakic P. The radial edifice of cortical architecture: from neuronal silhouttes to genetic engineering. Brain Res Rev. 2007;55(2):204–219. doi: 10.1016/j.brainresrev.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hebsgaard JB, Nelander J, Sabelström H, et al. Dopamine neuron precursors within the deveoping human mesencephalon show radial glial characteristics. Glia. 2009;57(15):1648–1658. doi: 10.1002/glia.20877. [DOI] [PubMed] [Google Scholar]
- [25].Ugrumov MV. Developing brain as an endocrine organ: a paradoxical reality. Neurochem Res. 2010;35(6):837–850. doi: 10.1007/s11064-010-0127-1. [DOI] [PubMed] [Google Scholar]
- [26].Callier S, Snapyan M, Le Crom S, et al. Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell. 2003;95(7):489–502. doi: 10.1016/s0248-4900(03)00089-3. [DOI] [PubMed] [Google Scholar]
- [27].Diaz J, Ridray S, Mignon V, et al. Selective expression of dopamine D3 receptor mRNA in proliferative zones during embryonic development of the rat brain. J Neurosci. 1997;17(11):4282–4292. doi: 10.1523/JNEUROSCI.17-11-04282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- [29].Kapsimali M, Vidal B, Gonzalez A, et al. Distribution of the mRNA encoding the four dopamine D (1) receptor subtypes in the brain of the european eel (Anguilla anguilla): comparative approach to the function of D(1) receptors in vertebrates. J Comp Neurol. 2000;419(3):320–343. doi: 10.1002/(sici)1096-9861(20000410)419:3<320::aid-cne5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [30].Meek J. Catecholamines in the brains of osteichtyes (bony fishes) In: Smetts WJ, Reiner A, editors. Phylogeny and development of Catecholamine Systems in the CNS of Vertebrates. Cambridge: Cambrifge University Press; 1994. [Google Scholar]
- [31].Wullimann MF, Rink E. Detailed immunohistology of Pax6 protein and tyrosine hydroxylase in the early zebrafish brain suggests role of Pax6 gene in development of dopaminergic diencephalic neurons. Brain Res Dev Brain Res. 2001;131(1-2):173–191. doi: 10.1016/s0165-3806(01)00270-x. [DOI] [PubMed] [Google Scholar]
- [32].Pushchina EV. Tyrosine hydroxylase in telencephalon and diencephalons of the Rhodeus sericeus (Cyprinidae) Tsitologyia. 2009;51(1):63–77. [PubMed] [Google Scholar]
- [33].Götz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21(5):1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- [34].Puelles L, Kuwana E, Puelles E, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424(3):409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [35].Kohwi M, Osumi N, Rubenstein JL. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25(30):6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ma PM. Tanycytes in the sunfish brain: NADPH-diaphorase histochemistry and regional distribution. J Comp Neurol. 1993;336(1):77–95. doi: 10.1002/cne.903360107. [DOI] [PubMed] [Google Scholar]
- [37].Bicker G. Stop and go with NO: nitric oxide as a regulator of cell motility in simple brains. BioEssays. 2005;27(5):495–505. doi: 10.1002/bies.20221. [DOI] [PubMed] [Google Scholar]
- [38].Pinto L, Götz M. Radial glial cell heterogeneity – the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83(1):2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- [39].Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hope ÂÒ, Vincent SR. Histochemical characterization of neuronal NADPH-diaphorase. J Histochem Cytochem. 1989;37(5):653–661. doi: 10.1177/37.5.2703701. [DOI] [PubMed] [Google Scholar]
- [41].Pushchina EV. Nitric oxide-ergic organization of medullar cranial nuclei in teleost fishes. Tsitologyia. 2007;49(6):471–483. [PubMed] [Google Scholar]


