Abstract
The present study assessed the influence of medium-intensity (treadmill at a speed of 19.3 m/min until exhaustion) and high-intensity (treadmill at a speed of 26.8 m/min until exhaustion) acute exhaustive exercise on rat hippocampal neural cell apoptosis. TUNEL staining showed significantly increased neural cell apoptosis in the hippocampal CA1 region of rats after medium- and high-intensity acute exhaustive exercise, particularly the medium-intensity acute exhaustive exercise, when compared with the control. Immunohistochemistry showed significantly increased expression of the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax in the hippocampal CA1 region of rats after medium- and high-intensity acute exhaustive exercise. Additionally, the ratio of Bax to Bcl-2 increased in both exercise groups. In particular, the medium-intensity acute exhaustive exercise group had significantly higher Bax and Bcl-2 protein expression and a higher Bax/Bcl-2 ratio. These findings indicate that acute exhaustive exercise of different intensities can induce neural cell apoptosis in the hippocampus, and that medium-intensity acute exhaustive exercise results in greater damage when compared with high-intensity exercise.
Keywords: neural regeneration, brain injury, hippocampus, apoptosis, neuron, different intensities, acute exhaustive exercise, Bax, Bcl-2, grant-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) Medium- and high-intensity acute exhaustive exercise can increase the expression of the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax in the hippocampal CA1 region, as well as the ratio of Bax/Bcl-2.
(2) Medium- and high-intensity acute exhaustive exercise induced neural cell apoptosis in the hippocampus, and medium-intensity acute exhaustive exercise resulted in more damage when compared with high-intensity exercise.
INTRODUCTION
Apoptosis, which aids in the regulation and maintenance of normal development and physiological processes, is a critical mechanism for regulating the cell number of multicellular organisms during their entire life. Abnormal changes to the apoptotic process can occur in a variety of diseases[1]. Aerobic glucose metabolism is known to be the source of 85–95% of the brain's energy requirements, while the capacity of the brain to store oxygen and glucose is almost zero, which results in poor tolerance to hypoxic and ischemic injury[2]. Of the various brain regions, hippocampal neural cells are the most sensitive to ischemic and hypoxic injury. The hippocampus functions via hypothalamic control of the pituitary gland and endocrine system, so if the hippocampus is damaged, the circadian rhythm release of adrenocorticotropic-hormone will be damaged[3,4,5]. The hippocampus plays a key role in learning and short-term memory processing. In addition, stimulation of the hippocampus may cause behavioral changes, such as static state, apathy, and loss of initiative[6]. During movement, the blood supply from various parts of the body is redistributed, and large amounts of blood are transferred to the skeletal muscle to maintain the blood supply during exercise[7]. Under these conditions, brain ischemia/hypoxia inevitably occurs. When movement stops, blood supply to the brain recovers, which is similar to the process of ischemia/reperfusion[8]. A previous study has shown that ischemia/reperfusion can cause hippocampal apoptosis[9], alter its function, and cause tissue necrosis[10]. Studies investigating the influence of exercise on neuronal number indicate that rats subjected to physical activity have an increased number of hippocampal neurons[11]. The mechanism controlling neuronal number may involve both regulation of cell proliferation and apoptosis[12]. Regular physical exercise such as wheel running and treadmill running have been shown to increase cell proliferation and survival in the hippocampal dentate gyrus of adult rodents[13,14,15,16]. However, it remains unknown how regular exercise influences the adolescent brain, and what role it plays in normal development and tissue homeostasis[17]. Strenuous exercise influences apoptosis in a variety of tissues[18,19]. Few studies have investigated the effects of exercise on apoptosis in the brain; however, a previous study has shown that medium-intensity exercise does not alter apoptosis in the brain of adult rats[20].
The present study examined the impact of acute exercise on hippocampal apoptosis in rats and monitored the effect of exercise training on apoptosis to provide scientific guidance for training athletes.
RESULTS
Quantitative analysis of animals and results of acute exhaustive exercise
Thirty rats were equally and randomly assigned to three groups: control group (no exercise), medium-intensity exercise (at a speed of 19.3 m/min) and high-intensity exercise (at a speed of 26.8 m/min). Both exercise groups finished the acute exhaustive exercise without resistance. On average, the exhaustive exercise time (minutes) in the medium-intensity exercise group and high-intensity exercise group were 234.6 ± 60.05 and 92.4 ± 34.61, respectively. All 30 rats were involved in the final analysis.
Effect of acute exhaustive exercise on apoptotic cell death in the rat hippocampal CA1 region
TUNEL staining showed that apoptotic cells were round or oval shaped, with brown or yellow stained nuclei of irregular size; while the nuclei of normal cells were blue following hematoxylin staining, and the size and appearance was consistent (Figure 1). Few apoptotic cells were found in the hippocampus of the control group, but the number of apoptotic cells significantly increased in the medium- and high-intensity exercise groups compared with the control group. In particular, apoptotic cells in the high-intensity exercise group significantly decreased when compared with the medium-intensity exercise group (P < 0.05; Figure 2).
Figure 1.
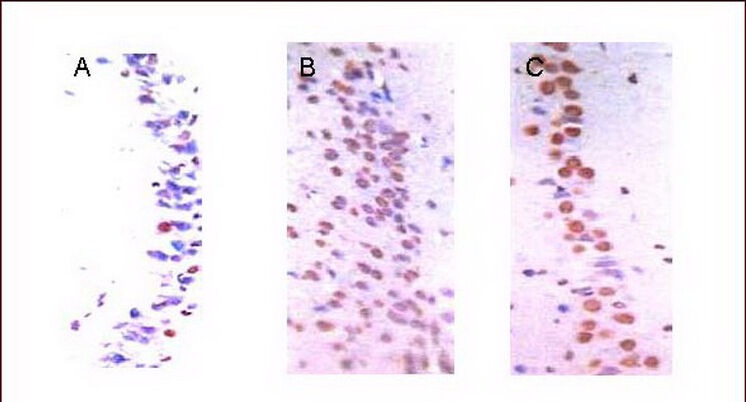
Apoptotic cell death in the hippocampal CA1 region (TUNEL staining, light microscopy, × 400).
(A) In the control group, there were few apoptotic cells (brown).
(B) There were more apoptotic cells (brown) in the medium-intensity exercise group.
(C) There were some apoptotic cells (brown) in the high-intensity exercise group.
Figure 2.
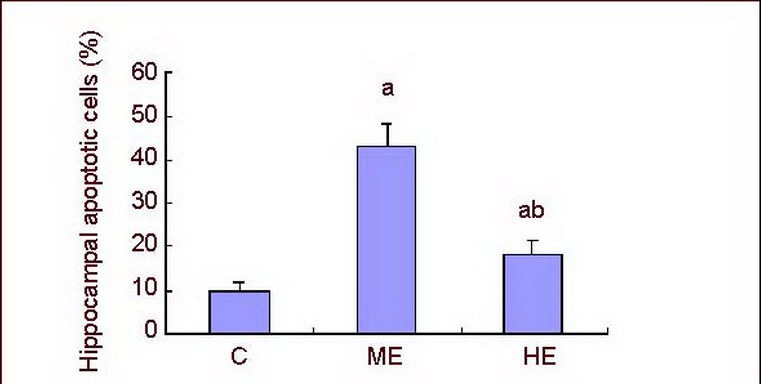
Effect of exhaustive exercise on apoptotic cell death in the CA1 region of the hippocampus.
Data are expressed as mean ± SD of 10 rats in each group. aP < 0.05, vs. control group; bP < 0.05, vs. ME group using one-way analysis of variance followed by Tukey's method.
C: Control group; ME: medium-intensity exercise group; HE: high-intensity exercise group. Apoptotic rate = number of TUNEL-positive cells/total cells × 100%.
Effect of acute exhaustive exercise on Bcl-2 and Bax expression in the rat hippocampal CA1 region
Neural cells in the rat hippocampal CA1 region were observed by light microscopy. The expression of Bcl-2 and Bax was brown/yellow or appeared as brown granules, and the cytoplasm and nucleus were also stained. There were few positive cells in the control group. The number of positive cells was significantly increased in the medium-intensity exercise and high-intensity exercise groups, particularly in the medium-intensity exercise group when compared with the control group (Figures 3, 4).
Figure 3.
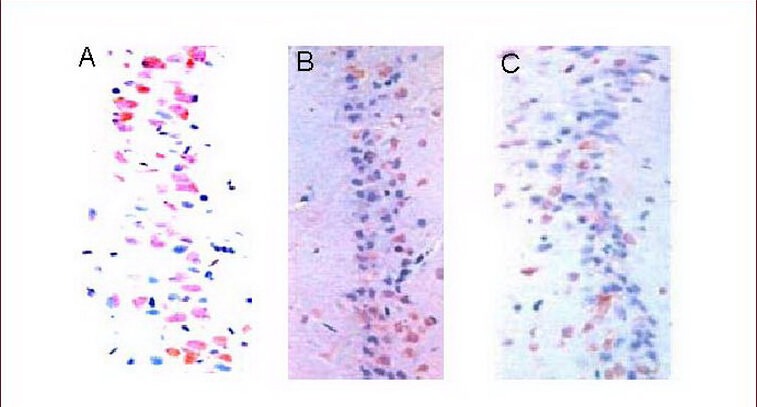
Expression of Bcl-2 in the rat hippocampal CA1 region (immunohistochemical staining, light microscopy, × 400).
(A) There were few Bcl-2 positive cells (brown) in the control group.
(B) There were a large number of Bcl-2 positive cells (brown) in the medium-intensity exercise group.
(C) There were some Bcl-2 positive cells (brown) in the high-intensity exercise group.
Figure 4.
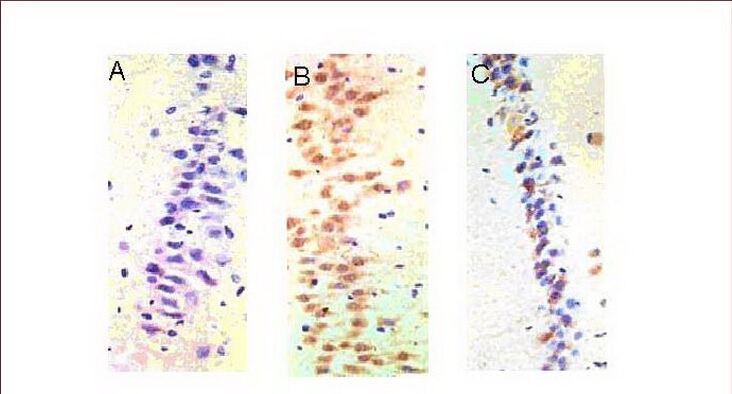
Expression of Bax in the rat hippocampal CA1 region (immunohistochemical staining, light microscopy, × 400).
(A) There was almost no expression of Bax in the control group.
(B) A large amount of Bax expression (brown cells) was observed in the medium-intensity exercise group.
(C) Bax expression (brown cells) was less in the high-intensity exercise group when compared with the medium-intensity exercise group.
Compared with the control group, Bcl-2 and Bax expression was significantly increased in the medium-intensity exercise and high-intensity exercise groups (P < 0.01). Expression was particularly obvious in the medium-intensity exercise group when compared with the high-intensity exercise group (P < 0.01; Figure 5). In addition, the ratio of Bax to Bcl-2 expression significantly increased in the medium-intensity exercise group when compared with the control group (P < 0.01). However, the ratio of Bax to Bcl-2 expression was significantly decreased in the high-intensity exercise group (P < 0.05) when compared with the control group. The ratio of Bax to Bcl-2 expression was significantly lower in the high-intensity exercise group when compared with the medium-intensity exercise group (P < 0.01; Figure 6).
Figure 5.
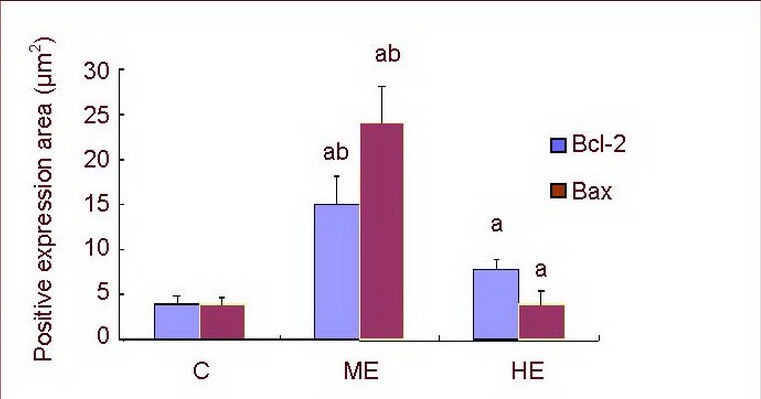
Bcl-2 and Bax protein expression in the rat hippocampal CA1 region.
Data are expressed as mean ± SD of 10 rats in each group. aP < 0.01, vs. control group; bP < 0.01, vs. HE group (one-way analysis of variance followed by Tukey's test).
C: Control group; ME: medium-intensity exercise group; HE: high-intensity exercise group.
Figure 6.
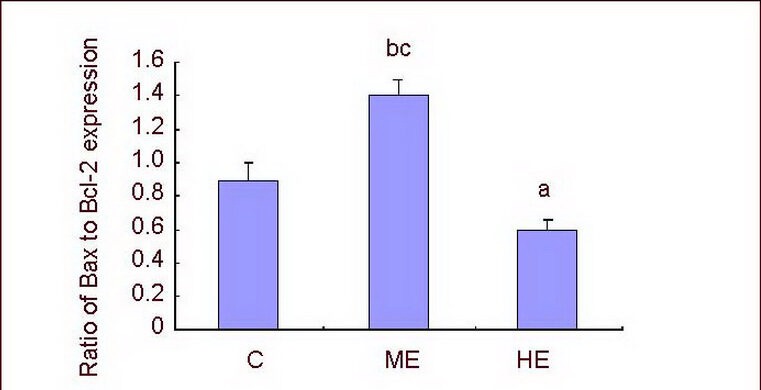
Ratio of Bax to Bcl-2 expression in the hippocampal CA1 region.
Data are expressed as mean ± SD of 10 rats in each group. aP < 0.05, bP < 0.01, vs. control group; cP < 0.01, vs. HE group (one-way analysis of variance followed by Tukey's test).
C: Control group; ME: medium-intensity exercise group; HE: high-intensity exercise group.
DISCUSSION
Apoptosis is considered a vital component of various processes, including normal cell turnover, proper development, immune system function, hormone-dependent atrophy, embryonic development, and chemical agent-induced cell death[21]. The proteins of the Bcl-2 family are important factors in the apoptotic process as they are key regulators of apoptosis that act on mitochondria[22]. This protein family includes anti-apoptotic molecules, such as Bcl-2, and pro-apoptotic molecules, such as Bax[23]. The Bcl-2 family members play a major role in regulating cell death in many cell types, including neurons[24]. Bcl-2 family protein expression may play an important role in determining whether neurons survive or die. Expression of Bax, which induces apoptosis, is up-regulated in many neurodegenerative diseases[25]. Bcl-2 family proteins are necessary components and essential regulators of apoptotic cell death. Recent studies have revealed that dysregulation of Bcl-2 family proteins could exacerbate ischemic neuronal injury[26].
Results from the present study showed that in the medium-intensity exercise group, Bcl-2 protein expression levels were significantly increased, which led to increased apoptosis. Meanwhile, Bax expression significantly increased, resulting in an imbalance of cells expressing Bcl-2 and Bax. In the high-intensity exercise group, Bcl-2 expression was higher than that in the control group, and Bax protein expression was lower, which resulted in the reduction of apoptotic cell death and fewer apoptotic cells. After acute exercise, Bcl-2 immunoreactivity was higher than that of Bax. It is possible that acute exercise induced the overexpression of Bcl-2 protein and down-regulated the expression of Bax to exert its anti-apoptotic effect, thus promoting cell survival[27] and neuroprotection[28]. In contrast, after a long period of medium-intensity exercise, mitochondrial permeability can change[22], which leads to the mitochondrion-mediated activation of the pro-apoptotic proteins Bax and Bak. Conformational changes to Bax and Bak lead to changes in mitochondrial inner membrane permeability, decreased membrane potential, and reduced energy synthesis, resulting in swelling of mitochondria and changes to mitochondrial permeability transition.
Following acute exhaustive exercise at different intensities, the amount of apoptosis increased in the hippocampal CA1 region of rats. Interestingly, medium-intensity exercise induced more apoptosis when compared with the high-intensity exercise group. Bax and Bcl-2 were involved in the regulation of apoptosis, and the balance between Bax and Bcl-2 expression was an important factor for apoptosis progression.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The experiment was performed at the School of Biological Science and Technology, Central South University, China from September 2011 to March 2012.
Materials
Thirty adult male Sprague-Dawley rats were provided by the Department of Experimental Animals, Xiangya School of Medicine, Central South University, China (license No. SYXK (Hunan) 2011-0001). They were aged 8 weeks and weighed 219.2 ± 19.5 g. They were raised separately, allowed free access to food and water, and were maintained at 20–24°C. The use of animals for experimental procedures was in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[29].
Methods
Exercise patterns
According to the Berdford model[30,31], treadmill exercise (Zhejiang Hangzhou Thai Science and Technology, Zhejiang, China) was performed for the exercise groups. Before experimentation, preparation involved 3-day-long exercise to adapt (velocity: 10 m/min, gradient: 0°) and then a 3-day-long rest period. The experiment started on the 7th day according to the following method: (1) In the medium-intensity exercise group, the gradient was 10 at an initial velocity of 10 m/min, accelerated to 19.3 m/min (equal to 76% VO2max) within 12 minutes. (2) In the high-intensity exercise group, the gradient was 10 at an initial velocity of 10 m/min, and then accelerated to 26.8 m/min (equal to 92.3% VO2max) within 12 minutes.
Exhaustion was observed when the rat could not maintain the anticipated velocity and lagged during the last one third of the treadmill exercise over 3 attempts. Rats would appear breathless, showed lassitude and lethargy, and would lie down.
Sample preparation
Rats were sacrificed immediately after acute exhaustion exercise using Zoletil 50 (10 mg/kg, intraperitoneal injection)[32]. The rats were transcardially perfused with 0.05 M PBS, and fixed with a freshly prepared solution consisting of 4% (w/v) paraformaldehyde in 0.5 M PBS (pH 7.4). Brains were dissected, post-fixed in the same fixative overnight, and transferred to 30% (w/v) sucrose for cryoprotection. Coronal sections (40 μm thick) were made using a freezing microtome (Leica, Nussloch, Germany). Ten sections on average in the hippocampus were collected from each rat. The sections of 2.5 mm to 2.7 mm posterior from the bregma were used for immunohistochemical staining.
TUNEL staining
Apoptosis was detected by TUNEL[33] using the in situ cell death detection kit (Shenzhen Jinmei BioEngineering Co. Ltd., Shenzhen, China). Shortly after deparaffinization and rehydration, hippocampal sections were rinsed briefly in 0.1 M PBS twice, digested in Proteinase K working solution (10 μg/mL in 10 mM Tris/HCl, pH 7.4–8.0), and incubated at 37°C for 15 minutes. Sections were next treated with TdT enzyme solution, label solution, and converter-peroxidase. The sections were developed with diaminobenzidine substrate for 10 minutes. Negative controls were produced using 0.1 M PBS rather than TUNEL reaction mixture. Apoptotic cells that were TUNEL-positive had brown yellow particles in the nucleus. At a magnification of × 400, 10 microscopic fields in the hippocampal CA1 region were taken randomly, and all cells including TUNEL-positive cells were quantified. The ratio of the number of TUNEL-positive cells to total cells was expressed as a percentage. The sections were observed under a light microscope (Nikon, Tokyo, Japan).
Immunohistochemical staining
Paraffin embedded sections were placed in xylene for 5 minutes × 2 times, and treated with a series of graded ethanol solutions. Sections were incubated in 3% (v/v) H2O2 for 10 minutes, rinsed with distilled water for 5 minutes, washed with PBS, retrieved in citric acid salt antigen retrieval liquid (pH 6.0) using a microwave oven, cooled, and blocked in normal goat serum at room temperature for 10 minutes. The sections were incubated with Bcl-2 or Bax antibody at a dilution of 1:50 (rabbit polyclonal Bcl-2 antibody and mouse anti-rat Bax monoclonal antibody from Shenzhen Jinmei Biological Engineering Co., Ltd., Shenzhen, China) at 4°C in a wet box overnight. The next day, sections were washed with PBS and incubated with horseradish peroxidase-labeled sheep anti-rabbit/mouse (10 μg/mL) in 10 mM Tris/HCl, pH 7.4–8.0 (Shenzhen Jinmei Biological Engineering Co., Ltd.) at 37°C for 30 minutes. Sections were washed, incubated with diaminobenzidine, and counterstained with hematoxylin for 4 minutes. Tissue was examined using optical microscopy (× 400; Nikon), and six fields of view from each slice were analyzed using a Nikon microscope camera system and MIAS medical image analysis system.
Statistical analysis
All data were expressed as mean ± SD and analyzed by SPSS 12.0 statistical software (SPSS, Chicago, IL, USA). One-way analysis of variance followed by Tukey's method was used for multiple comparisons. A P level less than 0.05 was considered statistically significant.
Footnotes
Shanni Li, Master, Lecturer.
Funding: This study was supported by the National Natural Science Foundation of China, No. 30500269.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Animal Ethics Committee of the Second Xiangya Hospital of Central South University, No. S080.
(Edited by Xiang R, Liu J/Su LL/Song LP)
REFERENCES
- [1].Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shao SY, Zhang JK, Zhang L, et al. Expression of Bcl-2 and Bax in mice developing hippocampus. Disan Junyi Daxue Xuebao. 2011;33(17):1831–1833. [Google Scholar]
- [3].Chai XB, Liu XB. Effects of MK801 on the expression of c-fos gene and the nerves in hippocampus CA1 region after ischemic-reperfusion injury. Shengwu Jishu Tongxun. 2011;22(4):532–535. [Google Scholar]
- [4].LaRusch GA, Jackson MW, Dunbar JD, et al. Nutlin3 blocks vascular endothelial growth factor induction by preventing the interaction between hypoxia inducible factor 1alpha and Hdm2. Cancer Res. 2007;67(2):450–454. doi: 10.1158/0008-5472.CAN-06-2710. [DOI] [PubMed] [Google Scholar]
- [5].Villa P, Bigini P, Mennini T, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198(6):971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sirén AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98(7):4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weber A, Maier RF, Hoffmann U, et al. Erythropoietin improves synaptic transmission during and following ischemia in rat hippocampal slice cultures. Brain Res. 2002;958(2):305–311. doi: 10.1016/s0006-8993(02)03604-1. [DOI] [PubMed] [Google Scholar]
- [8].Liu B, Ma YY, Mao WJ, et al. Effects of shenxiong huayu capsule on Apoptosis and Expression of Bcl-2 and Bax Protein in CA1 area of hippocampus in vascular dementia rats. Zhongguo Shiyan Fangjixue Zazhi. 2011;17(6):176–179. [Google Scholar]
- [9].Lock RB, Murphy KM. Immunodetecting members of the Bcl-2 family of proteins. Methods Mol Med. 2005;111:83–96. doi: 10.1385/1-59259-889-7:083. [DOI] [PubMed] [Google Scholar]
- [10].Zhang M. Fatigue training on hippocampus NOS activity and the intervention of Chinese medicine. Liaoning Zhongyiyao Daxue Xuebao. 2011;13(3):187–189. [Google Scholar]
- [11].Kim SH, Kim HB, Jang MH, et al. Treadmill exercise increases cell proliferation without altering of apoptosis in dentate gyrus of Sprague-Dawley rats. Life Sci. 2002;71(11):1331–1340. doi: 10.1016/s0024-3205(02)01849-0. [DOI] [PubMed] [Google Scholar]
- [12].Kim YP, Kim HB, Jang MH, et al. Magnitude- and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int J Sports Med. 2003;24(2):114–117. doi: 10.1055/s-2003-38202. [DOI] [PubMed] [Google Scholar]
- [13].Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- [14].Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58(1):76–87. [PubMed] [Google Scholar]
- [15].MacRae PG, Spirduso WW, Walters TJ, et al. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology (Berl) 1987;92(2):236–240. doi: 10.1007/BF00177922. [DOI] [PubMed] [Google Scholar]
- [16].Morris RG, Garrud P, Rawlins JN, et al. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- [17].Hudon C, Doré FY, Goulet S. Spatial memory and choice behavior in the radial arm maze after fornix transection. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(6):1113–1123. doi: 10.1016/s0278-5846(02)00245-2. [DOI] [PubMed] [Google Scholar]
- [18].Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rosenzweig MR, Krech D, Bennett EL, et al. Variation in environmental complexity and brain measures. J Comp Physiol Psychol. 1962;55:1092–1095. doi: 10.1037/h0042758. [DOI] [PubMed] [Google Scholar]
- [20].Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [21].Sutoo D, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13(1):1–14. doi: 10.1016/s0969-9961(03)00030-5. [DOI] [PubMed] [Google Scholar]
- [22].Kaneko D, Nakamura N, Ogawa T. Cerebral infarction in rats using homologous blood emboli: development of a new experimental model. Stroke. 1985;16(1):76–84. doi: 10.1161/01.str.16.1.76. [DOI] [PubMed] [Google Scholar]
- [23].Yu J, Liu C, Zhang X, et al. Acupuncture improved cognitive impairment caused by multi-infarct dementia in rats. Physiol Behav. 2005;86(4):434–441. doi: 10.1016/j.physbeh.2005.07.015. [DOI] [PubMed] [Google Scholar]
- [24].Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- [25].Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- [26].Lu J, Moochhala S, Kaur C, et al. Changes in apoptosis-related protein (p53, Bax, Bcl-2 and Fos) expression with DNA fragmentation in the central nervous system in rats after closed head injury. Neurosci Lett. 2000;290(2):89–92. doi: 10.1016/s0304-3940(00)01307-0. [DOI] [PubMed] [Google Scholar]
- [27].Chen J, Simon RP, Nagayama T, et al. Suppression of endogenous bcl-2 expression by antisense treatment exacerbates ischemic neuronal death. J Cereb Blood Flow Metab. 2000;20(7):1033–1039. doi: 10.1097/00004647-200007000-00002. [DOI] [PubMed] [Google Scholar]
- [28].Gillardon F, Lenz C, Waschke KF, et al. Altered expression of Bcl-2, Bcl-X, Bax, and c-Fos colocalizes with DNA fragmentation and ischemic cell damage following middle cerebral artery occlusion in rats. Brain Res Mol Brain Res. 1996;40(2):254–260. doi: 10.1016/0169-328x(96)00059-9. [DOI] [PubMed] [Google Scholar]
- [29].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [30].Prakasa Babu P, Yoshida Y, Su M, et al. Immunohistochemical expression of Bcl-2, Bax and cytochrome c following focal cerebral ischemia and effect of hypothermia in rat. Neurosci Lett. 2000;291(3):196–200. doi: 10.1016/s0304-3940(00)01404-x. [DOI] [PubMed] [Google Scholar]
- [31].Berdford TG, Tipton CM, Wilson NC, et al. Maximal oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol. 1979;47(6):1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- [32].Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- [33].Sim YJ, Kim SS, Kim JY, et al. Treadmill exercise improves short-term memory by suppressing ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci Lett. 2004;372(3):256–261. doi: 10.1016/j.neulet.2004.09.060. [DOI] [PubMed] [Google Scholar]


