Abstract
This study established a dog model of acute multiple cauda equina constriction by experimental constriction injury (48 hours) of the lumbosacral central processes in dorsal root ganglia neurons. The repair effect of intrathecal injection of brain-derived neurotrophic factor with 15 mg encapsulated biodegradable poly(lactide-co-glycolide) nanoparticles on this injury was then analyzed. Dorsal root ganglion cells (L7) of all experimental dogs were analyzed using hematoxylin-eosin staining and immunohistochemistry at 1, 2 and 4 weeks following model induction. Intrathecal injection of brain-derived neurotrophic factor can relieve degeneration and inflammation, and elevate the expression of brain-derived neurotrophic factor in sensory neurons of compressed dorsal root ganglion. Simultaneously, intrathecal injection of brain-derived neurotrophic factor obviously improved neurological function in the dog model of acute multiple cauda equina constriction. Results verified that sustained intraspinal delivery of brain-derived neurotrophic factor encapsulated in biodegradable nanoparticles promoted the repair of histomorphology and function of neurons within the dorsal root ganglia in dogs with acute and severe cauda equina syndrome.
Keywords: neural regeneration, peripheral nerve injury, cauda equina syndrome, dorsal root ganglion, brain-derived neurotrophic factor, multiple cauda equina constrictions, neurotrophic factors, neural protection, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) Intrathecal injection of encapsulated biodegradable poly(lactide-co-glycolide) nanoparticles carrying brain-derived neurotrophic factor can elevate the expression of brain-derived neurotrophic factor in sensory neurons of dorsal root ganglia in dogs with acute multiple cauda equina constriction.
(2) Injection also lessens neuronal degeneration, and contributes to the repair of neurological function in dogs with acute and severe cauda equina syndrome.
Abbreviations
DRG, dorsal root ganglion; BDNF, brain-derived neurotrophic factor
INTRODUCTION
Dorsal root ganglia (DRG) should not be overlooked when considering the mechanism of lower back pain, sciatica and sensory disturbance in the legs. It is therefore important to understand the morphologic and functional changes that occur in primary sensory neurons of the DRG as a result of nerve root compression[1]. In models of inflammatory and neuropathic pain, brain-derived neurotrophic factor (BDNF) synthesis is greatly increased in different populations of DRG neurons[2]. This has been well demonstrated in the rat rubrospinal system (a motor control system) after acute and chronic spinal cord injury. Following a cervical spinal cord injury in which the rubrospinal tract is cut, the immediate administration of BDNF into the site of spinal cord injury promoted significant rubrospinal axonal regeneration and prevented axotomy-induced atrophy and/or death of rubrospinal neurons[3,4]. The acutely injured rubrospinal axons at the injury site are responsive to BDNF and therefore initiate the appropriate intracellular signaling pathways to augment the intrinsic growth propensity of these central nervous system neurons. The development of effective therapeutic strategies that use the administration of neurotrophic factors (such as BDNF) will require an understanding of the biologic responsiveness of the target tissue. Furthermore, this responsiveness may in fact change with time and thus differ between the acute and chronic injury settings.
To verify the involvement of BDNF in the repair of injury to sensory neurons of the DRG, an experimental constriction injury of the lumbosacral central processes of DRG neurons, resulting in the cauda equina syndrome, was performed in dogs. Fully developed cauda equina syndrome is accompanied by sensory and motor disorders such as low-back pain, saddle anesthesia, and motor weakness of lower extremities, sometimes leading to paraplegia or bladder dysfunction[5]. These clinical symptoms are associated with a sustained stimulation of the cutaneous, muscular and visceral nociceptive afferents[6,7,8]. Thus, BDNF protein expression in the neurons of corresponding DRG induced by multiple cauda equina constrictions could be expected. This hypothesis was tested in the present study using immunocytochemical analysis of BDNF expression, after surgery, in DRG cells following 4 weeks of cauda equina syndrome. Since BDNF, not nerve growth factor, has been known to be one of the powerful survival factors for spinal motoneurons[9], we investigated the levels of BDNF protein in compressive DRG cells using histopathologic studies (hematoxylin-eosin staining). Immunohistochemical methods were also used after 1, 2, and 4 weeks of acute and severe cauda equina syndrome in experimental dogs. Thus, BDNF expression in neurons of the DRG and neuroprotection and prevention of apoptosis could be expected in this experimental model of multiple cauda equina constrictions. BDNF may play an active role in neuroprotective processes, partly by maintaining intracellular protein integrity and preventing neuronal degeneration in this experimental paradigm.
RESULTS
Quantitative analysis of experimental animals
A total of 18 dogs were equally and randomly divided into the sham surgery group (laminectomy only), control group [which removed multiple cauda equina constrictions (ligations) from the cauda equina after 48 hours] and experimental group (model established as the control group plus intrathecal injection of BDNF). One dog from the control group died at 13 days postoperation and another from the experimental group died at 21 days postoperation because of diarrhea. A total of 16 dogs were involved in the final analysis.
General morphology
Control and experimental group animals, in which the cauda equina had been constricted by 75% and the four ligations removed after 48 hours, had significant weakness of the posterior limbs, paralysis of the tail and urinary incontinence, although no clinically important changes were noted in respiration and heart rate. However, activity of the lower limbs in dogs of the experimental group improved after 2 and 4 weeks compared with control group animals. Severe arterial narrowing, venous congestion and inflammatory reaction were present at the level of the constriction and the corresponding dural sac of the cauda equina, roots and dorsal root ganglia.
Pathological changes of neurons in compressed DRG
Light micrographs of DRG at the level of L7 in the sham surgery group showed that nuclei were round, lightly colored, and centrally located. The nucleoli were distinct; and the striped Nissl granules in the cytoplasm were almost normal. After 1 week of removing compression, control and experimental group samples displayed more obviously central chromatolysis of DRG neurons.
Neuronal nuclei with chromatolysis were present at the periphery and the number of Nissl bodies in the central cytoplasm decreased more visibly, with extensive infiltration of inflammatory reaction. After 2 and 4 weeks following the removal of compression, the number of neurons with central chromatolysis was increased. There was a certain Wallerian degeneration of the neurons and nerve roots in the control and experimental groups, as well as extensive inflammatory cell infiltration. Degeneration of sensory neurons and inflammatory reaction in the control group were significantly more visible than the experimental group (Figure 1). The rank order of animals according to severity of pathology score is shown in Table 1. This ranking revealed statistically significant ordering based on intrathecal injection of BDNF and multiple cauda equina constrictions, thus indicating that intrathecal injection of BDNF prevented pathological changes of DRG at the L7 level.
Figure 1.
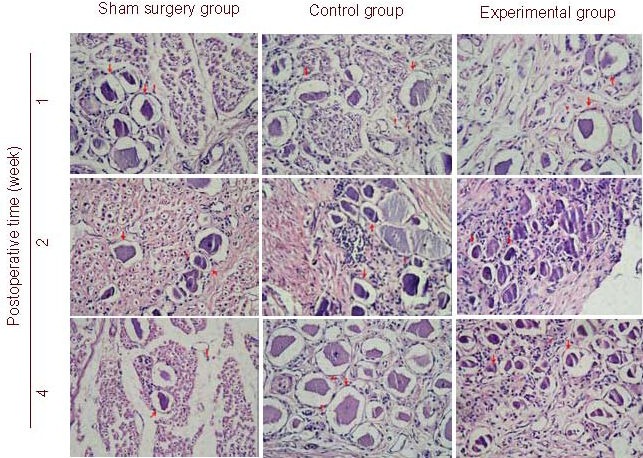
Morphology of dorsal root ganglion (DRG) from level L7 in dogs with acute and severe cauda equina constrictions following intrathecal injection of brain-derived neurotrophic factor nanoparticles at postoperative weeks 1, 2, and 4 (hematoxylin-eosin staining, light microscope, × 100).
Examination of the sham surgery group showed almost normal sensory neuron structure (red arrow) and surrounding tissue morphology. A small quantity of inflammatory reaction (red triangle) was observed.
Experimental and control group samples displayed central chromatolysis in DRG neurons. Neuronal nuclei with chromatolysis were located in the periphery and the number of Nissl bodies in the central cytoplasm decreased. The control group had more significant evidence of neuronal degeneration compared with the experimental group. Extensive inflammatory reaction was observed in the control and experimental groups.
Table 1.
Rank order of histopathology in dogs from the sham surgery, control and experimental groups after 1, 2 and 4 weeks postsurgery

BDNF expression in neurons of compressed DRG
Acute and severe multiple cauda equina constrictions in experimental dogs could induce extensive and varied expression of BDNF-immunoreactivity in sensory neurons of L7 DRGs. This immunoreactivity could persist for at least 4 weeks, with the BDNF expression in the cytoplasm of sensory neurons from the experimental group being significantly greater than that from the control group. BDNF-immunoreactivity in the sham surgery group was negative (Figure 2). All three groups had different expression of BDNF in neuroglia and nerve fibers of DRG at L7, but all immunoreactivity was strong.
Figure 2.
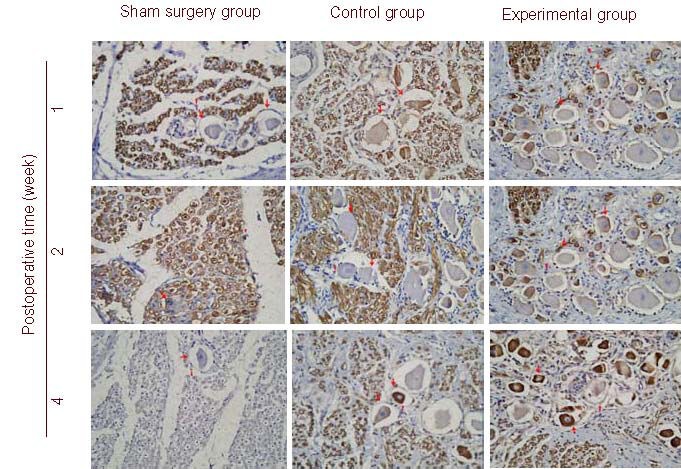
Brain-derived neurotrophic factor (BDNF) expression in the dorsal root ganglion (DRG) from level L7 in dogs with acute and severe cauda equina constrictious following intrathecal injection of BDNF nanoparticles at postoperative weeks 1, 2, and 4 (immunohistochemical staining, light microscope, × 100).
Arrows indicate BDNF immunoreactivity.
BDNF immunoreactivity was not seen in the cytoplasm of the sensory neurons in the sham surgery group, but present in the control and experimental groups.
BDNF immunoreactivity in the experimental group was more intense than that in the control group. BDNF expression after 4 weeks was essentially similar to that observed after 2 weeks, but immunostaining was more intense. BDNF expression in neuroglial cells and nerve fibers of the L7 DRG varied among all three groups.
Neurological function in experimental animals
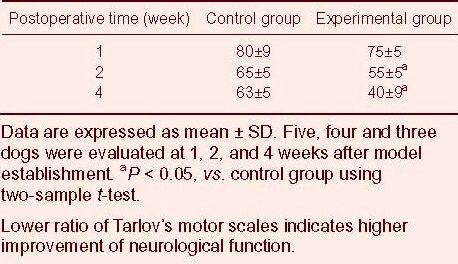
Given that the neurofunctional assessment of the sham surgery group was normal, the ratio of Tarlov's motor scales was performed between control and experimental groups. The results of the neurofunctional assessment after 1, 2 and 4 weeks of removing compression are presented in Table 2. No significant difference in neurofunctional assessment between control and experimental groups was observed after 1 week of removing compression. However, there were significant differences (P < 0.05) in motor disturbance between control and experimental groups after 2 and 4 weeks of removing compression. This observation showed that the neuronal function of the experimental group had greater improvement than the control group, and prophylactic intrathecal injection of BDNF could improve the neurological function in the experimental dogs experiencing acute and severe cauda equina syndrome.
Table 2.
Effect of intrathecal injection of brain-derived neurotrophic factor nanoparticles on motor function [ratio of Tarlov's motor scale (%)] of rats in control and experimental groups
DISCUSSION
While the cause of cauda equina syndrome still remains obscure, mechanistic analyses favored by some authors[10,11,12,13] may underlie the basis for the persistent and unvarying background symptoms of paresthesia and numbness of the feet and legs observed in some patients at rest due to a mechanical compression of the cauda equina. To verify the involvement of BDNF in injury repair of sensory neurons, experimental constriction injury of the lumbosacral central processes of DRG neurons resulting in cauda equina syndrome was studied in dogs. Thus, BDNF expression in sensory neurons of corresponding DRG cells could be expected. This hypothesis was tested in experimental dogs that had sustained severe constriction of the cauda equina for 48 hours. BDNF expression was performed, using immunochemical analysis, in DRG cells from L7 after 1, 2 and 4 weeks of removing constriction.
Establishment of a canine model
Several animal models mimicking cauda equina syndrome have been used to study and explain the pathophysiology of the polyradicular symptomatology of the syndrome[14,15]. A model of lumbar spinal stenosis in dogs[16] was developed consisting of the constriction of entire cauda equina at the seventh lumbar level with a nylon electrical-cable tie, 2.8 mm wide, placed circumferentially around the dura and, after a laminectomy of the sixth and seventh lumbar vertebrae, the cauda equina was constricted by 25%, 50% or 75% to produce chronic compression. The symptoms of intermittent neurogenic claudication are most possibly the result of stenosis at two levels[17]. This view is strongly supported by the circulatory anatomy of the cauda equina and with myeloscopic and experimental studies[18,19]. As a result, the chronic double-level cauda equina compression model in the dog[20] is a modification of the earlier presented model for chronic compression in the dog[21,22] to allow for compression at two levels. Double-level cauda equina compression closely resembles two-level stenosis and induces more symptoms[17,23].
Multiple protracted cauda equina constrictions are characterized as a model of somato-visceral pain in dogs[24], and are more comparable with pain models using peripheral nerve ligation. Lumbar laminectomy of the sixth and seventh laminae is carried out in multiple cauda equina constrictions, thus gaining access to the cauda equina. Constrictions of the dural sac are produced by tying four loosely constrictive ligatures with 2 mm spacing causing protracted constrictions of the central processes of the DRG cells of L7, S1-3, and Co1-5 segments along with the ventral roots of the same segments. In dog models of multiple cauda equina constriction-induced cauda equina syndrome, constrictions of entire cauda equina with different degrees can cause different neurological deficits, cortical evoked potentials and histological abnormalities. For example, in dogs, in which the cauda equina had been constricted, 75% had significant weakness, paralysis of the tail, and urinary incontinence. Dramatic changes of cortical evoked potentials and complete nerve-root atrophy at the level of the constriction were also observed. There was blockage of axoplasmic flow and Wallerian degeneration of the motor nerve roots distal to the constriction and of the sensory roots proximal to the constriction, as well as degeneration of the posterior column. This experiment confirmed that dogs of the control and experimental groups had significant weakness of posterior limbs, paralysis of the tail, urinary incontinence, severe arterial narrowing, venous congestion, and inflammatory reaction of the constricted dural sac and nerve roots. Therefore, an inadequate blood supply acting as a dynamic factor of severe cauda equina syndrome may intervene and, more probably, both mechanisms, mechanical and vascular, might be operating in combination, and also may have other participative pathogenesis. As a result, different constrictions of cauda equina may present different motor and sensory deficits occurring as well as blockage of axoplasmic flow. However, constriction of more than 50% was the critical point that resulted in complete loss of cortical evoked potentials and in neurological deficits and histological abnormalities[16]. From an analysis of the pressure changes within the cauda equina following constriction of the dural sac, it was found that pressure started to build up at a cross-sectional area of the dural sac, ranging between 60 and 80 mm2[25,26]. Once this critical size was reached, even a minimal further reduction of the area caused a distinct pressure increase among the nerve roots[25].
This study has proven that cauda equina injury will be at a significantly greater ischemic risk from a multi-level compression than from a one-level or two-level lesion. This investigation has also identified that the cauda equina had significant disorders of blood and cerebrospinal fluid circulation, and pathological changes of Wallerian degeneration and demyelination after multiple cauda equina constrictions in experimental dogs from control and experimental groups. Mechanical and vascular factors may participate in the development of cauda equina syndrome.
Role of DRG in acute and severe cauda equina syndrome
DRG has an important role when considering the mechanism of lower back pain, sciatica and sensory disturbance in the legs, so it is important to understand the morphologic and functional changes that occur in primary sensory neurons of the DRG as a result of nerve root compression in experimental models of multiple cauda equina constrictions. This study investigated the changes and development of BDNF protein in primary sensory neurons of the DRG after cauda equina compression using immunohistochemical methods. The characteristic axonal transport system of DRG neurons, which comprises anterograde and retrograde flow, plays an important role in the movement of neurotransmitters and neurotrophic factors, as well as in the transmission of information relating to environmental changes in the axon itself and the target organ. Disturbance of axonal flow therefore threatens the survival of neurons and appears to be one cause of neurological dysfunction. Axotomy of the peripheral branches of primary sensory neurons induces retrograde cell death in DRG neurons[27,28], which is more abundant in neonatal rats than in adult rats. Groves et al[27] showed that a reduction of 7–14% in the number of both L4 and L5 DRG neurons occurred 1 to 6 months following axotomy. Schmalbruch[29] transected the sciatic nerve and found reductions in the number of DRG neurons after 19–22 weeks. Certainly, the expression of neuropeptides on primary sensory neurons can be influenced by several factors.
This study investigated the effect of acute and severe compression of the central branches of primary sensory neurons in the seventh lumbar nerve root of dogs and resulted in the impairment of axonal flow and central chromatolysis in the neurons of the DRG, where the central branches of these neurons originated.
Kobayashi et al[30] noted a decrease in the number of ribosomes attached to the endoplasmic reticulum and an increase in free ribosomes in these neurons due to fragmentation of the rough endoplasmic reticulum making up the Nissl bodies. Nathaniel et al[31] observed an increase in nuclear clefts in rat dorsal root ganglia after direct trauma, and found that nuclei from dorsal root ganglion neurons began to show increased numbers of membrane clefts 2 to 4 days after a crush injury to the nerve root, and that these clefts contained increased numbers of nuclear pores. Nathaniel et al[31] speculated that these clefts developed in response to injury to facilitate the transport of ribonucleoproteins from the nucleus to the synthetic mechanisms of the cytoplasm. The triad of increased nuclear clefts, increased density of nuclear pores, and the aggregation of metabolic organelles suggest a relationship between mechanisms of cellular metabolism and, possibly, peptide or neuropeptide synthesis[30].
This experiment proved that severe compression of the central branches of primary sensory neurons in the seventh lumbar nerve root of dogs can cause pathological changes of neurons in the dorsal root ganglion because of disturbances of axonal flow and axonal reaction, such as neuronal degeneration, central chromatolysis in neurons and degeneration of glia and nerve fibers. If the disturbance of axonal flow caused by compression and the resulting central chromatolysis are mild, neurons can recover fully after compression is relieved. However, it seems likely that sustained mechanical compression of nerve roots could result in irreversible damage to DRG neurons.
BDNF promotes the repair of injured DRG neurons
BDNF has very extensive neurotrophy and can maintain the survival of various kinds of neurons and directly promote their axon growth[32]. The mRNA and protein for both BDNF and its major receptor, tyrosine protein kinase B receptor (TrkB), are made by discrete populations of neurons in the adult central nervous system. BDNF, which is synthesized in primary sensory neurons, is anterogradely transported to the central terminals of the primary afferents in the spinal dorsal horn, where it is involved in the modulation of painful stimuli[2]. In models of inflammatory and neuropathic pain, BDNF synthesis is greatly increased in different populations of DRG neurons[33]. BDNF plays a neuromodulatory role in spinal cord dorsal horn via the post-synaptic TrkB receptor to facilitate pain transmission[34]. Certainly, the activation of mitogen-activated protein kinases occurs in sensory neurons and contributes to persistent inflammatory and neuropathic pain by regulating BDNF expression. In fact, BDNF upregulation in the DRG and spinal cord contributes to chronic pain hypersensitivity[2,34]. The present study showed that acute and severe multiple cauda equina constrictions in experimental dogs could induce extensive and different expression of BDNF-immunoreactivity in the sensory neurons of L7 DRGs, and the immunoreactivity could persist for at least 4 weeks. BDNF expression in the cytoplasm of sensory neurons of the experimental group was significantly higher than the control group. Simultaneously, this study also suggested that the primary sensory neurons in DRG not only are effector cells of BDNF, but also can synthesize and express BDNF as a neurotrophic factor. This BDNF, coming from autocrine and paracrine secretion of neurons, can stimulate unactivated neuroblasts to proliferate, differentiate and play an important role in neuronal survival and regeneration. We believe that BDNF binds to full-length TrkB and then undergoes retrograde transport to cell bodies to produce biological effects. BDNF can also undergo anterograde transport from the cell body to the axonal extremity to be released to participate in synaptic plasticity after absorption and utilization by secondary neurons[35]. The normal DRG has mechanisms of anterograde and retrograde transport to increase the activity of sensory neurons.
Previous studies showed that deficiency of endogenous neurotrophins is associated with poor neuronal survival and cell death[36,37]. Thus, it is conceivable that chronic mechanical compression of the spinal cord induces both neuronal regeneration, mediated by neurotrophins, and cell death[38]. Several investigators have suggested that direct neurotrophin delivery provides neuroprotective effects to the spinal cord that has sustained traumatic injury[39,40,41]. These studies demonstrated the effectiveness of neurotrophin delivery in promoting neuronal cell survival, alleviation of neuronal atrophy, and facilitation of axonal regeneration. At 7, 14 and 28 days following surgery, the degeneration and necrosis in the primary sensory neurons of DRG in the control group were more remarkable than that of the experimental group, which received intrathecal injection of BDNF with persistent and slow-moving copolymer nanoparticles. Meanwhile, BDNF expression in the cytoplasm of the sensory neurons from the experimental group was significantly greater than the control group, while DRGs from sham-operated dogs did not reveal any specific neuronal staining of BDNF. The results of semiquantitative scoring and ratio of Tarlov's motor scale also suggested that intrathecal injection of BDNF could effectively prevent the degeneration and necrosis of primary sensory neurons in the DRG. The present study is considerably consistent with previous studies[42,43] and clearly shows that a certain dose of intrathecal BDNF which cannot cross the blood-brain barrier may effectively prevent cell death (apoptosis) of sensory neurons and protect against experimentally induced lesions or damage. BDNF can adjust gene expression (such as c-fos and c-jun) in neurons by modulation of upstream elements to inhibit development of cell death and necrosis, and this effect may be completed by increasing the expression of the TrkB receptor[44]. Our findings showed the remarkable positive reaction of BDNF-immunoreactivity in the cytoplasm of the sensory neurons in the experimental group, which may represent enhancement of certain neuronal activities to compensate the compromised function of sensory neurons at the level of mechanical compression. This experiment also confirmed the expression of BDNF protein and TrkB in neuroglial cells using immunohistochemical analysis. Astroglial cells produce BDNF and other nerve growth factors. In a model of spinal cord injury, Frisén et al[45] demonstrated that trkB mRNA, the receptor for BDNF, is strongly positive in motoneurons and astroglial cells, and axonal regeneration was more marked at the site with significant increase in trkB mRNA immunoreactivity within the white matter. Uchida et al[46,47] speculated that the presence of BDNF and neurotrophin-3 in neurons and astrocyte-like cells is proportionate to the severity of chronic mechanical compression and may contribute to the heterotropic neuronal reserve and survival. In summary, BDNF may play an active role in neuroprotective processes partly by maintaining intracellular protein integrity and preventing neuronal degeneration. Intrathecal injection of BDNF with persistent and slow-moving copolymer nanoparticles had obvious therapeutic effects in experimental dogs modeling acute and severe cauda equina syndrome. Nevertheless, when BDNF is considered as a therapeutic agent for the treatment of neurological disorders, it is important to recognize the extreme diversity of neurotrophic factors and their functions. In addition to the complex cytoarchitecture of the spinal cord, it would be unrealistic to expect that the administration of a single trophic factor will, by itself, elicit a comprehensive regenerative response in all relevant neuronal and glial cell populations necessary for full recovery after spinal cord injury[48].
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The experiment was performed at the Animal Care and Use Department of the Navy Institute, China, from September 2005 to June 2006.
Materials
A total of 18 adult mongrel male dogs, aged 18–48 months and weighing 10–15 kg, were purchased from the Institutional Animal Care and Use Committee of the Institute of Navy (license No. SYXK (Hu) 2007-0003). Animals were housed in individual runs, given free access to water and fed a dry certified canine diet. Animal room temperature and light cycle were controlled (targeted conditions: temperature range 18.3–25.5°C, 12-hour light/dark cycle). Humidity was not controlled but recorded regularly. Dogs were allowed to acclimate for a minimum of 7 days after receipt and conditioned to vests for 3 days prior to surgery. All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[49].
Methods
Model establishment and intervention
Each animal received intramuscular injection (i.m.) of penicillin G procaine (20 000 U/kg) and atropine (0.04 mg/kg) before surgery. Dogs were anesthetized with a parenteral solution of Su-mian-xin II (0.08–0.10 mL/kg, i.m., made by the Military Veterinarian Institute of the Academy of Military Medical Sciences, Changchun, Jilin Province, China), endotracheally intubated and artificially ventilated on a respirator with halothane (1–2%) in a mixture of oxygen and nitrous oxide (1:1). Lumbar laminectomy was performed and the dural sac of the exposed cauda equina comprising dorsal and ventral roots of L7, S1-3, Co1-5 segments was tied by four loose 4-0 ligatures (Shanghai Jade Weaver Co., Ltd., Shanghai, China) with about 2 mm spacing. The entire cauda equina was constricted by 50–75% by the first tightened constriction, and the lower cauda equina was constricted by 25–50% by the other three tightened constrictions (Figure 3).
Figure 3.
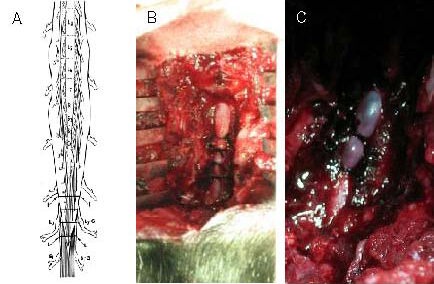
Procedure for the establishment of multiple protracted cauda equina constrictions model.
(A) Schematic drawing depicting the position of four tightened constrictions (about 2.0 mm wide) around the cauda equina (L, L). L7–G and S1–G point to the corresponding dorsal root ganglia to produce acute and severe compression, and the entire cauda equina was constricted by 50–75% by the first tightened constriction. The lower cauda equina was constricted by 25–50% by the other three tightened constrictions.
(B) Protracted multiple cauda equina constrictions during surgery on an experimental animal.
(C) Severe arterial narrowing at the level of the constriction and venous congestion of the nerve roots and dura mater of the corresponding lumbar and sacral levels were present after 48 hours of multiple cauda equina constrictions.
The central processes of the L7–Co5 DRG neurons were permanently constricted. The sham surgery group underwent cauda equina exposure, without ligation. Forty-eight hours after surgery, dogs from both control and experimental groups were again deeply anesthetized with the Su-mian-xinII (0.08–0.10 mL/kg, i.m.). The four constrictions on the cauda equina were removed through the original operative incision, and dogs in the experimental group were infused through intrathecal injection with 15 mg of encapsulated biodegradable poly(lactic-co-glycolic acid) nanoparticles carrying 2.5 mg of active BDNF (Pharmacy College of the Second Military Medical University, Shanghai, China).
DRG (L7) preparation
Two animals, one from the control and experimental groups separately, were deeply anesthetized with the Su-mian-xin II (0.08–0.10 mL/kg, i.m.) after 1, 2 and 4 weeks following the second operation. Animals were transcardially perfused through the heart with 2 L PBS followed by 2 L of 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The corresponding DRG (L7) was removed and sliced into 5-μm transverse paraffin sections for histological and immunohistochemical study.
Neuronal changes and damage in compressed DRG, observed by hematoxylin-eosin staining
Samples were taken from DRG at L7 and embedded in paraffin, before preparing 5-μm transverse sections. The sections were stained with hematoxylin-eosin and examined for the density of neurons in the DRG, and then observed using a light microscope (Olympus, Tokyo, Japan). To count the neurons, we chose a slice thickness of 5 μm and gap interval of more than 8 μm. Cell nuclei stained blue-black and cytoplasm stained salmon pink[50].
Semiquantitative scoring of pathological sections was performed independently by two pathological investigators who were blinded to the animal groups. Thus, tissue sections from each block were described qualitatively with local reactions, local inflammatory cells, and pathological change, such as chromatolysis, neuronal loss, edema or necrosis being noted. For comparison purposes, material from each animal was rated on a semiquantitative scale of 0 to IV, where 0 represented a lack of pathological findings and IV represented a severe inflammatory reaction that involved corresponding tissues and structures. These values were reported and submitted for a rank order comparison[51].
Immunohistochemical staining for BDNF expression in DRG neurons
Samples were immersed in PBS containing 30% sucrose for 24 hours at 4°C for cryoprotection. Free floating sections were immediately sliced into 5-μm paraffin sections, deparaffinized and dehydrated. Sections were subsequently washed in distilled water and then PBS, followed by microwave retrieval with citrate buffer solution (pH 6.0) for 3 minutes and 30 seconds. Sections were then rinsed in distilled water and PBS again after natural cooling. Specimens were exposed to 0.3% H2O2 in PBS for 10 minutes to inactivate endogenous peroxidase, and then rinsed in PBS. Sections were subsequently incubated with a rabbit monoclonal anti-BDNF antibody of low-density lipoprotein (1:50; Boster Biotech Corp, Wuhan, Hubei Province, China) for 1 hour at 37°C. BDNF was immunostained using the ENVISION system (DAKO, Carpinteria, CA, USA) according to the manufacturer's instructions. Staining therefore continued by rinsing in the PBS, incubation with secondary sheep anti-rabbit IgG and labeled with horseradish peroxidase (1:250; Sigma, St. Louis, MO, USA). After washing in PBS, the sections were developed with 0.05% 3,3′ diaminobenzidine tetrahydrochloride (Sigma) and 0.006% H2O2 in PBS for 10 minutes. The slides were counterstained with Meyer's hematoxylin for 10 minutes, differentiated with 75% hydrochloric acid-alcohol for 30 seconds, and then the sections were washed, dried, dehydrated and mounted on neutral resin. Positive signal was located in the cytoplasm of neurons and gliocytes and stained with buffy[50] under light microscopy (Toshiba, Tokyo, Japan).
Neurofunctional evaluation
Neurological evaluation of motor function in the posterior limbs of each animal was performed independently by two investigators blinded to the animal groups. Each animal was graded three times for 40 minutes each time according to the Tarlov's scoring system[51]. The average value was obtained before deep anesthesia and transcardial perfusion.
The ratio of Tarlov's motor scales was applied to assess neurological function. The calculation used is shown below[52]:

Statistical analysis
Nonparametric Wilcoxon's signed-ranks test was used to compare the semiquantitative scoring of the three groups using SPSS 10.0 software (SPSS, Chicago, IL, USA), and two-sample t-test was used for the analysis of Tarlov's motor scale between control and experimental groups. Data are expressed as mean ± SD. A value of P < 0.05 was considered statistically significant.
Footnotes
Junming Tan, M.D., Associate chief physician, Associate professor, Master's supervisor.
Funding: This study was financially supported by grants from the Medical Scientific Fund and Intensive Research of Nanjing Military Area Command of Chinese PLA, No. Nan 2007-13 and Nan 08Z003; and the Medical Scientific Fund and Research of Chinese PLA during the 12th Five-Year Plan Period, No. CWS11J260.
Conflicts of interest: None declared.
Ethical approval: This experimental protocol was approved by the Institutional Animal Care and Use Committee of the Naval Institute, Shanghai, China.
(Edited by Yu DW, He ZY/Qiu Y/Song LP)
REFERENCES
- [1].Hasue M. Pain and the nerve root. An interdisciplinary approach. Spine (Phila Pa 1976) 1993;18(14):2053–2058. doi: 10.1097/00007632-199310001-00022. [DOI] [PubMed] [Google Scholar]
- [2].Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55(1):1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [3].Liu Y, Kim D, Himes BT, et al. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19(11):4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu Y, Himes BT, Murray M, et al. Grafts of BDNF-producing fibroblasts rescue axotomized rubrospinal neurons and prevent their atrophy. Exp Neurol. 2002;178(2):150–164. doi: 10.1006/exnr.2002.7977. [DOI] [PubMed] [Google Scholar]
- [5].Fraser S, Roberts L, Murphy E. Cauda equina syndrome: a literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009;90(11):1964–1968. doi: 10.1016/j.apmr.2009.03.021. [DOI] [PubMed] [Google Scholar]
- [6].Marsala J, Sulla I, Jalc P, et al. Multiple protracted cauda equina constrictions cause deep derangement in the lumbosacral spinal cord circuitry in the dog. Neurosci Lett. 1995;193(2):97–100. doi: 10.1016/0304-3940(95)11676-n. [DOI] [PubMed] [Google Scholar]
- [7].Orendácová J, Cízková D, Kafka J, et al. Cauda equina syndrome. Prog Neurobiol. 2001;64(6):613–637. doi: 10.1016/s0301-0082(00)00065-4. [DOI] [PubMed] [Google Scholar]
- [8].Orendácová J, Marsala M, Cízková D, et al. Fos protein expression in sacral spinal cord in relation to early phase of cauda equina syndrome in dogs. Cell Mol Neurobiol. 2001;21(4):413–419. doi: 10.1023/a:1012610407025. [DOI] [PubMed] [Google Scholar]
- [9].Kasahara K, Nakagawa T, Kubota T. Neuronal loss and expression of neurotrophic factors in a model of rat chronic compressive spinal cord injury. Spine (Phila Pa 1976) 2006;31(18):2059–2066. doi: 10.1097/01.brs.0000231893.21964.f2. [DOI] [PubMed] [Google Scholar]
- [10].van Gelderen C. Ein orthotisches (lordotisches) kaudasyndrom. Acta Psychiat Scand. 1948;23(1-2):57–68. [PubMed] [Google Scholar]
- [11].Verbiest H. Further experiences on the pathological influence of a developmental narrowness of the bony lumbar vertebral canal. J Bone Joint Surg Br. 1955;37-B(4):576–583. doi: 10.1302/0301-620X.37B4.576. [DOI] [PubMed] [Google Scholar]
- [12].Brish A, Lerner MA, Braham J. Intermittent claudication from compression of cauda equina by a narrowed spinal canal. J Neurosurg. 1964;21:207–211. doi: 10.3171/jns.1964.21.3.0207. [DOI] [PubMed] [Google Scholar]
- [13].Graveleau J, Guiot G. Congenital narowness of the lumbar spinal canal and sensitivo-motor intermittent claudication syndrome of the cauda equina. Presse Med. 1964;72:3344–3348. [PubMed] [Google Scholar]
- [14].Vacca VM., Jr Cauda equina syndrome. Nursing. 2012;42(5):72. doi: 10.1097/01.NURSE.0000413619.44238.5f. [DOI] [PubMed] [Google Scholar]
- [15].Vanelderen P, Rouwette T, Kozicz T, et al. The role of brain-derived neurotrophic factor in different animal models of neuropathic pain. Eur J Pain. 2010;14(5):473.e1–9. doi: 10.1016/j.ejpain.2009.09.006. [DOI] [PubMed] [Google Scholar]
- [16].Delamarter RB, Bohlman HH, Bodner D, et al. Urologic function after experimental cauda equina compression. Cystometrograms versus cortical-evoked potentials. Spine (Phila Pa 1976) 1990;15(9):864–870. doi: 10.1097/00007632-199009000-00005. [DOI] [PubMed] [Google Scholar]
- [17].Porter RW, Ward D. Cauda equina dysfunction. The significance of two-level pathology. Spine (Phila Pa 1976. 1992;17(1):9–15. [PubMed] [Google Scholar]
- [18].Olmarker K, Holm S, Rydevik B. Importance of compression onset rate for the degree of impairment of impulse propagation in experimental compression injury of the porcine cauda equina. Spine (Phila Pa 1976) 1990;15:416–419. doi: 10.1097/00007632-199005000-00013. [DOI] [PubMed] [Google Scholar]
- [19].Ooi Y, Mita F, Satoh Y. Myeloscopic study on lumbar spinal canal stenosis with special reference to intermittent claudication. Spine (Phila Pa 1976) 1990;15(6):544–549. doi: 10.1097/00007632-199006000-00021. [DOI] [PubMed] [Google Scholar]
- [20].Mao GP, Konno S, Arai I, et al. Chronic double-level cauda equina compression. An experimental study on the dog cauda equina with analyses of nerve conduction velocity. Spine (Phila Pa 1976. 1998;23(15):1641–1644. doi: 10.1097/00007632-199808010-00004. [DOI] [PubMed] [Google Scholar]
- [21].Konno S, Yabuki S, Sato K, et al. A model for acute, chronic, and delayed graded compression of the dog cauda equina. Presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine (Phila Pa 1976. 1995;20:2758–2764. doi: 10.1097/00007632-199512150-00019. [DOI] [PubMed] [Google Scholar]
- [22].Sato K, Konno S, Yabuki S, et al. A model for acute, chronic, and delayed graded compression of the dog cauda equina. Neurophysiologic and histologic changes induced by acute, graded compression. Spine (Phila Pa 1976) 1995;20(22):2386–2391. doi: 10.1097/00007632-199511001-00003. [DOI] [PubMed] [Google Scholar]
- [23].Sato K, Kikuchi S. Clinical analysis of two-level compression of the cauda equina and the nerve roots in lumbar spinal canal stenosis. Spine (Phila Pa 1976) 1997;22(16):1898–1903. doi: 10.1097/00007632-199708150-00018. [DOI] [PubMed] [Google Scholar]
- [24].Marsala J, Kafka J, Lukácová N, et al. Cauda equina syndrome and nitric oxide synthase immunoreactivity in the spinal cord of the dog. Physiol Res. 2003;52(4):481–496. [PubMed] [Google Scholar]
- [25].Schönström N, Bolender NF, Spengler DM, et al. Pressure changes within the cauda equina following constriction of the dural sac. An in vitro experimental study. Spine (Phila Pa 1976) 1984;9(6):604–607. doi: 10.1097/00007632-198409000-00011. [DOI] [PubMed] [Google Scholar]
- [26].Schonstrom NS, Bolender NF, Spengler DM. The pathomorphology of spinal stenosis as seen on CT scans of the lumbar spine. Spine (Phila Pa 1976) 1985;10(9):806–811. doi: 10.1097/00007632-198511000-00005. [DOI] [PubMed] [Google Scholar]
- [27].Groves MJ, Christopherson T, Giometto B, et al. Axotomy-induced apoptosis in adult rat primary sensory neurons. J Neurocytol. 1997;26(9):615–624. doi: 10.1023/a:1018541726460. [DOI] [PubMed] [Google Scholar]
- [28].Vestergaard S, Tandrup T, Jakobsen J. Effect of permanent axotomy on number and volume of dorsal root ganglion cell bodies. J Comp Neurol. 1997;388(2):307–312. [PubMed] [Google Scholar]
- [29].Schmalbruch H. Motoneuron death after sciatic nerve section in newborn rats. J Comp Neurol. 1984;224(2):252–258. doi: 10.1002/cne.902240206. [DOI] [PubMed] [Google Scholar]
- [30].Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression. Part 2: morphological and immunohistochemical changes of dorsal root ganglion. J Orthop Res. 2004;22(1):180–188. doi: 10.1016/S0736-0266(03)00132-3. [DOI] [PubMed] [Google Scholar]
- [31].Nathaniel EJ, Nathaniel DR. Electron microscopic studies of spinal ganglion cells following crushing of dorsal roots in adult rat. J Ultrastruct Res. 1973;45(3):168–182. doi: 10.1016/s0022-5320(73)80045-0. [DOI] [PubMed] [Google Scholar]
- [32].McCall J, Weidner N, Blesch A. Neurotrophic factors in combinatorial approaches for spinal cord regeneration. Cell Tissue Res. 2012;349(1):27–37. doi: 10.1007/s00441-012-1388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Matsuoka Y, Yang J. Selective inhibition of extracellular signal-regulated kinases 1/2 blocks nerve growth factor to brain-derived neurotrophic factor signaling and suppresses the development of and reverses already established pain behavior in rats. Neuroscience. 2012;206:224–236. doi: 10.1016/j.neuroscience.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lin YT, Ro LS, Wang HL, et al. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J Neuroinflammation. 2011;8:126. doi: 10.1186/1742-2094-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tonra JR, Curtis R, Wong V, et al. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18(11):4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bregman BS. Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 1987;431(2):265–279. doi: 10.1016/0165-3806(87)90214-8. [DOI] [PubMed] [Google Scholar]
- [37].Nakamura M, Bregman BS. Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp Neurol. 2001;169(2):407–415. doi: 10.1006/exnr.2001.7670. [DOI] [PubMed] [Google Scholar]
- [38].Xu K, Uchida K, Nakajima H, et al. Targeted retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene prevents loss of mouse (twy/twy) anterior horn neurons in vivo sustaining mechanical compression. Spine (Phila Pa 1976) 2006;31(17):1867–1874. doi: 10.1097/01.brs.0000228772.53598.cc. [DOI] [PubMed] [Google Scholar]
- [39].Kishino A, Nakayama C. Enhancement of BDNF and activated-ERK immunoreactivity in spinal motor neurons after peripheral administration of BDNF. Brain Res. 2003;964(1):56–66. doi: 10.1016/s0006-8993(02)04066-0. [DOI] [PubMed] [Google Scholar]
- [40].Blits B, Boer GJ, Verhaagen J. Pharmacological, cell, and gene therapy strategies to promote spinal cord regeneration. Cell Transplant. 2002;11(6):593–613. [PubMed] [Google Scholar]
- [41].Novikova LN, Novikov LN, Kellerth JO. Survival effects of BDNF and NT-3 on axotomized rubrospinal neurons depend on the temporal pattern of neurotrophin administration. Eur J Neurosci. 2000;12(2):776–780. doi: 10.1046/j.1460-9568.2000.00978.x. [DOI] [PubMed] [Google Scholar]
- [42].Acheson A, Conover JC, Fandl JP, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374(6521):450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- [43].Tong JX, Eichler ME, Rich KM. Intracellular calcium levels influence apoptosis in mature sensory neurons after trophic factor deprivation. Exp Neurol. 1996;138(1):45–52. doi: 10.1006/exnr.1996.0045. [DOI] [PubMed] [Google Scholar]
- [44].Liu M, Kang ND, Yu EH. Changs of expression of c-fos gene and BDNF in neocortex induced by audiogenic kindling. Shenjing Jiepou Xue Zazhi. 2002;18(3):243–246. [Google Scholar]
- [45].Frisén J, Verge VM, Cullheim S, et al. Increased levels of trkB mRNA and trkB protein-like immunoreactivity in the injured rat and cat spinal cord. Proc Natl Acad Sci U S A. 1992;89(23):11282–11286. doi: 10.1073/pnas.89.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Uchida K, Baba H, Maezawa Y, et al. Histological investigation of spinal cord lesions in the spinal hyperostotic mouse (twy/twy): morphological changes in anterior horn cells and immunoreactivity to neurotropic factors. J Neurol. 1998;245(12):781–793. doi: 10.1007/s004150050287. [DOI] [PubMed] [Google Scholar]
- [47].Uchida K, Baba H, Maezawa Y, et al. Increased expression of neurotrophins and their receptors in the mechanically compressed spinal cord of the spinal hyperostotic mouse (twy/twy) Acta Neuropathol. 2003;106(1):29–36. doi: 10.1007/s00401-003-0691-4. [DOI] [PubMed] [Google Scholar]
- [48].Kwon BK, Fisher CG, Dvorak MF, et al. Strategies to promote neural repair and regeneration after spinal cord injury. Spine (Phila Pa 1976) 2005;30(17 Suppl):S3–13. doi: 10.1097/01.brs.0000175186.17923.87. [DOI] [PubMed] [Google Scholar]
- [49].The Ministry of Science and Technology of the People s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [50].Tan JM, Shi JG, Shi GD, et al. Influence on dorsal root ganglion after acute and severe cauda equina constrictions and intrathecal injection of brain-derived neurotrophic factor in experimental dogs. Jizhu Waike Zazhi. 2009;7(4):201–204. [Google Scholar]
- [51].Yaksh TL, Rathbun ML, Dragani JC, et al. Kinetic and safety studies on intrathecally infused recombinant-methionyl human brain-derived neurotrophic factor in dogs. Fundam Appl Toxicol. 1997;38(1):89–100. doi: 10.1006/faat.1997.2314. [DOI] [PubMed] [Google Scholar]
- [52].Song YM, Yang Z, Lei J. Experimental study on effect of anlsodamine for traction injury of spinal cord in rabbit. Zhongguo Jizhu Jisui Zahzi. 1999;9(4):208–211. [Google Scholar]


