Abstract
In the present study, a rat model of chronic neuropathic pain was established by ligation of the sciatic nerve and a model of learning and memory impairment was established by ovariectomy to investigate the analgesic effect of repeated electroacupuncture stimulation at bilateral Zusanli (ST36) and Yanglingquan (GB34). In addition, associated synaptic changes in neurons in the paraventricular nucleus of the hypothalamus were examined. Results indicate that the thermal pain threshold (paw withdrawal latency) was significantly increased in rats subjected to 2-week electroacupuncture intervention compared with 2-day electroacupuncture, but the analgesic effect was weakened remarkably in ovariectomized rats with chronic constrictive injury. 2-week electroacupuncture intervention substantially reversed the chronic constrictive injury-induced increase in the synaptic cleft width and thinning of the postsynaptic density. These findings indicate that repeated electroacupuncture at bilateral Zusanli and Yanglingquan has a cumulative analgesic effect and can effectively relieve chronic neuropathic pain by remodeling the synaptic structure of the hypothalamic paraventricular nucleus.
Keywords: neural regeneration, acupuncture and moxibustion, chronic neuropathic pain, electroacupuncture, acupuncture analgesia, cumulative effect, synaptic plasticity, hypothalamus, learning and memory, neurobiology, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) Repeated electroacupuncture at bilateral Zusanli (ST36) and Yanglingquan (GB34) relieved chronic neuropathic pain in a rat model of sciatic nerve injury.
(2) Hypothalamic paraventricular nucleus neural plasticity was associated with the cumulative analgesic effects of electroacupuncture.
Abbreviations
EA, electroacupuncture; CCI, chronic constrictive injury; OVX, ovariectomized; PVN, paraventricular nucleus
INTRODUCTION
Clinical studies have reported that in patients suffering from phantom pain, chronic back pain, irritable bowel syndrome, fibromyalgia or frequent headaches, local morphological alterations in brain regions that play a role in the transmission and regulation of pain, such as the cingulate cortex, orbitofrontal cortex, insula and dorsal pons, are present[1,2,3]. Experimental studies have also demonstrated that peripheral nerve injury may result in structural and functional plastic changes in the primary somatosensory cortex, anterior cingulate cortex and hypothalamus in macaque monkeys and rats[4,5].
The hypothalamus plays an important role in the homeostatic regulation of physiological functions, and helps link the body's nervous and endocrine systems. In particular, it stimulates the pituitary gland and initiates the tightly regulated stress response. Chronic pain patients often have disturbances of the hypothalamic-pituitary-adrenal axis[6,7,8]. Electroacupuncture (EA) intervention can effectively modulate chronic pain-induced structural changes in spinal cord and hippocampal neurons in rats[10,11]. However, there are few data on acupuncture-mediated modulation of synaptic changes in neurons in the hypothalamus.
Our previous studies[11,12,13] demonstrated that in rats with sciatic nerve chronic constrictive injury (CCI), repeated EA intervention at Zusanli (ST36) and Yanglingquan (GB34) could effectively relieve neuropathic pain. Concomitantly, upregulation of plasma cortisol, beta-endorphin and adrenocorticotropic hormone levels, and increased expression of hypothalamic intracellular protein kinase A, were found. In comparison, in ovariectomized (OVX) + CCI (estrogen-deprived) rats, the repeated EA intervention-induced analgesic effect was substantially weakened, suggesting an intimate association between the analgesic effect and the estrogen level[11,12,13]. Thus, it may be reasonable to conjecture that repeated EA stimulation-induced pain relief may result from favorable regulation of hypothalamic neuronal plasticity and changes in hormone and neurotransmitter levels.
In biology, structure and function are closely related to each other. Consequently, long-term functional changes often accompany structural remodeling[14]. However, the correlation between the analgesic effect of EA and plastic changes in hypothalamic neurons has not been fully investigated. Therefore, the present study was designed to analyze the relationship between the cumulative analgesic effect of repeated EA intervention and hypothalamic neuronal synaptic structural plasticity in CCI and OVX + CCI rats.
RESULTS
Quantitative analysis of experimental animals
A total of 84 female Wistar rats were randomized into control, CCI (ligation of the left sciatic nerve), CCI + EA 2 days (2D), CCI + EA 2 weeks (2W), OVX + CCI, OVX + CCI + EA 2D and OVX + CCI + EA 2W groups. Eight animals from each group were used for behavioral testing, and four from each group were used for electron microscopic observation. A model of OVX-induced learning-memory impairment was established before CCI. Rats in the EA 2D and EA 2W groups were subjected to EA at Zusanli and Yanglingquan beginning on the 4th and 16th day after surgery, respectively. Two rats in the OVX + CCI group died during OVX and were supplemented. Therefore, 84 rats were included in the final analysis.
EA alleviates pain reaction after CCI
Thermal pain threshold was represented by mean paw withdrawal latency, and the difference in paw withdrawal latency between the two limbs was used as a hyperalgesia score to assess the pain reaction. The pain scores were significantly greater in the CCI and OVX + CCI groups compared with the control group (P < 0.05), suggesting a decreased pain threshold. The pain scores were significantly lower in the CCI + EA 2W and OVX + CCI + EA 2W groups compared with the CCI and OVX + CCI groups, respectively (P < 0.05). No significant differences were found between the CCI + EA 2D and CCI groups, or between the OVX + CCI and OVX + CCI + EA 2D groups in pain scores (P > 0.05). In comparison with the CCI + EA 2W group, the pain scores in the OVX + CCI + EA 2W group were significantly higher (P < 0.05), suggesting an attenuation of analgesic effect of EA intervention in OVX rats compared with simple CCI rats (Table 1).
Table 1.
Comparison of hyperalgesia scores among the various groups
EA decreases synaptic cleft width in hypothalamic paraventricular nucleus (PVN) neurons
The ultrastructure of hypothalamic PVN neurons was observed using a transmission electron microscope (Figure 1). Compared with the control group, the synaptic cleft widths in the CCI and OVX + CCI groups were significantly larger (P < 0.05). In comparison with the CCI group, the synaptic cleft width was significantly decreased in the CCI + EA 2W group (P < 0.05), but not in the CCI + EA 2D group.
Figure 1.
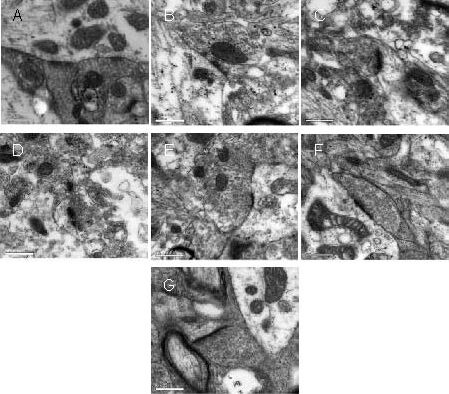
Hypothalamic paraventricular nucleus neurons in rats (transmission electron microscope, × 50 000; scale bars: 0.5 μm).
(A) Control group; (B) CCI group; (C) CCI + EA 2D group; (D) CCI + EA 2W group; (E) OVX + CCI group; (F) OVX + CCI + EA 2D group; (G) OVX + CCI + EA 2W group.
Widening of the synaptic cleft, and decreases in postsynaptic density, active zone length and synaptic interface curvature were observed after CCI and OVX + CCI.
In the CCI + EA 2W and OVX + CCI + EA 2W groups, these changes were noticeably suppressed because of the repeated EA intervention.
CCI: Chronic constrictive injury; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks; OVX: ovariectomy.
Compared with the OVX + CCI group, the synaptic cleft width was remarkably reduced in the OVX + CCI + EA 2W group (P < 0.05), but not in the OVX + CCI + EA 2D group (P > 0.05). The synaptic cleft width in the OVX + CCI + EA 2W group was significantly greater than that in the CCI + EA 2W group (P < 0.05; Figure 2).
Figure 2.
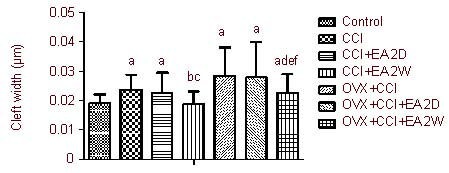
Influence of EA on the synaptic cleft in hypothalamic paraventricular nucleus neurons.
Data are expressed as mean ± SD of four rats in each group. aP < 0.05, vs. control group; bP < 0.05, vs. CCI group; cP < 0.05, vs. CCI + EA 2D group; dP < 0.05, vs. OVX + CCI group; eP < 0.05, vs. OVX + CCI + EA 2D group; fP < 0.05, vs. CCI + EA 2W group. Results were analyzed by one-way analysis of variance and least significant difference t-test.
CCI: Chronic constrictive injury; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks; OVX: ovariectomy.
EA increases postsynaptic density size in hypothalamic PVN neurons
Compared with the control group, postsynaptic density size in the CCI and OVX + CCI groups was significantly decreased (P < 0.05). The postsynaptic density size in the CCI + EA 2W group was significantly greater than that in the CCI group (P < 0.05). In comparison with the OVX + CCI group, the postsynaptic density size in the OVX + CCI + EA 2W group was significantly increased (P < 0.05). No significant differences were found between the CCI and CCI + EA 2D groups, or between the OVX + CCI and OVX + CCI + EA 2D groups (P > 0.05). Postsynaptic density sizes in the OVX + CCI + EA 2W group were significantly smaller than those in the CCI + EA 2W group (P < 0.05; Figure 3).
Figure 3.
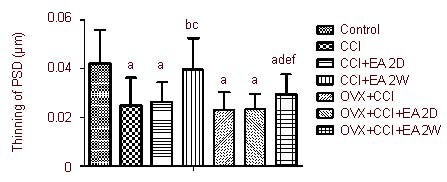
Influence of EA on postsynaptic density (PSD) of hypothalamic paraventricular nucleus neurons.
Data are expressed as mean ± SD of four rats in each group. aP < 0.05, vs. control group; bP < 0.05, vs. CCI group; cP < 0.05, vs. CCI + EA 2D group; dP < 0.05, vs. OVX + CCI group; eP < 0.05, vs. OVX + CCI + EA 2D group; fP < 0.05, vs. CCI + EA 2W group. Results were analyzed by one-way analysis of variance and least significant difference t-test.
CCI: Chronic constrictive injury; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks; OVX: ovariectomy.
EA increases synaptic active zone length in hypothalamic PVN neurons
Compared with the control group, the active zone lengths in the CCI and OVX + CCI groups were significantly decreased (P < 0.05; Figure 4). The synaptic active zones were significantly longer in the CCI + EA 2W group compared with the CCI group, and in the OVX + CCI + EA 2W group compared with the OVX + CCI group (both P < 0.05). The active zone lengths in the CCI + EA 2W group were significantly greater than those in the OVX + CCI + EA 2W group (P < 0.05). No significant differences were found between the CCI and CCI + EA 2D groups or between the OVX + CCI and OVX + CCI + EA 2D groups in active zone length (both P > 0.05; Figure 4).
Figure 4.
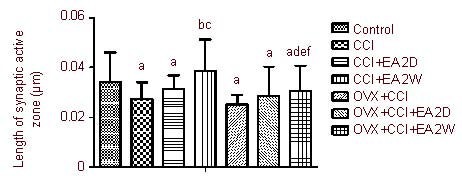
Influence of EA on the synaptic active zone of hypothalamic paraventricular nucleus neurons.
Data are expressed as mean ± SD of four rats in each group. aP < 0.05, vs. control group; bP < 0.05, vs. CCI group; cP < 0.05, vs. CCI + EA 2D group; dP < 0.05, vs. OVX + CCI group; eP < 0.05, vs. OVX + CCI + EA 2D group; fP < 0.05, vs. CCI + EA 2W group. Results were analyzed by one-way analysis of variance and least significant difference t-test.
CCI: Chronic constrictive injury; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks; OVX: ovariectomy.
EA increases the curvature of the synaptic interface in hypothalamic PVN neurons
Compared with the control group, the curvature of the synaptic interface in the CCI and OVX + CCI groups were significantly decreased (P < 0.05). The curvature of the synaptic interface was significantly increased in the CCI + EA 2W group compared with the CCI group, and in the OVX + CCI + EA 2W group compared with the OVX + CCI group (both P < 0.05). The curvature of the synaptic interface was significantly wider in the CCI + EA 2W group compared with the OVX + CCI + EA 2W group (P < 0.05). No significant differences were found between the CCI and CCI + EA 2D groups or between the OVX + CCI and OVX + CCI + EA 2D groups in the curvature of the synaptic interface (both P > 0.05; Figure 5).
Figure 5.
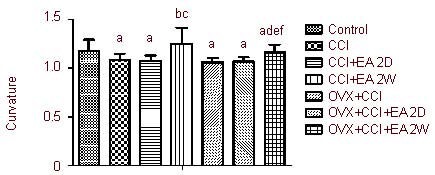
Influence of EA on the curvature of the synaptic interface of hypothalamic paraventricular nucleus neurons.
Data are expressed as mean ± SD of four rats in each group. aP < 0.05, vs. control group; bP < 0.05, vs. CCI group; cP < 0.05, vs. CCI + EA 2D group; dP < 0.05, vs. OVX + CCI group; eP < 0.05, vs. OVX + CCI + EA 2D group; fP < 0.05, vs. CCI + EA 2W group. Results were analyzed by one-way analysis of variance and least significant difference t-test.
CCI: Chronic constrictive injury; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks; OVX: ovariectomy.
DISCUSSION
In neuroscience, synaptic plasticity is the ability of the connection, or synapse, between two neurons to change in strength in response to either use or disuse of transmission over synaptic pathways[15]. Girardet obtained data highlighting the close cooperation between neurons and glia in the functional adaptation of the brain to the changing conditions of the internal and external environment. Structural plasticity of the adult central nervous system is related to the neuroendocrine activities of the hypothalamus[16]. Significant remodeling of synaptic contacts is found in corticotropin-releasing hormone neurons in response to chronic stress. This morphological plasticity might be related to hyperactivity of the hypothalamic-pituitary-adrenal axis and the development of stress-related psychopathologies[17]. Peripheral nerve injury results in increases in synaptic density or number, in the percentage of positively curved synapses[18,19], and in interface curvature, as well as in the number of curved spine synapses, spine synapses and perforation synapses in the dorsal horn of the spinal cord[20]. This morphological rearrangement may alter the organism's pain perception ability or result in abnormal sensory processing (including tactile allodynia) as an adaptive and protective response[21,22,23]. The synaptic cleft (the space between the presynaptic and postsynaptic endings) is a space for chemical transmission.
The postsynaptic density is a protein-dense specialization attached to the postsynaptic membrane, serving as a signaling apparatus. The synaptic active zone is a unique presynaptic membrane specialization that is believed to be the site of neurotransmitter release. Synaptic interface curvature is a sign of the state of transmitter release, and may even reflect changes in the structural organization of receptor protein molecules on the postsynaptic membrane. Increasing interface curvature may represent enlargement of the synaptic contact, or it may reflect a reduction in the area that the neurotransmitter must diffuse across[24].
Results of the present study demonstrate that in chronic neuropathic pain and in OVX + CCI rats, the pain threshold is significantly decreased. In parallel, hypothalamic synaptic cleft width is increased significantly, and the synaptic postsynaptic density size, active zone length and the curvature of the synaptic interface of PVN nerve cells were decreased considerably in CCI and OVX + CCI rats, indicating dysfunction of the synaptic signal transmission and abnormal plasticity changes. These results are basically identical to previous results[18,19,20].
After EA intervention, the pain threshold was increased significantly in EA-2W rats, being markedly higher than in EA-2D rats, suggesting a cumulative analgesic effect of repeated EA stimulation of Zusanli and Yanglingquan in rats with neuropathic pain. Moreover, when the cumulative analgesic effect of EA was induced, the increase in synaptic cleft width was considerably diminished, and the reductions in postsynaptic density size, active zone length and synaptic interface curvature were substantially mitigated in EA-2W (both CCI and OVX + CCI) rats. In comparison with the effects of EA-2W on simple CCI rats, the effects of EA-2W on OVX + CCI rats were substantially weaker, both in their ability to modulate the behavioral response and in their ability to induce plastic changes at the synapse; this is likely to be because of estrogen deprivation in OVX rats. These results reveal that repeated EA intervention-induced pain relief is accompanied with enhanced hypothalamic PVN neural synaptic plasticity, including narrowing of the synaptic cleft and increases in postsynaptic density size, active zone length and interface curvature, which favors release of neurotransmitters and their binding to cell membrane receptors, resulting in an increase in synaptic signal transmission efficiency in the PVN.
These observations are similar to our previous findings[11,12,13,25] and the results of other groups. For example, Yan et al[26] observed that EA of Huantiao (GB30) and Weizhong (BL40) for one week clearly alleviates neuropathic pain in CCI rats. Kim et al[27] reported that acupuncture treatment at acupoint Yanglingquan increases dopamine release, which in turn may lead to the enhancement of dopamine availability in the synaptic cleft, increase postsynaptic dopamine neurotransmission and facilitate the normalization of basal ganglia activity. Luo et al[28] found that in rats with cerebral ischemia, the injury-induced reduction in synaptic number density, postsynaptic density size, synaptic interface curvature and synaptic cleft width were improved remarkably following EA treatment. These synaptic ultrastructural changes in the cerebral ischemic marginal zone were closely related to increases in expression of excitatory amino acid transporter-2, connexin 43 and glial fibrillary acidic protein, and a decrease in average Ca2+ fluorescence intensity in astrocytes on days 3 and 7 after EA. These observations suggest that EA might modulate the activation state of astrocytes and promote a beneficial interaction between astrocytes and synapses; this may underlie the ability of EA to induce synaptic reorganization. Lu et al[29] and Shao et al[30] also observed similar effects of repeated EA intervention in reversing aging-induced thinning of the hippocampal neuronal postsynaptic density and in reversing the increase in synaptic cleft width in senescence- accelerated mice. In OVX + CCI rats, the effect of EA-2W in improving synaptic plasticity might be associated with its functions in regulating neuronal activity in the hypothalamic PVN related to hormone secretion[31] and synaptophysin expression[25].
There are a number of reports on the cumulative effects of EA analgesia[32,33]. However, a systematic study on the mechanisms underlying the analgesic effect of repeated EA intervention has been lacking. The present study suggests that hypothalamic PVN neural plasticity mediates the cumulative analgesic effect of EA and provides insight into the underlying synaptic ultrastructural changes. It is possible that repeated EA intervention promotes the storage of pain-relief information in the brain, which is consolidated gradually after multiple treatments, resulting in the establishment of the cumulative analgesic effect.
In conclusion, repeated EA intervention of Zusanli and Yanglingquan has a cumulative analgesic effect in rats with chronic neuropathic pain. This effect is intimately associated with EA's ability to suppress the chronic paininduced increase in synaptic cleft width, thinning of the postsynaptic density and reduction in active zone length and interface curvature in hypothalamic PVN neurons.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
The experiments were performed in the Department of Physiology, Institute of Acu-moxibustion, China Academy of Chinese Medical Sciences, China from November 2007 to April 2009.
Materials
A total of 84 clean-grade, female Wistar rats, aged 3–4 months, weighing 240–250 g, were purchased from the Experimental Animal Center of Chinese Academy of Medical Sciences (License No. SCXX-Army 2007-004). Animal care and experimental process were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[34].
Methods
Establishment of memory impairment model
After anesthesia, rats in the OVX + CCI, OVX + CCI + EA 2D and OVX + CCI + EA 2W groups underwent OVX. Briefly, bilateral mid-abdominal dorsolateral incisions (about 2 cm long) were made, and both ovaries were removed. Four weeks after OVX, four animals from each group (except the control group) were subjected to a vaginal smear test for verifying OVX success. Forty-five days after OVX, the rats’ learning-memory ability was analyzed by escape latency (place navigation test), swimming distance in the target quadrant and target quadrant crossing times (spatial probe test) in the Morris water maze test for 7 days using a Morris water maze device (DigBehv-MWM Morris Water Maze Video Analysis System, Shanghai Jiliang Co. Ltd., Shanghai, China) as previously described[35]. Results indicated that the OVX rats had a defect in learning and memory (results not shown).
Establishment of CCI models
Chronic neuropathic pain model was established by ligation of the unilateral sciatic nerve according to a modification of previously published methods[36]. Under intraperitoneal anesthesia with a mixed solution of 28 mg/100 g urethane (Beijing Chemistry Reagent, Beijing, China) and 3.3 mg/100 g chloralose (Sigma-Aldrich, St. Louis, MO, USA) and routine sterilization, the left sciatic nerve was exposed at the mid-thigh level by blunt dissection through the biceps femoris muscle. Four constrictive ligatures (4-0 surgical suture) were tied around the nerve at the distal end close to the nerve bifurcation at spaces about 1.0 mm apart. The ligature was considered to be suitable if local and moderate muscular contraction of the leg was clearly observed. Following local application of antibiotics (sodium penicillin, 9 000–10 000 U/rat), the muscle and skin tissues were sutured in layers. To reduce experimental variability, all surgeries were performed by the same operator. For OVX + CCI rats, CCI surgery was performed 4–5 days after the Morris water maze test.
EA intervention
Bilateral Zusanli (5 mm beneath the capitulum fibulae and lateral-posterior to the knee-joint) and Yanglingquan (about 5 mm superior-lateral to Zusanli) were punctured with filiform needles (Hua Tuo acupuncture needle, Suzhou Medical Appliance Factory, Suzhou, China; Gauge 28, 0.5 cun) to a depth of 2–3 mm and electrically stimulated using a HAN's EA Apparatus (LH202, Beijing Huawei Industrial Developing Company, Beijing, China). EA (2/15 Hz, 1 mA) was administered for 30 minutes, once daily beginning on the 4th day after CCI surgery for 2 weeks for rats in the CCI + EA 2W and OVX + CCI + EA 2W groups, and on the 16th day after CCI surgery for 2 days for rats in the CCI + EA 2D and OVX + CCI + EA 2D groups.
Thermal pain threshold detection
Each rat was placed into a black cloth bag with the hindlimbs and tail exposed to move freely. A mobile radiant heat source (high-intensity light beam with radiant heat dolorimeter; Thermotron, Holland, Michigan, USA) was focused on the plantar surface of the hind paw. Paw withdrawal latency (i.e., pain threshold) of the bilateral footplates was measured three times with an interval of 3–5 minutes between detections. To avoid potential tissue damage, the cutoff time of the radiant heat radiation was set to 20 seconds. Thermal pain threshold detection was conducted prior to as well as 4, 8, 12, 16 and 18 days after CCI. Pain thresholds were measured in rats from the CCI + EA and OVX + CCI + EA groups on the following morning for observing the post-effects of EA.
Transmission electron microscopy observation of hypothalamic ultrastructure
At the end of each experiment and under deep anesthesia, the rat was perfused transcardially by using a solution of 2% paraformaldehyde + 2% glutaraldehyde. Then, the brain tissue containing the PVN of the hypothalamus[37] was cut into small cubic pieces (about 1 mm3), fixed with 3% glutaraldehyde (EMS Company, Shanghai, China) and 1% osmium tetroxide, sequentially, for 2 hours, dehydrated with ethanol, submerged in acetone, embedded in 812 Epon-Araldite, sectioned at 250-nm thickness, and stained using uranyl acetate and lead citrate. The brain sections were then examined under a JEM-1230 transmission electron microscope (JEOL Ltd, Tokyo, Japan) and areas of interest that contained synapses were imaged using a NIS-Elements BR2.30 (Nikon, Tokyo, Japan) operated at 200 keV and a Gatan 2K × 2K CCD camera at a magnification of 50 000 ×. The average synaptic cleft width, the thickness of the postsynaptic density and the length of the active zone in 10 optional visual fields of sections of each brain (four rats/group) were assessed. The values for the synaptic active zone (the length of the postsynaptic density, the thickness of the postsynaptic density and the synaptic cleft width) were measured separately according to previously published methods[38] (Figure 6). The curvature of the synaptic interface was also measured[39] (Figure 7).
Figure 6.
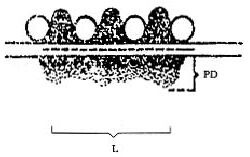
Schematic illustration of a synaptic connection showing the measured indexes in the present study.
PD: Postsynaptic density; L: length of postsynaptic density.
Figure 7.
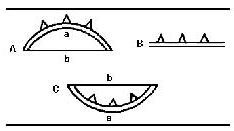
Schematic illustration of a synaptic connection showing the measured indexes in the present study[37].
A: Forward bending synaptic membrane; B: flat synaptic membrane; C: negative bending synaptic membrane. Curvature = Arc length/Chord length = a/b.
Statistical analysis
Data were expressed as mean ± SD. Differences in paw withdrawal latency were assessed using one-way analysis of variance with repeated measures when appropriate. Least significant difference, t-test was used to compare data between two groups. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Yuanshen Wang and Qing Cai from the Beijing Capital Medical University, China for technical assistance.
Footnotes
Qiuling Xu, M.D., Associate professor.
Funding: This work was supported by the National Natural Science Foundation of China, No. 30472241, 90709031 and 30973796; the National Basic Research Program of China for Traditional Chinese Medicine Theory (“973” Program), No. 2007CB512505; the Natural Foundation of Hainan Province (No. 310054); and a grant from the Health Department of Hainan Province (QiongWei 2010-45).
Conflicts of interest: None declared.
Ethical approval: All procedures were approved by the Animal Ethical Committee, Institute of Acu-moxibustion of China Academy of Chinese Medical Sciences, China.
(Edited by Qi XJ, Liu JL/Su LL/Wang L)
REFERENCES
- [1].May A. Chronic pain alters the structure of the brain. Schmerz. 2009;23(6):569–575. doi: 10.1007/s00482-009-0842-1. [DOI] [PubMed] [Google Scholar]
- [2].Peyron R, Schneider F, Faillenot I, et al. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63(10):1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- [3].Seifert F, Maihöfner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 2009;66(3):375–390. doi: 10.1007/s00018-008-8428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult Macaque monkeys. Science. 1998;282(5391):1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- [5].Jaggi AS, Singh N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res. 2011;1381:187–201. doi: 10.1016/j.brainres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- [6].Blackburn-Munro G. Hypothalamo-pituitar-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Curr Pain Headache Rep. 2004;8(2):116–124. doi: 10.1007/s11916-004-0025-9. [DOI] [PubMed] [Google Scholar]
- [7].Ulrich-Lai YM, Xie W, Meij JT, et al. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav. 2006;88(1-2):67–76. doi: 10.1016/j.physbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- [8].Rouwette T, Vanelderen P, de Reus M, et al. Experimental neuropathy increases limbic forebrain CRF. Eur J Pain. 2012;16(1):61–71. doi: 10.1016/j.ejpain.2011.05.016. [DOI] [PubMed] [Google Scholar]
- [9].Xing GG, Liu FY, Qu XX, et al. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol. 2007;208(2):323–332. doi: 10.1016/j.expneurol.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [10].Yang ZX, Zhuo YY, Yu HB, et al. Effect of electroacupuncture on cellular structure of hippocampus in splenic asthenia pedo-rats. Zhen Ci Yan Jiu. 2010;35(1):37–42. [PubMed] [Google Scholar]
- [11].Liu JL, Chen SP, Gao YH, et al. Effects of repeated electroacupuncture on beta-endorphin and adrencorticotropic hormone levels in the hypothalamus and pituitary in rats with chronic pain and ovariectomy. Chin J Integr Med. 2010;16(4):315–323. doi: 10.1007/s11655-010-0503-3. [DOI] [PubMed] [Google Scholar]
- [12].Wang JY, Chen SP, Li YH, et al. Observation on the accumulative analgesic effect of electroacupuncture and the expression of protein kinase A in hypothalamus and hippocampus in chronic pain or/and ovariectomized rats. Zhen Ci Yan Jiu. 2008;33(2):80–87. [PubMed] [Google Scholar]
- [13].Liu JL, Chen SP, Gao YH, et al. Observation on the analgesic effect of repeated electroacupuncture and its relation to changes of plasma beta-EP, ACTH and COR levels. Zhen Ci Yan Jiu. 2007;32(5):306–312. [PubMed] [Google Scholar]
- [14].Durmowicz AG, Stenmark KR. Mechanisms of structural remodeling in chronic pulmonary hypertension. Pediatr Rev. 1999;20(11):e91–102. [PubMed] [Google Scholar]
- [15].Hughes JR. Post-tetanic potentiation. Physiol Rev. 1958;38(1):91–113. doi: 10.1152/physrev.1958.38.1.91. [DOI] [PubMed] [Google Scholar]
- [16].Girardet C, Bosler O. Structural plasticity of the adult central nervous system: insights from the neuroendocrine hypothalamus. Biol Aujourdhui. 2011;205(3):179–197. doi: 10.1051/jbio/2011018. [DOI] [PubMed] [Google Scholar]
- [17].Miklós IH, Kovács KJ. Reorganization of synaptic inputs to the hypothalamic paraventricular nucleus during chronic psychogenic stress in rats. Biol Psychiatry. 2012;71(4):301–308. doi: 10.1016/j.biopsych.2011.10.027. [DOI] [PubMed] [Google Scholar]
- [18].Bakkum BW, Henderson CN, Hong SP, et al. Preliminary morphological evidence that vertebral hypomobility induces synaptic plasticity in the spinal cord. J Manipulative Physiol Ther. 2007;30(5):336–342. doi: 10.1016/j.jmpt.2007.04.007. [DOI] [PubMed] [Google Scholar]
- [19].Peng B, Yang ZW, Min S. Number of synapses increased in the rat spinal dorsal horn after sciatic nerve transection: a stereological study. Brain Res Bull. 2011;84(6):430–433. doi: 10.1016/j.brainresbull.2011.01.007. [DOI] [PubMed] [Google Scholar]
- [20].Peng ZG, Wang YJ, Wu XY, et al. Changes in synaptic structural plasticity in lamine II of spinal dorsal horn of rats with neuropathic pain. Shenjing Sunshang yu Gongneng Chongjian. 2011;6(3):161–165. [Google Scholar]
- [21].McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- [22].Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hama AT, Pappas GD, Sagen J. Adrenal medullary implants reduce transsynaptic degeneration in the spinal cord of rats following chronic constriction nerve injury. Exp Neurol. 1996;137(1):81–93. doi: 10.1006/exnr.1996.0009. [DOI] [PubMed] [Google Scholar]
- [24].Baron MK, Boeckers TM, Vaida B, et al. An architectural framework that may lie at the core of the postsynaptic density. Science. 2006;311(5760):531–535. doi: 10.1126/science.1118995. [DOI] [PubMed] [Google Scholar]
- [25].Xu QL, Chen SP, Gao YH, et al. Observation on the analgesic effect of repeated electroacupuncture and its relation to the expression of synaptophysin in rat's hippocampus and hypothalamus. Zhongguo Kangfu Yixue Zazhi. 2009;24(6):498–501. [Google Scholar]
- [26].Yan LP, Wu XT, Yin ZY, et al. Effect of electroacupuncture on the levels of amino acid neurotransmitters in the spinal cord in rats with chronic constrictive injury. Zhen Ci Yan Jiu. 2011;36(5):353–356. 379. [PubMed] [Google Scholar]
- [27].Kim SN, Doo AR, Park JY, et al. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson's disease. PLoS One. 2011;6(11):e27566. doi: 10.1371/journal.pone.0027566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luo Y, Xu NG, Yi W, et al. Study on the correlation between synaptic reconstruction and astrocyte after ischemia and the influence of electroacupuncture on rats. Chin J Integr Med. 2011;17(10):750–757. doi: 10.1007/s11655-011-0754-7. [DOI] [PubMed] [Google Scholar]
- [29].Lu SF, Shao X, Tang Y, et al. Effects of electroacupuncture on neural cell adhesion of synaptic plasticity in the hippocampus of SAMP8. Zhongguo Kangfu Yixue Zazhi. 2008;23(12):1057–1060. [Google Scholar]
- [30].Shao X, Yu SG, Lu SF, et al. Influence of electro-acupuncture therapy on synaptic ultrastructure of hippocampal neuron of SAMP8 rats. Zhongguo Laonian Xue Zazhi. 2009;29(7):780–782. [Google Scholar]
- [31].Ma S, Wu J, Feng Y, et al. Elevated estrogen receptor expression in hypothalamic preoptic area decreased by electroacupuncture in ovariectomized rats. Neurosci Lett. 2011;494(2):109–113. doi: 10.1016/j.neulet.2011.02.070. [DOI] [PubMed] [Google Scholar]
- [32].Dong ZQ, Ma F, Xuan CT, et al. Cumulative electroacupuncture enhances expression of GDNF mRNA in dorsal root ganglions of neuropathic pain rats. Shanghai Zhenjiu Zazhi. 2005;24(2):33–36. [Google Scholar]
- [33].Takahashi H. Effects of scalp acupuncture and auricular therapy on acute herpetic pain and postherpetic neuralgia: a case series. Acupunct Med. 2007;19(2):113–120. [Google Scholar]
- [34].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [35].Nunez J. Morris Water Maze Experiment. J Vis Exp. 2008;19:pii:897. doi: 10.3791/897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bennett GJ, Xie YK. A peripheralmononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- [37].Paxions G, Watson C. Beijing: People's Medical Publishing House; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [38].Güldner FH, Ingham CA. Increase in postsynaptic density material in optic target neuron of the rat supyachiasmatic necleus after bilateral enucleation. Neurosci Lett. 1980;17:27–31. doi: 10.1016/0304-3940(80)90056-7. [DOI] [PubMed] [Google Scholar]
- [39].Jones DG, Devon RM. An ultrastructrural study into the effect of pentobarbition on synaptic organization. Brain Res. 1978;147(1):47–63. doi: 10.1016/0006-8993(78)90771-0. [DOI] [PubMed] [Google Scholar]


