Abstract
Oligodendrocyte lineage gene-1 expressed in oligodendrocytes may trigger the repair of neuronal myelin impairment, and play a crucial role in myelin repair. Hypoxia-inducible factor 1α, a transcription factor, is of great significance in premature infants with hypoxic-ischemic brain damage. There is little evidence of direct regulatory effects of hypoxia-inducible factor 1α on oligodendrocyte lineage gene-1. In this study, brain slices of Sprague-Dawley rats were cultured and subjected to oxygen-glucose deprivation. Then, slices were transfected with hypoxia-inducible factor 1α or oligodendrocyte lineage gene-1. The expression levels of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 were significantly up-regulated in rat brains prior to transfection, as detected by immunohistochemical staining. Eight hours after transfection of slices with hypoxia-inducible factor 1α, oligodendrocyte lineage gene-1 expression was upregulated, and reached a peak 24 hours after transfection. Oligodendrocyte lineage gene-1 transfection induced no significant differences in hypoxia-inducible factor 1α levels in rat brain tissues with oxygen-glucose deprivation. These experimental findings indicate that hypoxia-inducible factor 1α can regulate oligodendrocyte lineage gene-1 expression in hypoxic brain tissue, thus repairing the neural impairment.
Keywords: neural regeneration, brain injury, biological factors, hypoxia-inducible factor 1α, oligodendrocyte lineage gene-1, oxygen-glucose deprivation, brain slice culture, immunohistochemistry, oligodendrocyte, myelin repair, premature delivery, rat, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) Brain slices in premature rats were subjected to oxygen-glucose deprivation to generate human brain slice culture models of premature infants with hypoxic-ischemic brain damage.
(2) The expression levels of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 were dynamically observed in a broader attempt to explore their expression during hypoxic-ischemic brain damage and their roles in nerve repair.
(3) Hypoxia-inducible factor 1α can regulate oligodendrocyte lineage gene-1 in hypoxic brain tissue, thus repairing the neural impairment.
INTRODUCTION
Despite recent developments in the fields of perinatal and neonatal medicine, there is still less improvement in treatments for abnormal cognition and behavior in premature infants[1,2,3]. Repair mechanisms and treatments for brain injury in prematurity are increasingly attracting the attention of neonatal and perinatal medicine experts[4,5]. However, the molecular biological mechanisms underlying brain injury in premature infants are also poorly understood.
Prenatal, perinatal and postnatal brain injury is regarded as the leading cause of long-term severe neurological defects[6,7]. Early brain injury results from many potential causes, rather than a single factor[8]. The main mechanisms of pathogenesis proposed include hypoxia/ischemia and infection[9,10,11]. Ischemia causes the activation of microglial cells, triggers an inflammatory reaction, increases local blood flow, and eventually changes the functions of glial cells and neurons[12]. Endothelial cells are also involved in the inflammatory reaction through generating proinflammatory mediators; these mediators alter local blood flow and vascular permeability, and promote leukocyte adhesion[13]. Cytokines are toxic to cerebral white matter by inhibiting differentiation of oligodendrocyte progenitor cells, inducing glial cell apoptosis and triggering myelin degradation[14].
Most studies on functional rehabilitation after brain injury have been confined to the protection of neurons, while few have focused on glial cells such as oligodendrocytes. Oligodendrocytes play important roles in the development and prognosis of periventricular leukomalacia[15]. Therefore, knowledge of the mechanism underlying oligodendrocyte injury could contribute to better treatment of white matter damage, provide a new therapeutic strategy for neurological defects, and serve as a new target for promoting the recovery of neurological functions.
Transcription factor oligodendrocyte lineage gene-1 expression in oligodendrocytes may trigger the repair of damaged neuronal myelin[16,17,18]. Therefore, oligodendrocyte lineage gene-1 plays a crucial role in myelin repair[16,17,18]. Oligodendrocyte lineage gene-1 is expressed in oligodendrocytes and repairs myelin after brain demyelination injury[16]. Oligodendrocyte lineage gene-1 is essential for the development of myelin, and knockout mice for this gene show shortened survival[17]. Oligodendrocyte lineage gene-1 knockout mice also show glial cell retardation[18]. In addition, oligodendrocyte lineage gene-1 is of importance in the later stages of oligodendrocyte development[19]. Expression of oligodendrocyte lineage gene-1 also influences myelin repair in the brain tissues of rodents and multiple sclerosis patients[20]. Oligodendrocyte lineage gene-1 is necessary for myelin regeneration after neural progenitor cells transplantation in virus-induced demyelination models[21]. Oligodendrocyte lineage gene-1 is also expressed during the maturation and regeneration of human oligodendrocytes[22].
Similarly, hypoxia-inducible factor 1 plays an extremely key protective role following hypoxic-ischemic brain damage. Hypoxia-inducible factor 1α and tissue adaptability-related protein expressed in oligodendrocytes may protect against neurological impairment[23]. Hypoxia-inducible factor 1α promotes the proliferation and differentiation of endogenous neural stem cells[24]. Hypoxia-inducible factor 1α is also involved in the functions of neural stem cells and post-stroke regeneration[25]. Hypoxia-inducible factor 1α exerts neuroprotective effects and neurotoxic effects based on the cell type and severity of hypoxia/ischemia[26].
In this study, brain slices of immature rats were subjected to oxygen-glucose deprivation to simulate human premature infants with hypoxic-ischemic brain damage. Expression of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 protein expression was detected using immunohistochemistry. The goal of this study was to detect the expression of these two proteins after hypoxic-ischemic brain damage, and to explore the roles of the two in nerve repair.
RESULTS
Brain slice groupings
Brain slices from 3-day-old rats were randomly divided into four groups: a blank control group (brain slices were cultured in complete medium, without hypoxia and glucose deprivation); an oxygen-glucose deprivation group [brain slices were cultured in hypoxia (8% oxygen + 92% nitrogen) and deprived from glucose (glucose-free medium) for 60 minutes, and then oxygen glucose supply was restored]; a hypoxia-inducible factor 1α transfection group (brain slices were deprived from oxygen-glucose and transfected with hypoxia-inducible factor 1α); and an oligodendrocyte lineage gene-1 transfection group (brain slices were deprived from oxygen-glucose and transfected with oligodendrocyte lineage gene-1). Five brain slices in each group were collected at 4 hours, 8 hours, 1 day and 3 days after transfection.
General observation of cultured brain slices
The cultured brain slices grew well and became thinner as the culture advanced. The slice thickness decreased from 450 μm to 150 μm at 1 week under an inverted microscope, and was maintained at 150 μm for 30 days. The brain slice structure could be clearly seen (Figure 1).
Figure 1.
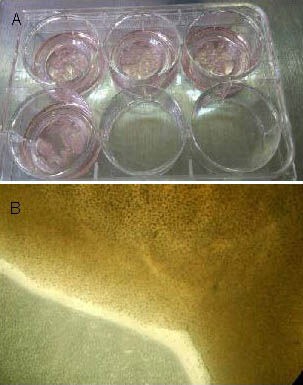
Morphology of cultured brain slices in neonatal rats with hypoxic-ischemic brain damage (before transfection).
Visual inspection (A) and optical microscope (B, × 100) observations showing that the cultured brain slices grew well, with no necrosis.
Identification of oxygen-glucose deprived models
At 4 hours after modeling, the blank control group showed clear cell structure, intact nuclear membrane, clear nucleoli, and evenly distributed chromatin (Figures 2A–D). In the oxygen-glucose deprivation group, cytoplasm rupture, nuclear pyknosis, karyorrhexis, and karyolysis were observed (Figures 2E–H).
Figure 2.
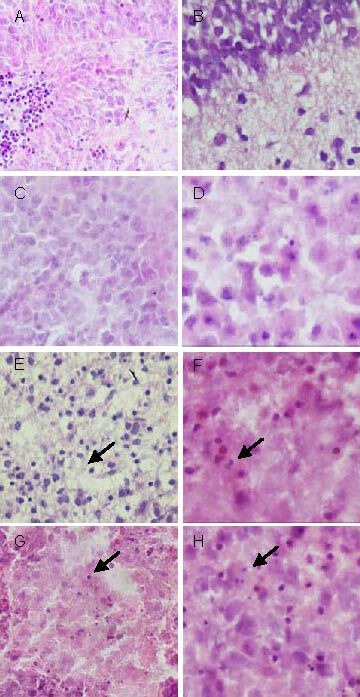
Hypoxic/ischemic animal models and cultured brain slices (subcortical) models before transfection (hematoxylin-eosin staining).
(A–D) Blank control group: cells grew well; (E–H) oxygen-glucose deprivation group: cells exhibited nuclear pyknosis and karyorrhexis (arrows); (A, B, E, F: × 400) frozen sections of brain slices in vivo; (C, D, G, H: × 1 000) brain slices 4 hours after modeling. Hypoxic-ischemic animal models are consistent with cultured brain slice models with hematoxylin-eosin staining.
Compared with the blank control group, the neuronal nuclear pyknosis and karyorrhexis were seen in rat cerebral cortex, hippocampus and lateral ventricle were significantly attenuated after oxygen-glucose deprivation (Figure 3).
Figure 3.
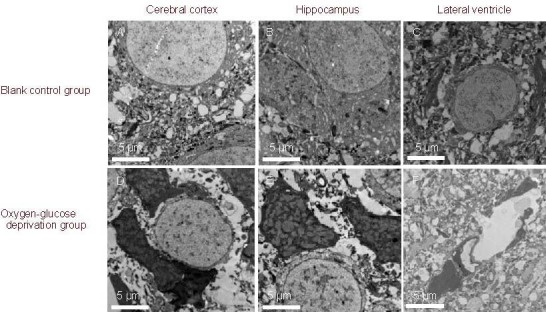
Ultrastructure of rat brain slices in oxygen-glucose deprived rats before transfection (uranyl acetate-lead citrate staining, transmission electron microscopy, × 3 000).
Compared with the blank control group, nuclear pyknosis, karyorrhexis, and myelinated axons in rat cerebral cortex, hippocampus and lateral ventricle were significantly attenuated after oxygen-glucose deprivation. Neuropil edema and mitochondrial swelling were visible.
Expression of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 in hypoxic-ischemic brain damage
After oxygen-glucose deprivation, hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 expression levels were both up-regulated in the brain tissue of oxygen-glucose deprived rats, with a more obvious increase in the expression level of hypoxia-inducible factor 1α (Figures 4, 5).
Figure 4.
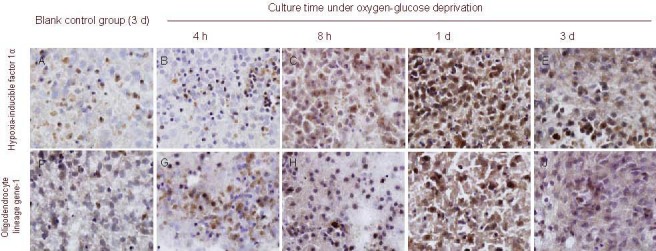
Expression of hypoxia-inducible factor 1α (A–E) and oligodendrocyte lineage gene-1 (F–J) in brain tissue of oxygen-glucose deprived rats prior to transfection (immunohistochemical staining, × 1 000).
Expression levels of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 were up-regulated at each time point, especially hypoxia-inducible factor 1α.
Figure 5.
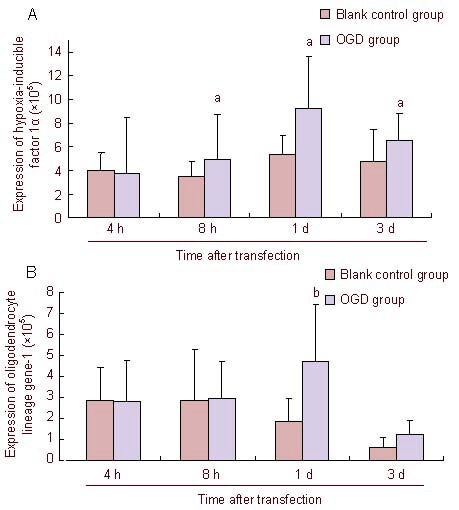
Immunohistochemical semiquantitative results for hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 in brain tissue of oxygen-glucose deprived (OGD) rats.
Expression of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 is expressed as absorbance value. aP < 0.01, bP < 0.05, vs. OGD group. Data are expressed as means ± SD, n = 5, Mann-Whitney U test.
As shown in Figures 4 and 5, the expression levels of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 were the same after oxygen-glucose deprivation. Their expression levels began to increase at 8 hours after oxygen-glucose deprivation, reaching a peak at 1 day, and gradually decreased at 3 days. The expression of hypoxia-inducible factor 1α in the oxygen-glucose deprivation group was significantly higher than that in the control group at 8 hours, 1 day and 3 days (P < 0.05), while oligodendrocyte lineage gene-1 expression in the oxygen-glucose deprivation group was significantly higher than that in the control group at 1 day (P < 0.01).
Expression of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 in rat brains after exogenous transfection
At 8 hours after exogenous transfection of slice cultures, hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 expression in brain slices began to rise, reaching a peak at 1 day. The expression of oligodendrocyte lineage gene-1 at each time point was significantly increased after transfection, compared with the oxygen-glucose deprivation group (P = 0.018, 0.001; Figure 6).
Figure 6.
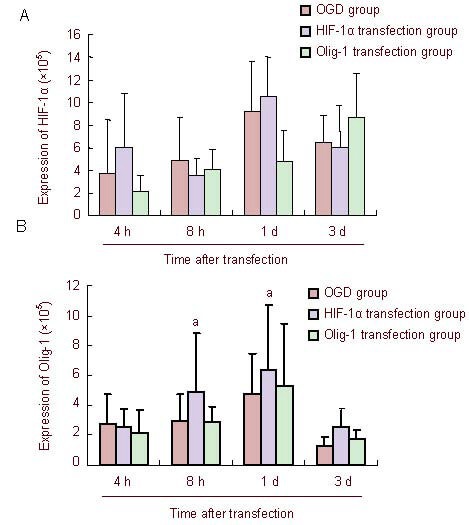
Immunohistochemical semiquantitative results for hypoxia-inducible factor 1α (HIF-1α) and oligodendrocyte lineage gene-1 (Olig-1) after exogenous transfection.
The expression of HIF-1α and Olig-1 is represented as the mean absorbance value. aP < 0.01, vs. oxygen-glucose deprived (OGD) group. Data are expressed as mean ± SD, n = 5, Mann-Whitney U test.
DISCUSSION
The survival rates of premature infants with very low birth weight increase. However, neurodevelopmental sequelae are evident in the growth and development process, mainly due to brain injury in infants.
Periventricular leukomalacia is one of the most common brain injuries in premature infants, and the leading cause of premature death and neurological sequelae. Brain injury in premature infants is often induced by hypoxia/ischemia and septicemia[27,28,29,30]. The selective vulnerability of oligodendrocyte precursor cells to hypoxic/ischemic injury provides a pathological explanation for the white matter damage in preterm infants. Free radicals and glutamate-mediated toxicity and infection may cause oligodendrocyte injury. Oligodendrocyte precursor cells injury hinders myelin formation in white matter, and results in cerebral palsy and mental retardation. Therefore, understanding of the repair mechanisms after oligodendrocyte injury could provide a new strategy for the prevention of the neurological defects associated with periventricular leukomalacia. In addition, it can be regarded as a new target for promoting the recovery of neurological function. Hypoxia-inducible factor 1 is a transcription factor that regulates the expression of more than 70 target genes. Many genes such as vascular endothelial growth factor have been confirmed as target genes of hypoxia-inducible factor 1[31,32,33,34]. When hypoxia/ischemia occurs, hypoxia-inducible factor 1 can strongly modulate target gene expression[35,36,37,38], improve energy metabolism disorder, promote cerebral hemodynamic recovery, inhibit excitatory amino acid toxicity, and reduce apoptosis[39]. The hypoxia-inducible factor 1α expression level decreases as hypoxia is attenuated[40,41,42,43]. Hypoxia-inducible factor 1α gene knockout (hypoxia-inducible factor 1α+/−) aggravated cerebral ischemia and reduced survival rates in mice[36]. When brain white matter injury occurs, accompanying myelin-associated glycoprotein loss and oligodendrocyte apoptosis, hypoxia-inducible factor 1 is expressed in oligodendrocyte cell nuclei[44]. Hypoxia-inducible factor 1α and tissue adaptability-related protein expression in oligodendrocytes play a neuroprotective role, and antagonize the injury[23]. In addition, hypoxia-inducible factor 1α promotes the proliferation and differentiation of endogenous neural stem cells[24]. Therefore, hypoxia-inducible factor 1α is of great significance in the prevention of hypoxic-ischemic brain damage. Hypoxia and ischemia mediate nerve demyelination, and expression of oligodendrocyte lineage gene-1 is closely correlated with myelin repair. In the myelin repair process, oligodendrocyte lineage gene-1 expression undergoes a dynamic change. Under normal circumstances, oligodendrocyte lineage gene-1 is located in the cytoplasm of stationary oligodendrocyte precursor cells. After myelin is damaged, oligodendrocyte lineage gene-1 expressed in the nuclei of oligodendrocyte precursor cells. When cells begin to differentiate, oligodendrocyte lineage gene-1 returns to the cytoplasm[20].
No work to date has tried to explore the correlation between hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 in premature infants with hypoxic-ischemic brain damage. Studies on hypoxia-inducible factor 1α expression in astrocytes[37,45] have shown a rapid rise in hypoxia-inducible factor 1α expression at 30–60 minutes after the onset of hypoxia. By contrast, in this study we observed increased expression at 8 hours after the onset of hypoxia. The variation depends on the study subject: the former study focused on astrocytes, while our study focused on brain slices. Generally, organs will show changes later than cells. The rapidly expressed hypoxia-inducible factor 1α can quickly regulate target gene expression, and allows adaptive and protective changes in cells and tissues.
Brain slice culture is a new method for nervous system research. The methods for establishment of cell culture and animal models have developed considerably, and are widely used for physiological and pathological studies in vivo. However, the shortcomings are inevitable. Cell culture is characterized by easy operation, clear sample features, and economic efficiency. But in vitro culture environment may lose links between tissues and cells, as well as related biochemical properties. Although animal models can reflect pathological and physiological reactions, experimental operations are limited and affected by many factors in vivo. Therefore, brain slice culture represents a platform between cell culture and animal models, being suitable for in vitro experiments owing to easy operation and easy observation, but providing a tissue structure close to the in vivo tissue structure.
We used 3-day-old Sprague-Dawley rats to establish brain slice culture models. On one hand, 3-day-old rats are equivalent to human infants at metaphase pregnancy, namely premature infants at 23–32 weeks[46]; on the other hand, 3-day-old rat brains are highly plastic and highly tolerant to mechanical damage, thus increasing culture success rates. In this study, growing concerns were paid to the following. (1) Brain slices were cultured using a microporous membrane, because of the simplicity of operation, long neuronal survival, and good cell reactivity. (2) Brain slices were placed at the gas-liquid interface, immersing them in medium so they could absorb nutrition and exposing them to the air to obtain a sufficient oxygen supply. (3) Because of mechanical damage in the process of preparing brain slices, too thin brain slices are not conducive to cell survival. Too thick brain slices may hinder oxygen and nutrient supply, and decrease cell survival rates. Therefore, the thickness of brain slices in this study was 450 μm. (4) To ensure the survival of brain slices, slices should be harvested as quickly as possible and maintained at low temperatures in aseptic conditions.
In this study, we determined the viability of brain slices by gross and microscopic observations, and then compared frozen sections between conventional brain tissue and brain tissue cultured for 3 days using hematoxylin-eosin staining. As shown in Figure 2, the cultured brain tissue exhibited good viability and clear structure. Brain injury in premature infants usually involves damage to hippocampal neurons and around the lateral ventricle, so here we choose to use whole brain slices for culture. Our experimental findings showed that hypoxia-inducible factor 1α expression was increased at 8 hours after oxygen-glucose deprivation and virus transfection, reaching a peak at 1 day, and then gradually decreasing. The expression level at day 3 was still significantly higher than that in the control group. Compared with the peak times in previous animal models (4–8 hours), the peak time of hypoxia-inducible factor 1α expression in this study was slightly delayed. This difference possibly resulted from the development and metabolism disorders following brain slice culture. After oxygen-glucose deprivation and transfection, oligodendrocyte lineage gene-1 expression remained unchanged at 8 hours, reached a peak at 1 day and began to decrease at 3 days. There was no significant difference in the expression levels of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 following hypoxia/ischemia. Oligodendrocyte lineage gene-1 expression began to rise at 8 hours and reached a peak at 1 day after exogenous transfection of slices with hypoxia-inducible factor 1α. In addition, oligodendrocyte lineage gene-1 expression in the hypoxia-inducible factor 1α transfection group was significantly higher than that in the oxygen-glucose deprivation group at each time point. Conversely, exogenous transfection of oligodendrocyte lineage gene-1 had no impact on the expression level of hypoxia-inducible factor 1α relative to that in the oxygen-glucose deprivation group. This evidence suggested that hypoxia-inducible factor 1α may up-regulate oligodendrocyte lineage gene-1 expression. Our research group previously analyzed the gene sequence of oligodendrocyte lineage gene-1 and found several possible hypoxia-inducible factor 1α binding sites in the promoter region, within the gene and downstream of the gene. Therefore, we speculate that hypoxia-inducible factor 1α regulates oligodendrocyte lineage gene-1 expression, and accelerates the repair process after hypoxic-ischemic brain damage. In summary, hypoxia-inducible factor 1α, a transcription factor involved in the rapid response to tissue hypoxia, shows increased expression following hypoxic-ischemic brain damage and in turn up-regulates oligodendrocyte lineage gene-1 expression. Oligodendrocyte lineage gene-1 plays an important role in oligodendrocyte development and differentiation, and is also necessary for remyelination, eventually triggering neuroprotective mechanisms and providing a favorable environment for tissue repair.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
Experiments were performed in the Department of Pediatrics, Beijing Friendship Hospital Affiliated to Capital Medical University from May 2009 to May 2010.
Materials
Twelve 3-day-old Sprague-Dawley specific pathogen-free rats, irrespective of gender, weighing 8–10 g, were provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., China. The license number is SCXK (Beijing) 2007-0001. Experimental disposals were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[47].
Methods
Preparation of brain slices
According to the methods of Adamchik et al[48,49] with some modifications, brain slices were prepared. In brief, Sprague-Dawley rats were disinfected with 75% alcohol and killed. The brains were quickly placed in precooled anatomical buffer for 15 minutes. The buffer contained 64% Dulbecco's modified Eagle's medium, 32% Hank's balanced salt solution (Gibco, New York, NY, USA), 6.5 g/L D-glucose, 2.98 mg/L hydroxyethyl piperazine ethanesulfonic acid (Sigma, St. Louis, MO, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin (Gibco). The meninges were removed under an anatomical microscope (Leica, Wetzlar, Germany). Brain tissues were cut into slices using a microtome (TED PELLA, Redding, CA, USA), at 450 μM thickness. Brain slices were cultured in 1 mL of complete culture medium (containing 50% Dulbecco's modified Eagle's medium, 25% Hank's balanced salt solution, 25% equine serum (Gibco), 2.98 mg/L hydroxyethyl piperazine ethanesulfonic acid, 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin, and 6.5 g/L D-glucose) in 6-well culture plates (Costar, Vernon, CA, USA). Then, cultured slices were transferred to Millicell culture plates (Gibco) and incubated at 37°C in an incubator (Heraeus, Wetzlar, Germany) in 95% O2 + 5% CO2 and saturated humidity. Half of the culture medium was changed twice per week.
Establishment of oxygen-glucose deprived models
Brain slices cultured for 36 hours were respectively placed in 92% nitrogen + 8% oxygen, glucose-free culture medium, in a 37°C incubator for the oxygen-glucose deprivation group, hypoxia-inducible factor 1α transfection group and oligodendrocyte lineage gene-1 transfection group. Oxygen and glucose supply was restored 60 minutes later[50]. Hematoxylin-eosin staining was applied to observe nuclear pyknosis, karyorrhexis, and fusion.
Target gene transfection
Brain slices were transfected with 2 × 107 IU Ad5-oligodendrocyte lineage gene-1 and Ad5-hypoxia-inducible factor 1α virus (Vector Gene Technology Co., Ltd., Beijing, China). In brief, 2 × 107 IU virus was diluted to 400 μL complete culture medium and transfected for 4 hours. Then, virus was discarded and fresh normal medium was added to Millicell culture plates[51]. The blank control group and oxygen-glucose deprivation group received no treatment.
Morphology of brain slices
Cultured brain slices were observed twice a week under an inverted microscope (Olympus, Tokyo, Japan), to detect brain slice viability, growth and structure.
Frozen sections of brain slices
The cultured brain slices were fixed with 10% formaldehyde and incubated with 30% sucrose overnight. Each brain slice was then cut into 2–3 frozen sections at 20 μm thickness.
Hematoxylin-eosin staining for brain slices and histological changes under light microscope
After 4 hours of transfection, brain slices were stained with 10% Harris hematoxylin for 3 minutes and differentiated with 0.5% hydrochloric acid alcohol. Blue dye was developed with saturated lithium carbonate water. Slices were rinsed with distilled water for several seconds and stained with 0.5% eosin for 1 minute. Afterwards, slices were dehydrated in graded ethanol, cleared in xylene, and mounted with gum. Cell morphology and nuclei were observed under an optical microscope (Olympus).
Electron microscope observation
Rat cerebral cortex, hippocampus and lateral ventricle tissue were fixed with 2.5% glutaraldehyde for 2 hours, rinsed with PBS three times, and fixed with 1% osmic acid for additional 2 hours, and finally dehydrated in gradient alcohol, for epoxy propane/resin replacement. Slices were embedded in pure resin, trimmed, and cut into semithin sections. Sections were stained with azure-methylene, and positioned under light microscope. Then semithin sections were further sliced into ultrathin sections and stained with uranyl acetatelead citrate, finally observed under an H-7650 electron microscope.
Expression of hypoxia-inducible factor 1α and oligodendrocyte lineage gene-1 in rat brain tissues as detected by immunohistochemical staining
Immunohistochemical analysis was performed at 4 hours, 8 hours, 1 day, and 3 days after transfection. Frozen sections were soaked in distilled water for 10 minutes and treated with 3% H2O2 for 10 minutes. Then, sections were rinsed with distilled water twice for 5 minutes each; citrate buffer was added and sections were microwaved for 1–2 minutes, to make the solution boiling. Afterwards, sections were soaked in cold water to cool them to room temperature, and rinsed with PBS twice for 5 minutes. Sections were blocked with 5% bovine serum albumin at room temperature for 20 minutes, and incubated with rabbit anti-oligodendrocyte lineage gene-1 polyclonal antibody (1:3 000; Chemicon, CA, USA) or rabbit anti-hypoxia-inducible factor 1α polyclonal antibody (1:100; Boster, Wuhan, China) at 4°C overnight. Sections were then rinsed with PBS three times for 5 minutes each time, and incubated with goat anti-rabbit IgG (Boster) at 37°C for 20 minutes. After three 5-minute PBS washes, sections were incubated with SABC at 37°C for 20 minutes, and again rinsed with PBS four times, for 5 minutes each time. Sections were developed with 3,3’-diaminobenzidine, to 50 seconds, and counterstained with hematoxylin for 40 seconds, followed by a series of procedures: dehydration, transparency and mounting. Five to ten visual fields (1 000 × magnification) randomly selected from each frozen section were used for image analysis. Immunohistochemical images were analyzed using Image Pro Plus 6 software (Media, Cybernetics, Baltimore, MD, USA), and the average absorbance value was measured.
Statistical analysis
Measurement data are expressed as mean ± SD and were statistically analyzed using SPSS 16.0 statistical software (SPSS, Chicago, IL, USA). The mean differences between groups were compared using the Mann-Whitney U test. A P value less than 0.05 was regarded as indicating a significant difference.
Footnotes
Hong Cui, Studying for doctorate, Chief physician, Professor.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81241022 and the Natural Science Foundation of Beijing, No. 7072023, 7122045.
Conflicts of interest: None declared.
Ethical approval: This experiment was approved by the Ethics Committee of Capital Medical University in China.
(Edited by Zhang GR, Zhang JM/Yang Y/Song LP)
REFERENCES
- [1].Liao SM, Ferradal SL, White BR, et al. High-density diffuse optical tomography of term infant visual cortex in the nursery. J Biomed Opt. 2012;17(8):81414. doi: 10.1117/1.JBO.17.8.081414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Panigrahy A, Wisnowski JL, Furtado A, et al. Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome. Pediatr Radiol. 2012;42(Suppl 1):S33–61. doi: 10.1007/s00247-011-2239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Woodward LJ, Edgin JO, Thompson D, et al. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128(Pt 11):2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- [4].Holsti L, Grunau RV, Whitfield MF. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr. 2002;23(1):9–15. doi: 10.1097/00004703-200202000-00002. [DOI] [PubMed] [Google Scholar]
- [5].Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- [6].Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child. Fetal Neonatal Ed. 2008;93(2):F153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Degos V, Favrais G, Kaindl AM, et al. Inflammation processes in perinatal brain damage. J Neural Transm. 2010;117(8):1009–1017. doi: 10.1007/s00702-010-0411-x. [DOI] [PubMed] [Google Scholar]
- [8].Alvarez-Díaz A, Hilario E, de Cerio FG, et al. Hypoxic-ischemic injury in the immature brain-key vascular and cellular players. Neonatology. 2007;92(4):227–235. doi: 10.1159/000103741. [DOI] [PubMed] [Google Scholar]
- [9].Volpe JJ. Cerebral white matter injury of the premature infant more common than you think. Pediatrics. 2003;112(1 Pt 1):176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- [10].Leviton A, Dammann O, Durum SK. The adaptive immune response in neonatal cerebral white matter damage. Ann Neurol. 2005;58(6):821–828. doi: 10.1002/ana.20662. [DOI] [PubMed] [Google Scholar]
- [11].Adén U, Favrais G, Plaisant F, et al. Systemic inflammation sensitizes the neonatal brain to excitotoxicity through a pro-/anti-inflammatory imbalance: key role of TNF-alpha pathway and protection by etanercept. Brain Behav Immun. 2010;24(5):747–758. doi: 10.1016/j.bbi.2009.10.010. [DOI] [PubMed] [Google Scholar]
- [12].Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28(9):1353–1365. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- [13].Dammann O, Leviton A. Inflammatory brain damage in preterm newborns-dry numbers, wet lab, and causal inferences. Early Hum Dev. 2004;79(1):1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- [14].Tekgul H, Yalaz M, Kutukculer N, et al. Value of biochemical markers for outcome in term infants with asphyxia. Pediatr Neurol. 2004;31(5):326–332. doi: 10.1016/j.pediatrneurol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- [15].Kinney HC, Haynes RL, Xu G, et al. Neuron deficit in the white matter and subplate in periventricular leukomalacia. Ann Neurol. 2012;71(3):397–406. doi: 10.1002/ana.22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arnett HA, Fancy SP, Alberta JA, et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306(5704):2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- [17].Xin M, Yue T, Ma Z, et al. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25(6):1354–1365. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu QR, Sun T, Zhu Z, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109(1):75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- [19].Ligon KL, Fancy SP, Franklin RJ, et al. Olig gene function in CNS development and disease. Glia. 2006;54(1):1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- [20].Burton A. Olig1 needed for remyelination. Lancet Neurol. 2005;4(2):80. doi: 10.1016/s1474-4422(05)00978-6. [DOI] [PubMed] [Google Scholar]
- [21].Whitman LM, Blanc CA, Schaumburg CS, et al. Olig1 function is required for remyelin ation potential of transplanted neural progenitor cells in a model of viral-induced demyelination. Exp Neurol. 2012;235(1):380–387. doi: 10.1016/j.expneurol.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Othman A, Frim DM, Polak P, et al. Olig1 is expressed in human oligodendrocytes during maturation and regeneration. Glia. 2011;59(6):914–926. doi: 10.1002/glia.21163. [DOI] [PubMed] [Google Scholar]
- [23].Stadelmann C, Ludwin S, Tabira T, et al. Tissue preconditioning may explain concentric lesions in Balo's type of multiple sclerosis. Brain. 2005;128(Pt 5):979–987. doi: 10.1093/brain/awh457. [DOI] [PubMed] [Google Scholar]
- [24].]Milosevic J, Maisel M, Wegner F, et al. Lack of hypoxia-inducible factor-1 alpha impairs midbrain neural precursor cells involving vascular endothelial growth factor signaling. J Neurosci. 2007;27(2):412–421. doi: 10.1523/JNEUROSCI.2482-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cunningham LA, Candelario K, Li L. Roles for HIF-1α in neural stem cell function and the regenerative response to stroke. Behav Brain Res. 2012;227(2):410–417. doi: 10.1016/j.bbr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fan X, Heijnen CJ, van der Kooij MA. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev. 2009;62(1):99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- [27].Deng W. Neurobiology of injury to the developing brain. Nat Rev Neurol. 2010;6(6):328–336. doi: 10.1038/nrneurol.2010.53. [DOI] [PubMed] [Google Scholar]
- [28].Guo R, Hou W, Dong Y, et al. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reproductive Sci. 2010;17(6):540–548. doi: 10.1177/1933719110364061. [DOI] [PubMed] [Google Scholar]
- [29].Wang LW, Chang YC, Lin CY, et al. Low-dose lipopolysaccharide selectively sensitizes hypoxic ischemia-induced white matter injury in the immature brain. Pediatric Res. 2010;68(1):41–47. doi: 10.1203/PDR.0b013e3181df5f6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang X, Rousset CI, Hagberg H, et al. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med. 2006;11(5):343–353. doi: 10.1016/j.siny.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [31].Shen K, Ji L, Gong C, et al. Notoginsenoside Ft1 promotes angiogenesis via HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and Raf/MEK/ERK signaling pathways. Biochem Pharmacol. 2012;84(6):784–792. doi: 10.1016/j.bcp.2012.05.024. [DOI] [PubMed] [Google Scholar]
- [32].Xiaowei H, Ninghui Z, Wei X, et al. The experimenttal study of hypoxia-inducible factor-1alpha and its target genes in spinal cord injury. Spinal Cord. 2006;44(1):35–43. doi: 10.1038/sj.sc.3101813. [DOI] [PubMed] [Google Scholar]
- [33].Zheng KY, Zhang ZX, Guo AJ, et al. Salidroside stimulates the accumulation of HIF-1α protein resulted in the induction of EPO expression: a signaling via blocking the degradation pathway in kidney and liver cells. Eur J Pharmacol. 2012;679(1-3):34–39. doi: 10.1016/j.ejphar.2012.01.027. [DOI] [PubMed] [Google Scholar]
- [34].Mu D, Chang YS, Vexler ZS, et al. Hypoxia inducible factor 1alpha and erythropoietin upregulation with deferoxamine salvage after neonatal stroke. Exp Neurol. 2005;195(2):407–415. doi: 10.1016/j.expneurol.2005.06.001. [DOI] [PubMed] [Google Scholar]
- [35].Zhang W, Petrovic JM, Callaghan D, et al. Evidence that hypoxia inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1β (IL-1β) in astrocyte cultures. J Neuroimmunol. 2006;174(1-2):63–73. doi: 10.1016/j.jneuroim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- [36].Baranova O, Miranda LF, Pichiule P, et al. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27(23):6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shein NA, Grigoriadis N, Alexandrovich AG, et al. Differential neuroprotective properties of endogenous and exogenous erythropoietin in a mouse model of traumatic brain injury. J Neurotrauma. 2008;25(2):112–123. doi: 10.1089/neu.2007.0358. [DOI] [PubMed] [Google Scholar]
- [38].Mojsilovic-Petrovic J, Callaghan D, Cui H, et al. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimu lated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee YM, Lim JH, Chun YS, et al. Nutlin-3, a Hdm2 antagonist, inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated inactivation of HIF-1 alpha. Carcinogenesis. 2009;30(10):1768–1775. doi: 10.1093/carcin/bgp196. [DOI] [PubMed] [Google Scholar]
- [40].Fandrey J, Gassmann M. Oxygen sensing and the activation of the hypoxia inducible factor 1 (HIF-1)-invited article. Adv Exp Med Biol. 2009;648:197–206. doi: 10.1007/978-90-481-2259-2_23. [DOI] [PubMed] [Google Scholar]
- [41].Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res. 2006;71(4):642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- [42].Demidenko ZN, Blagosklonny MV. The purpose of the HIF-1/PHD feedback loop: to limit mTOR-induced HIF-1α. Cell Cycle. 2011;10(10):1557–1562. doi: 10.4161/cc.10.10.15789. [DOI] [PubMed] [Google Scholar]
- [43].escador N, Cuevas Y, Naranjo S, et al. Identification of a functional hypoxia-responsive element that regulates the expressionof the egl nine homologue 3 (egln3/phd3) gene. Biochem J. 2005;390(Pt 1):189–197. doi: 10.1042/BJ20042121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Aboul-Enein F, Rauschka H, Kornek B, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62(1):25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- [45].Zhang W, Cui H, Naoum S, et al. Effects of hypoxia on inflammatory gene expression in primary human fetal astrocytes. J Cereb Blood Flow Metab. 2003;23:310. [Google Scholar]
- [46].Back SA, Luo NL, Borenstein NS, et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21(4):1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].The Ministry of Science and Technology of the People's Republic of China Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [48].Adamchik Y, Frantseva MV, Weisspapir M, et al. Methods to induce primary and secondary traumatic damage in organotypic hippocampal slice cultures. J Brain Res Brain Res Protoc. 2000;5(2):153–158. doi: 10.1016/s1385-299x(00)00007-6. [DOI] [PubMed] [Google Scholar]
- [49].Yang H, Shew WL, Roy R, et al. Maximal variability of phase synchrony in cortical networks with neuronal avalanches. J Neurosci. 2012;32(3):1061–1072. doi: 10.1523/JNEUROSCI.2771-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yang LJ, Cui H, Yang AJ, et al. Whole brain slices culture and anoxia/hypoglycaemia model building. Shiyan Dongwu yu Bijiao Yixue. 2009;29(51):283–285. [Google Scholar]
- [51].Keir SD, House SB, Li J, et al. Gene transfer into hypothalamic organotypic cultures using an adeno-associated virus vector. Exp Neurol. 1999;160(2):313–316. doi: 10.1006/exnr.1999.7236. [DOI] [PubMed] [Google Scholar]


