Abstract
Collagen protein is an ideal scaffold material for the transplantation of neural stem cells. In this study, rat neural stem cells were seeded into a three-dimensional collagen gel scaffold, with suspension cultured neural stem cells being used as a control group. Neural stem cells, which were cultured in medium containing epidermal growth factor and basic fibroblast growth factor, actively expanded and formed neurospheres in both culture groups. In serum-free medium conditions, the processes extended from neurospheres in the collagen gel group were much longer than those in the suspension culture group. Immunofluorescence staining showed that neurospheres cultured in collagen gels were stained positive for nestin and differentiated cells were stained positive for the neuronal marker βIII-tubulin, the astrocytic marker glial fibrillary acidic protein and the oligodendrocytic marker 2’,3’-cyclic nucleotide 3’-phosphodiesterase. Compared with neurospheres cultured in suspension, the differentiation potential of neural stem cells cultured in collagen gels increased, with the formation of neurons at an early stage. Our results show that the three-dimensional collagen gel culture system is superior to suspension culture in the proliferation, differentiation and process outgrowth of neural stem cells.
Keywords: neural regeneration, stem cells, neural stem cells, collagen gel, scaffold, central nervous system, proliferation, differentiation, neurosphere, photographs-containing paper, neuroregeneration
Research Highlights
(1) The effect of collagen gels on the process outgrowth of neural stem cells was observed in this study. The results confirmed that neural stem cells could be passaged in a three-dimensional collagen gel scaffold.
(2) After culture for 4, 7, and 14 days, the neural stem cells cultured in a three-dimensional collagen gel or in suspension were selected for induced differentiation experiments, which showed that three- dimensional collagen gel scaffolds can promote the differentiation of neural stem cells into neurons.
INTRODUCTION
The treatment of spinal cord injury has been the focus of much research[1]. One of the key issues with the treatment of spinal cord injury is that the damage is often irreversible because of neurons of the central nervous system being unable to spontaneously regenerate after injury[2].
Neural stem cell transplantation has shown a lot of promise for the treatment of spinal cord injury[3,4,5]. Recent studies have indicated that the transplantation of neural stem cells into the injured spinal cord can promote functional recovery; however, the effectiveness of this therapy is limited because of the lack of support in the microenvironment and a low survival rate of the cells after transplantation[6,7,8]. Thus, functional recovery of the injured spinal cord with neural stem cell transplantation might require combination with a biomaterial scaffold that provides microenvironmental support for the attachment and extension of neurites[9,10,11,12].
Collagen type I is a major class of insoluble fibrous protein present in the extracellular matrix that has good biocompatibility, degradability and low immunogenicity. Furthermore, it has been widely used in tissue engineering research and for three-dimensional cell culture[13,14,15,16,17]. However, the effect of a three-dimensional collagen gel scaffold on the behavior of neural stem cells is not very clear. This study aimed to evaluate whether a three-dimensional collagen gel scaffold could help the survival, proliferation, differentiation and process growth from neural stem cells in vitro.
RESULTS
Culture and identification of neural stem cells
Cortical cells were isolated from pregnant Sprague-Dawley rat embryos on embryonic day 14. After culture in suspension, some of the cortical cells proliferated into floating spherical aggregates, called neurospheres (Figure 1A). The neurospheres could be passaged by mechanical dissociation once every 7 days or induced by culture medium containing fetal bovine serum to differentiate after plating on poly-L-lysine-coated coverslips.
Figure 1.
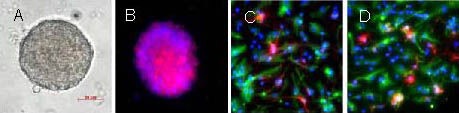
Morphology of primary cultured neural stem cells in the rat brain cortex.
(A) Inverted microscope: neural stem cells cultured for 5 days in serum-free medium (Bar: 50 μm).
(B) Immunofluorescence staining (× 400): neural stem cells stained with anti-nestin antibody (red). (C) The differentiated cells were stained with anti-βIII-tubulin antibody (red) and anti-GFAP antibody (green) (× 400). (D) The differentiated cells were stained with anti-GFAP (green) and anti-CNPase (red) (× 400). Nuclei were stained with 4’,6-diamidino-2-phenylindole (blue).
CNPase: 2’,3’-Cyclic nucleotide 3’-phosphodiesterase; GFAP: glial fibrillary acidic protein.
Immunocytochemical staining was performed on neurospheres on poly-L-lysine-coated coverslips, where neurospheres stained positive for the neural stem cell marker nestin (Figure 1B). After neurospheres were induced to differentiate in culture medium containing 1% fetal bovine serum, they were stained positive for the neuronal marker βIII-tubulin, astrocytic marker glial fibrillary acidic protein and oligodendrocytic marker 2’,3’-cyclic nucleotide 3’-phosphodiesterase (Figures 1C, D). Thus, the cells that were isolated were considered neural stem cells.
Behavior of neural stem cells cultured in the three-dimensional collagen gel and cell recovery
Cortical cells were seeded in a three-dimensional collagen gel. Only about 1% of isolated cells proliferated and formed neurospheres in suspension culture (Figure 2).
Figure 2.
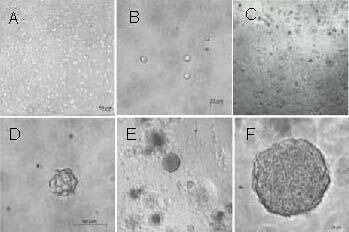
Rat neural stem cell expansion in a three-dimensional collagen gel (inverted microscope).
(A–F) Images of primary neural stem cells immobilized in a collagen gel for 1–7 days were obtained under an inverted microscope.
On day 1, dissociated cells can be seen scattered evenly in a collagen gel (A: × 100, B: × 400). On day 3, a number of neruospheres appeared in the collagen gel (C: × 100, D: × 400). On day 7, the clone-like clusters expanded like neurospheres in suspension culture (E: × 100, F: × 400).
When neurospheres were passaged, they reformed neurosphere-like clones in the three-dimensional collagen gel (Figure 3A). If the isolated cells were transferred to a suspension culture system, they still showed the capacity to form neurospheres (Figure 3B). After differentiation, the neurospheres were seeded in a three-dimensional collagen gel (Figure 3C) and on poly-L-lysine-coated coverslips (Figure 3D). The cells showed the same biological characteristics, where they lose their spherical structure and migrate away from clones.
Figure 3.
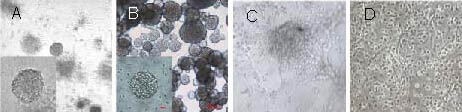
Passage and differentiation of neural stem cells cultured in a three-dimensional collagen gel (inverted microscope).
(A) The neurosphere-like clones in the collagen gel at passage 2 (× 100). (B) The dissociated cells from primary neurosphere-like clones in the collagen gel formed neurospheres in suspension culture (× 100). (A, B) A single amplifying neurosphere in the lower left of the figure (× 400).
(C) When the epidermal growth factor and basic fibroblast growth factor were removed and fetal bovine serum was added, the primary neurosphere-like clones in the collagen gel lose their spherical structure and began to differentiate (× 400).
(D) The cells from primary neurosphere-like clones induced by fetal bovine serum began to differentiate on poly-L-lysine-coated glass coverslips (× 400).
Quantification of process growth from neural stem cells
Figure 4 shows the quantification of process extension from neurospheres cultured on poly-L-lysine-coated coverslips or in three-dimensional collagen gels after 1, 4 and 7 days of culture in serum-free conditions.
Figure 4.
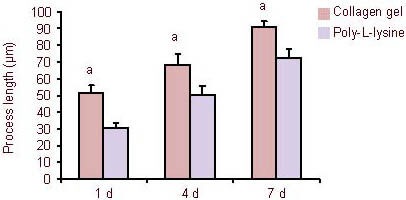
Quantification of process outgrowth from neural stem cells cultured in a three-dimensional collagen gel and poly-L-lysine-coated coverslips.
At 1, 4 and 7 days (d) after culture under serum-free conditions, the average process length in collagen gels were significantly longer than those on poly-L-lysine-coated coverslips. aP < 0.05, vs. process outgrowth of neural stem cells on poly-L-lysine-coated coverslips. Data are expressed as mean ± SD of three replicates (one-way analysis of variance, two-sample t-test).
With increased culture time, the growth of processes from neurospheres in both groups was continuously extended. The average process lengths on collagen gels at 1, 4 and 7 days were significantly longer than those on poly-L-lysine-coated coverslips (P < 0.05).
Expression of neural stem cell markers in the collagen gel
To characterize the neurospheres in the collagen gel and to evaluate the ability of the collagen gel to maintain the characteristics of neural stem cells, indirect immunochemical staining was used to analyze the expression of nestin, a marker of neural stem cells. After culturing in the three-dimensional collagen gel for 7 days, immunostaining showed that the primary cultured neurospheres in the collagen gel were homogeneously positive for nestin (Figure 5A). After differentiation, the cells migrating out from the neurospheres were positive for βIII-tubulin, glial fibrillary acidic protein and 2’,3’-cyclic nucleotide 3’-phosphodiesterase (Figures 5B, C). A similar staining profile was observed in neurospheres formed in suspension culture (Figure 1). These results indicate that most of the cells in the spheroids in the collagen gel have characteristics of neural stem cells, similar to the cells in neurospheres from suspension culture.
Figure 5.
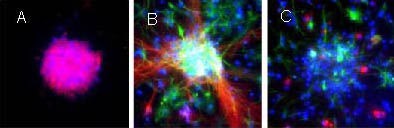
Neural stem cells cultured in a three-dimensional collagen gel (immunofluorescence staining, × 400).
(A) The neurosphere-like clones formed in collagen gels were positive for the neural stem cell marker nestin.
(B) The differentiated cells from neurosphere-like clones formed in the collagen gel were double-immunostained for βIII-tubulin (red) and glial fibrillary acidic protein (green).
(C) The differentiated cells from neurosphere-like clones formed in the collagen gel were double-immunostained for glial fibrillary acidic protein (green) and 2’,3’-cyclic-nucleotide 3’-phosphodiesterase (red). Nuclei were stained with 4’,6-diamidino-2-phenylindole (blue).
Effect of collagen gel on the differentiation potential of neural stem cells
To test whether the three-dimensional collagen gel influences the differentiation potential of neural stem cells, cortical cells were encapsulated in collagen gel and cultured in Dulbecco's modified Eagle's medium/F12 media containing epidermal growth factor and basic fibroblast growth factor. Cells in suspension culture were used as controls. Immunofluorescence staining showed that the percentage of neural stem cells differentiating into neurons in the three-dimensional collagen gel group was significantly higher than that of the suspension culture group on day 4 (P < 0.05), and there were no significant differences in the percentage of neural stem cells differentiating into glial cells and oligodendrocytes (P > 0.05). On days 7 and 14, the percentage of differentiated cells in the three-dimensional group was similar to that of the suspension group (Figures 6–8).
Figure 6.
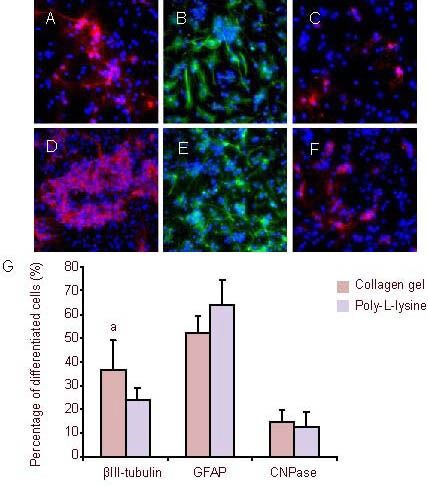
After culturing for 4 days, the neural stem cells were induced to differentiate (immunofluorescence staining, × 400).
Fluorescent photomicrographs represent differentiated cell phenotypes from neural stem cells cultured in a three-dimensional collagen gel (A–C) and in suspension (D–F): βIII-tubulin-positive neurons (A and D), GFAP (B and E, green) and CNPase (C and F, red). Nuclei were stained with 4’,6-diamidino-2-phenylindole (blue).
(G) Percentage of differentiated cells. aP < 0.05, vs. βIII-tubulin in suspension group. Data are expressed as mean ± SD of three replicates (one-way analysis of variance, two-sample t-test).
CNPase: 2’,3’-Cyclic-nucleotide 3’-phosphodiesterase; GFAP: glial fibrillary acidic protein.
Figure 8.
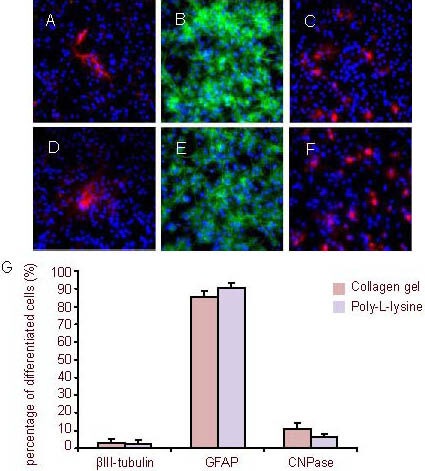
After culturing for 14 days, the neural stem cells were induced to differentiate (immunofluorescence staining, × 400).
Fluorescent photomicrographs represent differentiated cell phenotypes from neural stem cells cultured in a three-dimensional collagen gel (A–C) and in suspension (D–F): βIII-tubulin-positive neurons (A and D), GFAP (B and E, green) and CNPase (C and F, red). Nuclei were stained with 4’,6-diamidino-2-phenylindole (blue).
(G) Percentage of differentiated cells. Data are expressed as mean ± SD of three replicates (one-way analysis of variance, two-sample t-test).
CNPase: 2’,3’-Cyclic-nucleotide 3’-phosphodiesterase; GFAP: glial fibrillary acidic protein.
Figure 7.
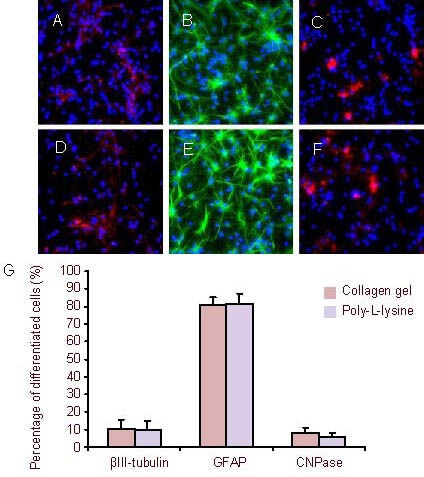
After culturing for 7 days, the neural stem cells were induced to differentiate (immunofluorescence staining, × 400).
Fluorescent photomicrographs represent differentiated cell phenotypes from neural stem cells cultured in three-dimensional collagen gels (A–C) and in suspension (D–F): βIII-tubulin-positive neurons (A and D), GFAP (B and E, green) and CNPase (C and F, red). Nuclei were stained with 4’,6-diamidino-2-phenylindole (blue).
(G) Percentage of differentiated cells. Data are expressed as mean ± SD of three replicates (one-way analysis of variance, two-sample t-test).
CNPase: 2’,3’-Cyclic-nucleotide 3’-phosphodiesterase; GFAP: glial fibrillary acidic protein.
DISCUSSION
In this study, we focused on collagen type-1 as a scaffold material as it is a biologically derived hydrogel and the major class of insoluble fibrous protein in the extracelluar matrix. Collagen gels can be used as a culture matrix for the growth of a variety of mammalian precursor cells. In our study, we found that by using medium containing epidermal growth factor and basic fibroblast growth factor, the collagen-trapped neural stem cells actively expanded and formed neurospheres. The cells derived from these neurospheres were capable of being passaged and reforming neurospheres in collagen gels or in suspension culture. We believe that the proliferation of neural stem cells in three-dimensional collagen gels is similar to that in suspension. The formation of neurospheres in suspension culture is a complicated process involving cell proliferation and aggregation[18,19].
However, the collagen gel culture enables neural stem cells to undergo clonal expansion to form single-cell-derived neurospheres. Clonal propagation of stem cells from primary culture has profound significances because it is the only way to determine the range of differentiation potential and self-renewing capacity of individual cells[20]. On 1, 4 and 7 days after culture in serum-free conditions, the process lengths extending from neurospheres in the collagen gel were significantly longer than those on poly-L-lysine-coated coverslips, which shows that the collagen gel favors increased axonal growth[21]. The neurospheres in the collagen gel were stained positive for nestin. When cultured under conditions facilitating spontaneous neural differentiation, the spheroid-forming cells derived from cortical cells showed a capacity to differentiate into neurons, astrocytes, and oligodendrocytes. This is consistent with previous findings[22,23]. However, we found that neural stem cells cultured in a three-dimensional collagen gel exhibited an increased differentiation percentage into neurons during the early stages, and with increased culture time. The reasons for this difference could be that neurospheres in suspension culture contain many dead cells and differentiated cells, some of which can secrete cytokines to induce the differentiation of neural stem cells into astrocytes, whereas the collagen gel culture enables neural stem cells to undergo clonal expansion to form single-cell-derived neurospheres. Research has shown that collagen, acting as a naturally extracellular matrix, could provide a comfortable environment to help promote neurite extension and guide axonal growth, and the stiffness of the collagen gel could affect neural stem cell proliferation and differentiation[24].
A potential limitation of this study is the use of immunofluorescence staining to estimate the differentiation potential of neural stem cells. Immunofluorescence staining might not accurately reflect the differentiation potential of neural stem cells as only a select number of cells are examined. To reduce the error, we used the method from Watanaba et al[22] and randomly selected 20 high-power fields from each of the three groups. There was also a number of other limitations, such as how long culture can be maintained and the determinants of neural stem cell differentiation. In summary, the present study confirms that collagen gel provides a good microenvironment to help the survival, proliferation, differentiation and process outgrowth from neural stem cells in vitro.
MATERIALS AND METHODS
Design
An in vitro cytological study.
Time and setting
The experiment was conducted between May 2010 and May 2011 at the Experiment Center of Shanghai First People's Hospital, Shanghai Jiao Tong University School of Medicine, China.
Materials
Two Sprague-Dawley rats on embryonic day 14, of clean grade, female, weighing 350 g, were provided by Shanghai Laboratory Animal Center, China (License No. SYXK (Hu) 2009-0086). All experiments were carried out in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[25].
Methods
Isolation and culture of rat neural stem cells
Neural stem cells were isolated and cultured as previously described[15]. Briefly, the neocortices of rat embryos on embryonic day 14 were dissected, cut into small pieces and mechanically triturated in cold PBS. The dissociated cells were collected by centrifugation for 10 minutes at 1 000 r/min, and re-suspended in serum-free medium containing Dulbecco's Modified Eagle's Medium /F12 medium (Gibco, New York, NY, USA) supplemented with 2% B27 (Gibco), 20 ng/mL epidermal growth factor and 20 ng/mL basic fibroblast growth factor (Peprotech, London, UK). The number of viable cells was counted by trypan blue exclusion assay in a hemocytometer (Corning, Steuben County, NY, USA). Cells were plated on untreated Petri dishes in the culture medium and incubated with 95% air/5% CO2 (Thermo Electron Corporation, USA) at 37°C. The culture medium was changed every 3–4 days. After 7 days, mechanically dissociated neural stem cells and undissociated neurospheres were replated in a new culture flask at a density of 1 × 105 cells/mL with fresh culture medium.
Neural stem cell immobilization in the collagen gel
Under sterile conditions, collagen type I collagen (BD Biosciences, San Jose, CA, USA) was dissolved in 0.001 M acetic acid to formulate a 0.3% collagen solution. To prepare the collagen gel, the collagen solution was diluted with 2 × Dulbecco's Modified Eagle's Medium/F12 and sterile H2O to achieve a final concentration of 1.0 mg/mL (maintaining physiological osmolarity, 250–300 mOsM). After adjusting the pH of the collagen solution to 7.4 by the addition of 1 M NaOH, the solution was chilled in an icebath to prevent gel formation. Cells were added at a concentration of 1 × 105/mL to obtain a final concentration of 0.5 mg/mL collagen. The cell-collagen solution was allowed to warm at room temperature and after approximately 10 minutes, 0.2 mL aliquots of the collagen-cell suspension were placed into wells of 48-well tissue culture plates. The gels were placed in an incubator (37°C, 5% CO2, 20% O2, 99% Rh) for 1–2 hours to allow gel formation. After neural stem cells were immobilized in the three-dimensional collagen gel, 0.4 mL of cell media was added to the top of the gels and the cell-gel culture was returned to the incubator. The cells were cultured in gels, with an exchange of culture media every 3–4 days.
Passaging of neural stem cells cultured in three-dimensional collagen gels
After neural stem cells were cultured in three-dimensional collagen gels for 6–7 days, the neurospheres were released from the cell-collagen constructs using collagenase type I (Sigma) treatment for 10 minutes at 37°C. The resulting suspension was centrifuged at 1 500 r/min for 10 minutes at room temperature. The supernatant was discarded and the cell pellet was treated with accutase for 10 minutes. Then, single cell suspension was reseeded in the three-dimensional collagen gel and suspension culture system at 1 × 105 cells/mL.
Quantification of process outgrowth of neural stem cells
At indicated experimental time points, images were taken from random fields of neurospheres, which were cultured on poly-L-lysine-coated coverslips or collagen gels. The length of the 20 longest processes on each neurosphere was measured from the edge of the neurospheres to the tip of the processes. The lengths of the processes were measured with DP71 Image software (Olympus, Tokyo, Japan). The length of the processes of 20 independent neurospheres was calculated for each group and the means and standard deviation were also determined.
Differentiation of neural stem cells cultured in the three-dimensional collagen gel
To evaluate the pattern of neural stem cell differentiation in the three-dimensional culture system, we measured the proportion of each phenotype that differentiated from neural stem cells in the three-dimensional cell-gel culture compared with the suspension culture. When the neural stem cells were cultured in a three-dimensional collagen gel and suspension for 4, 7 and 14 days, the neural stem cells from both were transferred onto poly-L-lysine-coated coverslips and incubated in culture medium containing 1% fetal bovine serum for 7 days. Cells were fixed in ice-cold 4% paraformaldehyde for 20 minutes at room temperature and washed twice in PBS. After incubation for 10 minutes with 0.3% Triton X-100, the cells were further incubated with 10% goat serum for another 15 minutes, followed by primary antibody incubation in PBS overnight at 4°C. The primary antibodies in this study were rabbit anti-nestin polyclonal antibody (1:500; Chemicon, Temecula, CA, USA), mouse anti-βIII-tubulin monoclonal antibody (1:100; Chemicon), rabbit anti-glial fibrillary acidic protein polyclonal antibody (1:200; Chemicon) and mouse anti-2',3'-cyclic nucleotide 3'-phosphodiesterase monoclonal antibody (1:200). Following three washing processes with PBS, AlexaFluor 488 goat anti-rabbit monoclonal antibody (green, 1:200; Invitrogen, Paisley, UK) and AlexaFluor 594 goat anti-mouse monoclonal antibody (red, 1:200; Invitrogen) were added at room temperature for an hour. After another three washes with PBS, all nuclei were stained with glial fibrillary acidic protein for 5 minutes at room temperature and examined under a fluorescence microscope (BX-51; Olympus). Twenty high power fields were randomly chosen from each of the three groups, then the total number of cells (glial fibrillary acidic protein-immunopositive cells) and the cells which were βIII-tubulin-, glial fibrillary acidic protein-and 2',3'-cyclic nucleotide 3'-phosphodiesterase-immunopositive were counted and the proportions of each phenotype were calculated.
Statistical analysis
All quantitative data are expressed as mean ± SD. SPSS 11.5 statistical software (SPSS, Chicago, IL, USA) was applied to process statistical analysis. Multi-group comparisons were performed by one-way analysis of variance and intergroup comparisons were performed by two-sample t-test. Differences were considered statistically significant at P < 0.05.
Acknowledgments:
We would like to thank Yan Hong from the Experiment Center of Shanghai First People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China for guiding the experiment.
Footnotes
Fei Huang, Master.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
(Edited by Zhou YF, Bian LG/Yang Y/Song LP)
REFERENCES
- [1].Shen Q, Jia L, Zhou X. Neurogenic motor evoked potential changes after acute experimental spinal cord injury. Chin J Traumatol. 2000;3(3):153–158. [PubMed] [Google Scholar]
- [2].Garbossa D, Boido M, Fontanella M, et al. Recent therapeutic strategies for spinal cord injury treatment: possible role of stem cells. Neurosurg Rev. 2012;35(3):293–311. doi: 10.1007/s10143-012-0385-2. [DOI] [PubMed] [Google Scholar]
- [3].Ketschek AR, Haas C, Gallo G, et al. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235(2):627–637. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sandner B, Prang P, Rivera FJ, et al. Neural stem cells for spinal cord repair. Cell Tissue Res. 2012;349(1):349–362. doi: 10.1007/s00441-012-1363-2. [DOI] [PubMed] [Google Scholar]
- [5].Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23(7):862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- [6].Lu P, Jones LL, Snyder EY, et al. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- [7].Guo JS, Zeng YS, Li HB, et al. Cotransplant of neural stem cells and NT-3 gene modified Schwann cells promote the recovery of transected spinal cord injury. Spinal Cord. 2007;45(1):15–24. doi: 10.1038/sj.sc.3101943. [DOI] [PubMed] [Google Scholar]
- [8].Zhang X, Zeng Y, Zhang W, et al. Co-transplantation of neural stem cells and NT-3-overexpressing Schwann cells in transected spinal cord. J Neurotrauma. 2007;24(12):1863–1877. doi: 10.1089/neu.2007.0334. [DOI] [PubMed] [Google Scholar]
- [9].Wong HL, Wang MX, Cheung PT, et al. A 3D collagen microsphere culture system for GDNF-secreting HEK293 cells with enhanced protein productivity. Biomaterials. 2007;28(35):5369–5380. doi: 10.1016/j.biomaterials.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [10].Horne MK, Nisbet DR, Forsythe JS, et al. Three-dimensional nanofibrous scaffolds incorporating immobilized BDNF promote proliferation and differentiation of cortical neural stem cells. Stem Cells Dev. 2010;19(6):843–852. doi: 10.1089/scd.2009.0158. [DOI] [PubMed] [Google Scholar]
- [11].Ortinau S, Schmich J, Block S, et al. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomed Eng Online. 2010;9(1):70. [Google Scholar]
- [12].Uemura M, Refaat MM, Shinoyama M, et al. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010;88(3):542–551. doi: 10.1002/jnr.22223. [DOI] [PubMed] [Google Scholar]
- [13].Leipzig ND, Wylie RG, Kim H, et al. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials. 2011;32(1):57–64. doi: 10.1016/j.biomaterials.2010.09.031. [DOI] [PubMed] [Google Scholar]
- [14].Lung AW, Stegemann JP, Plopper GE, et al. Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture. Stem Cells Dev. 2009;18(2):331–341. doi: 10.1089/scd.2008.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bhang SH, Lee TJ, Lim JM, et al. The effect of the controlled release of nerve growth factor from collagen gel on the efficiency of neural cell culture. Biomaterials. 2009;30(1):126–132. doi: 10.1016/j.biomaterials.2008.09.021. [DOI] [PubMed] [Google Scholar]
- [16].O’Connor SM, Stenger DA, Shaffer KM, et al. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci Lett. 2001;304(3):189–193. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- [17].Mahoney MJ, Krewson C, Miller J, et al. Impact of cell type and density on nerve growth factor distribution and bioactivity in 3-dimensional collagen gel cultures. Tissue Eng. 2006;12(7):1915–1927. doi: 10.1089/ten.2006.12.1915. [DOI] [PubMed] [Google Scholar]
- [18].Reynolds BA, Rietze RL. Neural stem cells and neurospheres re-evaluating the relationship. Nat Methods. 2005;2(5):333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- [19].Jessberge S, Clemenson GD, Jr, Gage FH. Spontaneous fusion and nonclonal growth of adult neural stem cells. Stem Cells. 2007;25(4):871–874. doi: 10.1634/stemcells.2006-0620. [DOI] [PubMed] [Google Scholar]
- [20].Yang XZ, Kataoka K, Medina R, et al. A novel three-dimensional culture system for isolation and clonal propagation of neural stem cells using a thermo-reversible gelation polymer. Tissue Eng Part C Methods. 2009;15(4):615–623. doi: 10.1089/ten.TEC.2008.0516. [DOI] [PubMed] [Google Scholar]
- [21].O’Connor SM, Stenger DA, Shaffer KM, et al. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci Lett. 2001;304(3):189–193. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- [22].Watanabe K, Nakamura M, Okano H, et al. Establishment of three-dimensional culture of neural stem/progenitor cells in collagen Type-1 Gel. Restor Neurol Neurosci. 2007;25(2):109–117. [PubMed] [Google Scholar]
- [23].Ma W, Fitzgerald W, Liu QY, et al. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp Neurol. 2004;190(2):276–288. doi: 10.1016/j.expneurol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- [24].Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30(36):6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- [25].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]


