Abstract
Porcine dentin sialophosphoprotein (DSPP) is the most abundant non-collagenous protein in dentin. It is processed by proteases into 3 independent proteins: dentin sialoprotein (DSP), dentin glycoprotein (DGP), and dentin phosphoprotein (DPP). We fractionated DPP and DSP along with TGF-β activity by ion exchange (IE) chromatography from developing pig molars and measured their alkaline phosphatase (ALP)-stimulating activity in human periodontal (HPDL) cells with or without TGF-β receptor inhibitor. We then purified TGF-β-unbound or -bound DPP and DSP by reverse-phase high-performance liquid chromatography (RP-HPLC) using the ALP-HPDL system. The TGF-β isoform bound to DPP and DSP was identified as being TGF-β1 by both ELISA and LC-MS/MS analysis. We incubated carrier-free human recombinant TGF-β1 (CF-hTGF-β1) with TGF-β-unbound DPP or DSP and characterized the binding on IE-HPLC using the ALP-HPDL system. When only CF-hTGF-β1 was incubated, approximately 3.6% of the ALP-stimulating activity remained. DPP and DSP rescued the loss of TGF-β1 activity. Approximately 19% and 10% of the ALP stimulating activities were retained by the binding of TGF-β to DPP and DSP, respectively. The type I collagen infrequently bound to CF-hTGF-β1. We conclude that both DPP and DSP help retain TGF-β1 activity in porcine dentin.
Keywords: tooth, HPLC, cell culture, extracellular matrix (ECM), isolation and purification, phosphophoryn
Introduction
Dentin is the predominant mineralized tissue comprising the body of a tooth. Collagen constitutes approximately 90% of the dentin organic matrix (Linde et al., 1989). The non-collagenous proteins in dentin are dominated by DSPP-derived proteins, which are generated by proteolysis of dentin sialophosphoprotein (DSPP) (MacDougall et al., 1997). Porcine DSPP is expressed and secreted by odontoblasts and is processed by BMP-1, MMP-20, and MMP-2 into 3 main components: dentin sialoprotein (DSP) (Butler et al., 1992; Ritchie et al., 1994), dentin glycoprotein (DGP) (Yamakoshi et al., 2005b), and dentin phosphoprotein (DPP) (Linde et al., 1980; Ritchie and Wang, 1996). Genetic studies have shown that DSPP mutations have been found in dentin dysplasia (DD) or dentinogenesis imperfecta (DGI) patients. DSPP is a multidomain protein with hundreds of post-translational modifications (Qin et al., 2004). Porcine DSP forms covalent dimers and is a highly glycosylated proteoglycan containing both chondroitin-4- and -6-sulfate chains (Yamakoshi et al., 2005a, 2011). The DGP has so far been characterized in pig and has a single N-glycosylation and 4 phosphoserines that are in a highly conserved region near its carboxyl-terminus (Yamakoshi et al., 2005b). DPP is the highly phosphorylated carboxyl-terminal domain of DSPP. The coding region of DPP varies in length among different individual pigs without affecting function (Yamakoshi et al., 2008). Expression of a DSP transgene in the Dspp null background partially recovered the null phenotype and showed that there are distinct roles for DSP and DPP in dentin mineralization, with DSP regulating the initiation of dentin mineralization, and DPP the maturation of dentin (Suzuki et al., 2009). Recently, it was demonstrated that mouse carboxy-terminal DSP enhanced the attachment, migration, proliferation, and differentiation of human periodontal ligament stem cells regulating gene expression of tooth and bone markers, growth factors, and transcription factors (Özer et al., 2013).
Besides DSPP-derived proteins, the bioactive component has been found in the extracellular matrix of dentin (Bègue-Kirn et al., 1992). This inductive activity was inhibited by the neutralizing transforming growth factor-β (TGF-β) antibody (Bègue-Kirn et al., 1992). The TGF-β isoforms have been extracted from the dentin matrices of both rabbit and human teeth (Cassidy et al., 1997); TGF-β1 is a predominant isoform, and approximately half of that is present in the active form (Smith et al., 1998). The dentin matrix sequesters TGF-β and acts as a reservoir for its activity. Decorin and biglycan have already been proposed as potential binding partners (Hildebrand et al., 1994; Iozzo and Murdoch, 1996; Cam et al., 1997; Schonherr et al., 1998; Markmann et al., 2000; Baker et al., 2009).
Our purpose in this study was to isolate TGF-β-bound DPP and DSP, identify the bound TGF-β isoform, and determine the binding ability of DPP and DSP to TGF-β.
Materials & Methods
All experimental procedures involving the use of animals were reviewed and approved by the Ethics Committee of the Institute of Tsurumi University, Yokohama, Japan.
Preparation of Porcine Dentin
Tooth germs of permanent molars were surgically extracted from the mandibles of five-month-old pigs obtained from the Meat Market of Metropolitan Central Wholesale Market (Tokyo, Japan). The enamel organ epithelia (EOE) and dental pulp were removed by means of tissue forceps. The remaining hard tissue on molars was reduced to ‘‘dentin powder’’ by means of a jaw crusher (Retsch Inc., Newtown, PA, USA).
Sequential Extraction of Proteins from Dentin Powder of Molars
The sequential extraction of proteins from dentin powder (40 g) of molars was carried out by the use of our previous demineralization method with 0.17 HCl and 0.95% formic acid (Tsuchiya et al., 2011). The supernatant obtained from 0.5 M acetic acid/2 M NaCl solution (AN extract) was used to isolate DSP and DPP.
Isolation of DPP and DSP from Molar Dentin
The AN extract (150 mg) was fractionated by IE chromatography in a Q-Sepharose Fast Flow column (1.6 x 20 cm; GE Healthcare UK Ltd, Little Chalfont, UK) by our previous method (Tsuchiya et al., 2011). The DPP and DSP in the third (ANQ3) and high-molecular-weight (HMW)-DSP in the fourth (ANQ4) fractions were dialyzed against water, lyophilized, and characterized by SDS-PAGE and ALP-HPDL system with or without the TGF-β specific inhibitor, SB431542.
Isolation of Bioactive Fractions in Porcine DPP and DSP
Both ANQ3 and ANQ4 samples (5 mg) and carrier-free recombinant human TGF-β1 (CF-hTGF-β1) (Cell Signaling Technology, Danvers, MA, USA) (1 µg) were fractionated by reverse-phase high-performance liquid chromatography (RP-HPLC) by our previous method (Yamakoshi et al., 2006). Protein fractions were collected at every 3 mL. Each fraction was lyophilized and characterized by the ALP-HPDL system.
Identification and Quantitative Analysis of TGF-β1 by Enzyme-linked Immunosorbent Assay (ELISA)
The bioactive fractions of ANQ3 and ANQ4 that enhanced ALP-inducing activity in HPDL cells were combined and lyophilized. Each lyophilized sample was dissolved in 200 µL of phosphate buffered saline (PBS), and aliquots (40 µL each) were used for ELISA. The identification and quantitative analysis of TGF-β1 was determined by a sandwich enzyme immunoassay method with a Quantikine ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA).
Mass Spectrometry (LC-MS/MS)
LC-MS/MS was performed by Japan Bio Services Co., LTD (Asaka, Saitama, Japan) on the 13-kDa protein bands in ANQ3 and ANQ4 samples. Samples were analyzed by nano-LC/MS/MS on a ThermoFisher LTQ Orbitrap XL (Thermo Fisher Scientific Inc., Waltham, MA, USA).
SDS-PAGE and Western Blots
SDS-PAGE was performed with Novex 4% to 20% Tris-glycine or NuPAGE 4% to 12% Bis-Tris gels (Life Technologies/Invitrogen, Carlsbad, CA, USA). The gels were stained with Simply Blue Safe Stain (Life Technologies/Invitrogen) or Stains-All (Sigma-Aldrich). The apparent molecular weights of the protein bands were estimated by comparison with SeeBlue Plus2 Pre-Stained Standard (Life Technologies/Invitrogen). Duplicate gels were transblotted onto Invitrolon PVDF membrane (Life Technologies/Invitrogen) and immunostained with porcine N-terminal DSP and DGP polyclonal antibodies.
In vitro Binding Experiments
TGF-β1-unbound DPP and DSP and neutral soluble type-I collagen (Nitta Gelatin, Osaka, Japan) (1 mg each) were incubated with 1 µg of CF-hTGF-β1 in 50 mM Tris-HCl buffer (pH 7.4) for 20 hr at 37°C. Each sample was fractionated by IE-HPLC in an Inertsil AX column (0.46 x 25 cm; GL Sciences Inc., Tokyo, Japan) run at a flow rate of 0.5 mL/min and monitored at 280 nm [buffer A, 50 mM Tris-HCl/6 M urea (pH 7.4); buffer B, 1 M NaCl/buffer A]. Proteins were eluted with a linear gradient of buffer B for 55 min at the flow rate of 0.5 mL/min, and 2-mL fractions were collected. Each fraction was de-salted and buffer-changed to 50 mM Tris-HCl buffer (pH 7.4) in an Amicon Ultra-3K (Merck KGaA, Darmstadt, Germany). Each fraction was concentrated to 20-µL volume, and aliquots (5 µL) were used for the ALP-HPDL system. TGF-β1-unbound DPP and DSP, neutral type-I collagen, and CF-hTGF-β1 only were incubated and fractionated by IE-HPLC as controls.
Enzyme Assay (ALP-HPDL System)
Human periodontal ligament fibroblasts (HPDL) were purchased from LONZA (LONZA, Walkersville, MD, USA). The cell culture and ALP activity were performed according to our previous method (Nagano et al., 2006). Positive controls included the use of recombinant hTGF-β1 with carrier (0.3 ng/mL) (R&D Systems). The TGF-β1 receptor inhibitor, SB431542, was applied to a final concentration of 1 mM into the ALP-HPDL system for examination of the influence against the ALP-inducing activity increased by the application of samples. In controls, the ALP-inducing activity in HPDL cells was enhanced by TGF-β1.
Results
DPP from Dentin of Molars
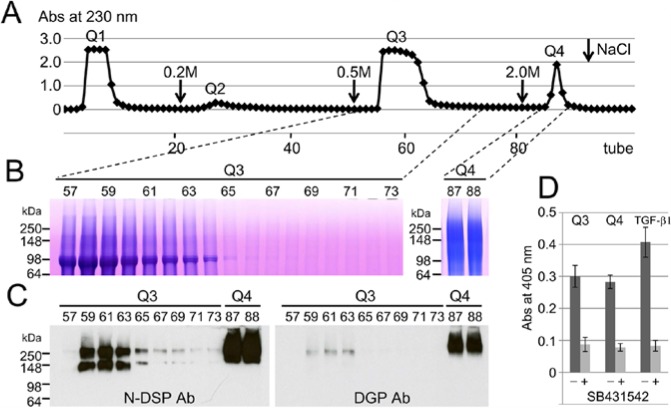
Since TGF-β has been found in rabbit and human incisor dentin (Cassidy et al., 1997), we first decided to use porcine incisor dentin for our preliminary study and found DPP containing TGF-β activity (Appendix Figs. 1-3). Next we used porcine molars to obtain DPP containing TGF-β activity in quantity from developing pig teeth and confirmed that DPP in porcine molars behaves the same as DPP isolated from the incisor. We used IE chromatography to separate the AN extract into 4 fractions (Fig. 1A). The DPP eluted in the third fraction (ANQ3) and migrated as triplet bands at 98 kDa on SDS-PAGE, while the high-molecular-weight DSP eluted in the fourth fraction (ANQ4) and migrated as a smear band at approximately 100 to 250 kDa on SDS-PAGE (Fig. 1B). Immunopositive bands at approximately 150 and 250 kDa, with the N-DSP antibody, were also identified in the ANQ3 fraction, while both N-DSP and DGP antibodies strongly recognized protein bands at over 250 kDa (Fig. 1C). Both ANQ3 and ANQ4 enhanced ALP-inducing activity in HPDL cells, and the SB431542 significantly decreased its activity (Fig. 1D).
Figure 1.
Isolation of DSPP-derived proteins in porcine molar dentin. (A) Q-Sepharose chromatograms showing absorbance at 230 nm for AN extracts from dentin of porcine developing molars (150 mg). Downward-pointing arrows are the starting point of the step gradient with 0.2, 0.5, and 2 M NaCl. (B) SDS-PAGE (4% to 20% gradient gel) stained with Stains-All showing each tube on a Q-Sepharose chromatogram. (C) Western blots showing each tube on a Q-Sepharose chromatogram with specific antibodies against N-terminal dentin sialoprotein (N-DSP Ab, left) and dentin glycoprotein (DGP Ab, right), showing isolated DSP in Q3 and Q4 fractions. (D) ALP-inducing activity of HPDL cells exposed by Q3 and Q4 fractions and TGF-β1 (0.3 ng/mL) without (-) or with (+) SB431542. Data are means ± SE of 3 culture wells.
Identification of TGF-β1 in ANQ3 and ANQ4 Fractions
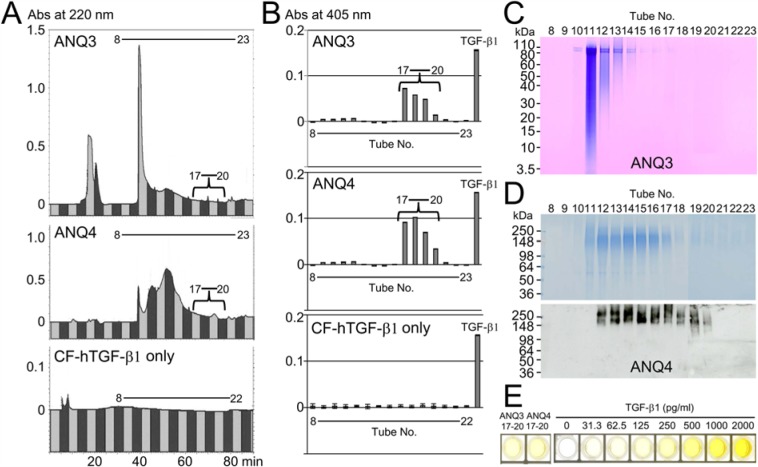
We further separated ANQ3 and ANQ4 samples into 23 fractions by RP-HPLC using a C18 column. The DSPP-derived proteins in ANQ3 and ANQ4 samples eluted within fractions 10 to 16 (Fig. 2A, top and middle), but the ALP-inducing activity in HPDL cells was in fractions 17 to 20 in both the ANQ3 and ANQ4 samples (Fig. 2B, top and middle). No ALP-inducing activity was detected when we ran CF-hTGF-β1 only under this isolation system (Figs. 2A and 2B, bottom). Both SDS-PAGE and Western blot analyses showed that the trace amount of DPP was detected in fraction 17 in ANQ3 (Fig. 2C), and DSP was identified as two bands within fractions 17 to 20 (Fig. 2D). We interpret these findings to indicate that an in vivo bioactive molecule, such as TGF-β in porcine dentin, binds to DPP and to DSP.
Figure 2.
Isolation of TGF-β1-unbound and -bound DPP and DSP in porcine molar dentin. (A) RP-HPLC chromatograms showing absorbance at 220 nm for ANQ3 and ANQ4 (5 mg each) fractionated by IE chromatography and for CF-hTGF-β1 (1 µg). (B) ALP-inducing activity of HPDL cells exposed by fractions 8-23 in ANQ3, ANQ4, and CF-hTGF-β1. TGF-β1 (0.3 ng/mL) is used as a positive control. Data are means ± SE of 3 culture wells. (C) SDS-PAGE (4% to 12% gradient gel) stained with Stains-All showing each tube in ANQ3 fractionated by RP-HPLC. (D) SDS-PAGE (4% to 12% gradient gel) stained with Simply Blue (top) and Western blots (bottom) used specific antibodies against N-terminal dentin sialoprotein, showing each tube in ANQ4 fractionated by RP-HPLC. (E) ELISA for the detection of TGF-β1 in combined fractions 17-20 in ANQ3 and ANQ4 enhanced ALP-inducing activity in HPDL cells. This figure is available in color online at http://jdr.sagepub.com.
To learn more about the TGF-β in tooth dentin, we performed ELISA. An aliquot of fractions 17 to 20 in ANQ3 and in ANQ4 was assayed by ELISA. Each sample in ANQ3 and ANQ4 was positive against 2 TGF-β1 antibodies and contained approximately 270 and 380 pg of TGF-β1 per mg of ANQ3 and ANQ4, respectively (Fig. 2E). We furthermore attempted to characterize TGF-β1 by LC-MS/MS analysis. The LC-MS/MS analysis gave a part of the TGF-β1 protein sequence corresponding to Q297-K315 (Appendix Fig. 4).
In vitro Binding Experiments
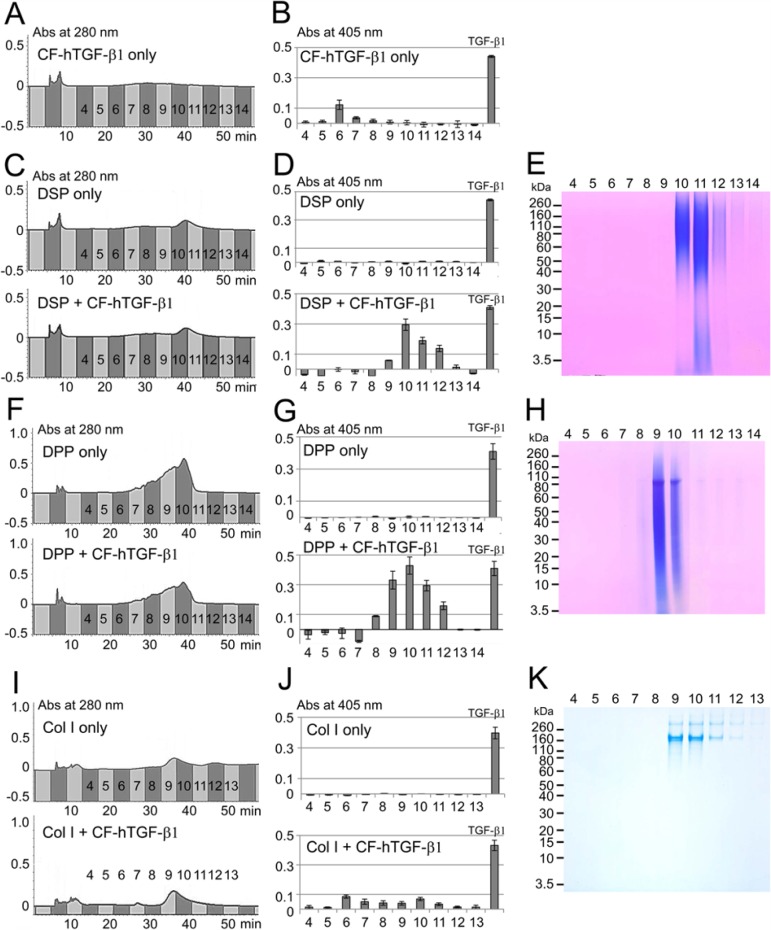
To gain more information about the binding potential of DPP and DSP to TGF-β1, we performed in vitro binding experiments. For this study, we used TGF-β1-unbound DPP and DSP obtained from fractions 10 to 12 in ANQ3 and 12 to 15 in ANQ4, respectively. Since the CF-hTGF-β1 lost its activity only during RP-HPLC (Fig. 2B, bottom), we changed the isolation system from RP-HPLC to IE-HPLC. The CF-hTGF-β1 eluted into fractions 6 to 8 by IE-HPLC, which enhanced ALP-inducing activity after the incubation with HPDL cells (Figs. 3A, 3B). The TGF-β1-unbound DSP eluted into fractions 10 to 12 and enhanced ALP-inducing activity after CF-hTGF-β1 binding (Figs. 3C, 3D). The DSP protein band on SDS-PAGE correlated with positions of ALP-inducing activity (Fig. 3E). The TGF-β1-unbound DPP eluted into fractions 8 to 11 by IE-HPLC. Those fractions enhanced ALP-inducing activity after CF-hTGF-β1 binding (Figs. 3F, 3G). The DPP band migrated at the same positions with ALP-inducing activity (Fig. 3H). We also tested the binding potential of the neutral soluble type I collagen against the CF-hTGF-β1. The type I collagen eluted into 9 to 12 by IE-HPLC (Fig. 3I). The CF-hTGF-β1 was also able to bind to type I collagen in fractions 9 to 12, and those fractions weakly enhanced ALP-inducing activity (Fig. 3J). The ALP-inducing activity from unbound CF-hTGF-β1 isolated by IE-HPLC was found in fractions 6 to 8, with no detectable collagen bands on SDS-PAGE (Fig. 3K). Total amounts (ng) of unbound or bound TGF-β1 in fractions obtained from IE-HPLC after binding experiments against one mg of DSP, DPP, and type I collagen were calculated from the standard CF-hTGF-β1 (0.3 ng/mL) and are shown in the Table. The CF-hTGF-β1 only as control retained its activity during IE-HPLC, but its amount after the dilution correction was 36.4 ± 3.01 ng. Both DPP and DSP were able to bind CF-hTGF-β1, and amounts of TGF-β1 bound to DSP or DPP after the dilution correction were 101.8 ± 2.90 and 190.5 ± 7.34 ng/mg of DSP or DPP, respectively. The type I collagen was able to bind CF-hTGF-β1 weakly, and its amount of TGF-β1 bound to type I collagen after the dilution correction was 23.8 ± 2.04 ng/mg of type I collagen. The unbound CF-hTGF-β1 against type I collagen was also found during IE-HPLC, and its amount was 24.7 ± 2.87 ng.
Figure 3.
In vitro binding study of CF-hTGF-β1 for DSP, DPP, and type I collagen. (A, C, F, I) IE-HPLC chromatograms showing absorbance at 280 nm for (A) CF-hTGF-β1 only, (C) TGF-β1-unbound-DSP only (DSP only, top) and TGF-β1-bound DSP after binding experiment (DSP + CF-hTGF-β1, bottom), (F) TGF-β1-unbound-DPP only (top) and TGF-β1-bound DPP after binding experiment (DPP + CF-hTGF-β1, bottom), and (I) type I collagen only (Col I only, top) and TGF-β1-bound collagen after binding experiment (Col I + CF-hTGF-β1, bottom). (B, D, G, J) ALP-inducing activity of HPDL cells exposed by (B) fractions 4 to 14 in CF-hTGF-β1 only, (D) TGF-β1-unbound-DSP only (DSP only, top) and TGF-β1-bound DSP (DSP + CF-hTGF-β1, bottom), (G) TGF-β1-unbound-DPP only (DPP only, top) and TGF-β1-bound DPP (DPP + CF-hTGF-β1), and (J) fractions 4 to 13 in type I collagen only (Col I only, top) and TGF-β1-bound collagen (Col I + CF-hTGF-β1, bottom). The recombinant human TGF-β1 (TGF-β1) (0.3 ng/mL) was used as positive control. Data are means ± SE of 3 culture wells. (E, H, K) SDS-PAGE (4% to 12% gradient gel) stained with Stains-All showing (E) DSP in fractions 4 to 14, (H) DPP in fractions 4 to 14, and (K) stained with Simply Blue showing type I collagen in fractions 4 to 13 isolated by IE-HPLC after binding to CF-hTGF-β1. This figure is available in color online at http://jdr.sagepub.com.
Table.
Amount of Unbound or Bound TGF-β1 for DSP, DPP, and Type I Collagen
| (ng) |
||
|---|---|---|
| Binding Protein | Unbound | Bound |
| CF-hTGF-β1 only | 36.4 ± 3.01 | Not detected |
| DSP | Not detected | 101.8 ± 2.90 |
| DPP | Not detected | 190.5 ± 7.34 |
| Col I | 24.7 ± 2.87 | 23.8 ± 2.04 |
The value indicates total ng of TGF-β1 obtained from IE-HPLC after binding experiments against 1 mg of protein.
Discussion
DPP from Dentin of Molars
DSPP-derived proteins are the most abundant non-collagenous proteins in porcine molar dentin (Yamakoshi et al., 2011). Both DPP and DSP are expressed in stoichiometric amounts as the N-terminal and C-terminal domains of DSPP (MacDougall et al., 1997) and are found in equal molar amounts in dentin (Yamakoshi et al., 2008; Zhu et al., 2010). Previously, we showed that porcine DPP and DSP can be found in the acid-NaCl or “AN” extract ( Yamakoshi et al., 2006, 2008; Tsuchiya et al., 2011). We demonstrated that TGF-β co-eluted with DPP and DSP into the ANQ3 fraction, and with HMW-DSP (i.e., DSP-DGP complex) in ANQ4. The ALP-inducing activity in ANQ3 and ANQ4 fractions obtained from the IE chromatography under denatured conditions is due to the TGF-β bound to both DPP and DSP in porcine molar dentin.
Identification of TGF-β1 in ANQ3 and ANQ4 Fractions
Using the porcine molar, which provides abundant dentin proteins, and having previously surveyed dentin extracts for the presence of DPP and DSP, we were able to quantify levels of TGF-β from porcine molars. Previously, we showed that DPP and DSP were well-separated by RP-HPLC with a C18 column (Yamakoshi et al., 2006). Our study demonstrated that the ALP-inducing activity in HPDL cells was retained under the acidic condition of 0.05% TFA and 80% acetonitrile (pH~2) during RP-HPLC and was detected in fractions 17 to 20 with minor amounts of DPP and DSP in ANQ3 and ANQ4 fractions, respectively. We first tried to identify TGF-β by using the CF-hTGF-β1 as standard and by evaluating the same elution time on RP-HPLC. However, the CF-hTGF-β1 alone lost its activity during RP-HPLC. This suggested that both DPP and DSP may function to preserve TGF-β activity. We determined that the approximate percentages of TGF-β-bound DPP and DSP in vivo are 1.5 and 2.6, from the calculation of the total area of TGF-β-unbound DPP and DSP (i.e., fractions 10-16 in ANQ3 and ANQ4) and TGF-β-bound DPP and DSP (i.e., fractions 17-20 in ANQ3 and ANQ4) on RP-HPLC.
The TGF-β family consists of 5 closely related isoforms, which are derived from structurally related genes (Massagué, 1990). TGF-β1 is the only isoform detected in human dentin and the major isoform in rabbit dentin, where small amounts of TGF-β2 and TGF-β3 isoforms have been detected (Cassidy et al., 1997). In this study, we were able to identify the TGF-β1 isoform bound to DPP and DSP in ANQ3 and ANQ4 fractions. In addition, the concentrated mixture of fractions 17 to 20 was enough to identify a part of the amino acid sequence of TGF-β1 by LC-MS/MS analysis.
In vitro Binding Experiments
We focused our in vitro binding study on the type I collagen- and DSPP-derived proteins because of the major protein and most abundant non-collagenous protein in dentin (Yamakoshi et al., 2011). It has been shown that the collagen alone does not interact with TGF-β (Schonherr et al., 1998). We demonstrated that the neutral type I collagen was not easy to bind to CF-hTGF-β1. When only 1 µg of CF-hTGF-β1 was incubated and run on IE-HPLC, approximately 3.6% of activity remained. DPP and DSP were able to rescue the loss of TGF-β1 activity, and approximately 19% and 10% of its activity were retained by binding to DPP and DSP, respectively. Thus, our in vitro study demonstrated that both DPP and DSP are necessary for maintaining TGF-β1 activity.
Other in vitro studies have demonstrated that both decorin and biglycan are able to bind to TGF-β and are candidates for the sequestration of TGF-β reservoirs (Hildebrand et al., 1994; Iozzo and Murdoch, 1996; Hyytiäinen et al., 2004; Baker et al., 2009). The decorin contains independent binding sites for TGF-β and type I collagen (Schonherr et al., 1998). DSP is the most abundant proteoglycan in dentin and is therefore likely to interact with collagen. Conversely, DPP binding with collagen improves its structural stability (Yamakoshi et al., 2008). Such DSP-collagen and DPP-collagen interactions may sterically hinder the binding of TGF-β isoforms in vivo. In this study, we demonstrated that both DPP and DSP provide a retention capability for bioactive molecules such as TGF-β. During dentinogenesis, the TGF-β1 bound to DPP and DSP is liberated in association with the degradation of DPP and DSP and may facilitate reparative dentin formation by affecting the odontoblast. It also may affect cell behavior in the dentin–pulp complex following tissue injury. Studies are now required to examine the interaction of DPP and DSP with TGF-β1 and collagen, and the bioavailability of such a complex for the future.
Supplementary Material
Acknowledgments
We thank Mr. Minoru Yasui of Japan Bio Services Co., LTD (Asaka, Saitama, Japan) for his help in LC-MS/MS analysis. We thank Dr. James P. Simmer, Department of Biologic and Materials Science, School of Dentistry, University of Michigan (Ann Arbor, MI, USA), who served as a scientific advisor.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by JSPS Grant-in-Aid for Research Activity Start-up Number 24890265, and by National Institute of Dental and Craniofacial Research (NIDCR, U.S. National Institutes of Health [NIH]) grant DE018020.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Baker SM, Sugars RV, Wendel M, Smith AJ, Waddington RJ, Cooper PR, et al. (2009). TGF-beta/extracellular matrix interactions in dentin matrix: a role in regulating sequestration and protection of bioactivity. Calcif Tissue Int 85:66-74. [DOI] [PubMed] [Google Scholar]
- Bègue-Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D, et al. (1992). Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol 36:491-503. [PubMed] [Google Scholar]
- Butler WT, Bhown M, Brunn JC, D’Souza RN, Farach-Carson MC, Happonen RP, et al. (1992). Isolation, characterization and immunolocalization of a 53-kDal dentin sialoprotein (DSP). Matrix 12:343-351. [DOI] [PubMed] [Google Scholar]
- Cam Y, Lesot H, Colosetti P, Ruch JV. (1997). Distribution of transforming growth factor beta1-binding proteins and low-affinity receptors during odontoblast differentiation in the mouse. Arch Oral Biol 42:385-391. [DOI] [PubMed] [Google Scholar]
- Cassidy N, Fahey M, Prime SS, Smith AJ. (1997). Comparative analysis of transforming growth factor-beta isoforms 1-3 in human and rabbit dentine matrices. Arch Oral Biol 42:219-223. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, et al. (1994). Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302 (Pt 2):527-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytiäinen M, Penttinen C, Keski-Oja J. (2004). Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci 41:233-264. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Murdoch AD. (1996). Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J 10:598-614. [PubMed] [Google Scholar]
- Linde A, Bhown M, Butler WT. (1980). Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem 255:5931-5942. [PubMed] [Google Scholar]
- Linde A, Lussi A, Crenshaw MA. (1989). Mineral induction by immobilized polyanionic proteins. Calcif Tissue Int 44:286-295. [DOI] [PubMed] [Google Scholar]
- MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. (1997). Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem 272:835-842. [DOI] [PubMed] [Google Scholar]
- Markmann A, Hausser H, Schonherr E, Kresse H. (2000). Influence of decorin expression on transforming growth factor-beta-mediated collagen gel retraction and biglycan induction. Matrix Biol 19:631-636. [DOI] [PubMed] [Google Scholar]
- Massagué J. (1990). The transforming growth factor-beta family. Annu Rev Cell Biol 6:597-641. [DOI] [PubMed] [Google Scholar]
- Nagano T, Oida S, Suzuki S, Iwata T, Yamakoshi Y, Ogata Y, et al. (2006). Porcine enamel protein fractions contain transforming growth factor-β1. J Periodontol 77:1688-1694. [DOI] [PubMed] [Google Scholar]
- Özer A, Yuan G, Yang G, Wang F, Li W, Yang Y, et al. (2013). Domain of dentine sialoprotein mediates proliferation and differentiation of human periodontal ligament stem cells. PloS One 8:e81655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Baba O, Butler WT. (2004). Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15:126-136. [DOI] [PubMed] [Google Scholar]
- Ritchie HH, Wang LH. (1996). Sequence determination of an extremely acidic rat dentin phosphoprotein. J Biol Chem 271:21695-21698. [DOI] [PubMed] [Google Scholar]
- Ritchie HH, Hou H, Veis A, Butler WT. (1994). Cloning and sequence determination of rat dentin sialoprotein, a novel dentin protein. J Biol Chem 269:3698-3702. [PubMed] [Google Scholar]
- Schonherr E, Broszat M, Brandan E, Bruckner P, Kresse H. (1998). Decorin core protein fragment Leu155-Val260 interacts with TGF-beta but does not compete for decorin binding to type I collagen. Arch Biochem Biophys 355:241-248. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Matthews JB, Hall RC. (1998). Transforming growth factor-beta1 (TGF-beta1) in dentine matrix. Ligand activation and receptor expression. Eur J Oral Sci 106 (Suppl 1):179-184. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, et al. (2009). Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol 28:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S, Simmer JP, Hu JC, Richardson AS, Yamakoshi F, Yamakoshi Y. (2011). Astacin proteases cleave dentin sialophosphoprotein (Dspp) to generate dentin phosphoprotein (Dpp). J Bone Miner Res 26:220-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC, Fukae M, Iwata T, Kim JW, Zhang H, et al. (2005a). Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J Biol Chem 280:1552-1560. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC, Fukae M, Zhang H, Simmer JP. (2005b). Dentin glycoprotein: the protein in the middle of the dentin sialophosphoprotein chimera. J Biol Chem 280:17472-17479. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC, Iwata T, Kobayashi K, Fukae M, Simmer JP. (2006). Dentin sialophosphoprotein is processed by MMP-2 and MMP-20 in vitro and in vivo. J Biol Chem 281:38235-38243. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Lu Y, Hu JC, Kim JW, Iwata T, Kobayashi K, et al. (2008). Porcine dentin sialophosphoprotein: length polymorphisms, glycosylation, phosphorylation, and stability. J Biol Chem 283:14835-14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi Y, Nagano T, Hu JC, Yamakoshi F, Simmer JP. (2011). Porcine dentin sialoprotein glycosylation and glycosaminoglycan attachments. BMC Biochem 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Sun Y, Prasad M, Wang X, Yamoah AK, Li Y, et al. (2010). Glycosaminoglycan chain of dentin sialoprotein proteoglycan. J Dent Res 89:808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.