Abstract
The Proteoglycan 4 (Prg4) product lubricin plays essential roles in boundary lubrication and movement in limb synovial joints, but its roles in temporomandibular joint (TMJ) are unclear. Thus, we characterized the TMJ phenotype in wild-type and Prg4 –/– mouse littermates over age. As early as 2 weeks of age, mutant mice exhibited hyperplasia in the glenoid fossa articular cartilage, articular disc, and synovial membrane. By 1 month of age, there were fewer condylar superficial tenascin-C/Col1-positive cells and more numerous apoptotic condylar apical cells, while chondroprogenitors displayed higher mitotic activity, and Sox9-, Col2-, and ColX-expressing chondrocyte zones were significantly expanded. Mutant subchondral bone contained numerous Catepsin K- expressing osteoclasts at the chondro-osseous junction, increased invasive marrow cavities, and suboptimal subchondral bone. Mutant glenoid fossa, disc, synovial cells, and condyles displayed higher Hyaluronan synthase 2 expression. Mutant discs also lost their characteristic concave shape, exhibited ectopic chondrocyte differentiation, and occasionally adhered to condylar surfaces. A fibrinoid substance of unclear origin often covered the condylar surface. By 6 months of age, mutant condyles displayed osteoarthritic degradation with apical/mid-zone separation. In sum, lubricin exerts multiple essential direct and indirect roles to preserve TMJ structural and cellular integrity over post-natal life.
Keywords: Prg4, hyaluronic acid, osteoarthritis, temporomandibular joint, Has2, superficial layer
Introduction
The temporomandibular joint (TMJ) is a diarthrodial articulation between the mandibular condyle and glenoid fossa and contains upper and lower synovial cavities separated by an intervening articular disc. These unique anatomical features enable the TMJ to undergo complex rearrangements, during mastication, that impart shearing and frictional loads (Nitzan, 2003; Milam, 2005). Furthermore, the mandibular condyle, articular disc, and glenoid fossa are fibrocartilaginous structures that are well-suited for resistance to compression and shearing forces. Fibrocartilage expresses collagen types I and II (Shibukawa et al., 2007; Wadhwa and Kapila, 2008).
Synovial fluid is primarily responsible for joint lubrication via macromolecules such as hyaluronic acid (HA) and lubricin (Tanaka et al., 2008). Hyaluronic acid, which is synthesized by chondrocytes and synovial cells, increases fluid viscosity and cartilage elasticity. Lubricin, also known as superficial zone protein (SZP), megakaryocyte stimulating factor (MSF) precursor, or proteoglycan-4 (PRG4), is a product of the gene Proteoglycan-4 (Prg4). It is synthesized by synovial or superficial zone cells and is secreted into joint cavities (Flannery et al., 1999; Rhee et al., 2005). It contains an extensive mucin-like region rich in O-linked oligosaccharides that reduce friction during boundary movement (Swann et al., 1985; Jay et al., 2001). It also contains a somatomedin B homology domain, a hemopexin-like domain, and a heparin-binding domain (Swann et al., 1985; Jay et al., 2001), suggesting that lubricin may have diverse biological activities.
Lubricin is essential for joint function and structure, since PRG4 mutations are responsible for camptodactyly-arthropathy-coxa vara-pericarditis syndrome (CACP) (Bahabri et al., 1998; Marcelino et al., 1999). The synovial joints in CACP patients appear normal at birth, but eventually undergo failure associated with non-inflammatory hyperplasia and fibrosis of the synovial membrane (Bahabri et al., 1998). Prg4 null mice recapitulate the CACP joint phenotype and display synovial joint deterioration, synovial membrane hyperplasia, articular cartilage fibrillations, abnormal protein deposition, and disappearance of superficial zone cells (Rhee et al., 2005; Novince et al., 2012a,b). Thus, in addition to boundary lubrication, lubricin may prevent cell/protein adhesion, regulate proliferation of synovial cells and protect superficial chondrocytes from death (Rhee et al., 2005; Waller et al., 2013). Recent studies showed that exogenously introduced recombinant lubricin or Prg4 overexpression provides chondroprotection and lubrication in mouse models of osteoarthritis (OA), suggesting that exogenous lubricin could be a joint therapeutic (Flannery et al., 2009; Ruan et al., 2013).
The importance of lubricin activity in joint function has been studied primarily in large synovial joints. However, little is known about its roles in TMJ, a joint that differs from other synovial joints in structure, function, biomechanical properties, developmental ontogeny, and molecular genetics. In this study, we performed detailed analyses of the TMJ in Prg4–/– mice to determine whether lubricin is required for the maintenance of TMJ structure and function. Our study demonstrated that Prg4–/– TMJ presents not only the phenotypic characteristics seen in mutant long bone synovial joints but also novel and unique changes, indicating that lubricin is essential for the maintenance of TMJ integrity
Materials & Methods
Mice
Prg4–/– mutant mice were kindly provided by Dr. Matthew Warman (Boston Children’s Hospital, MA, USA) (Rhee et al., 2005). Animals were maintained in accordance with the NIH Guide for Care and Use of Laboratory Animals, and protocols were approved by the Children’s Hospital of Philadelphia IACUC.
Histological, Histochemical, Histomorphometric, and in situ Hybridization Analyses
Prg4 mutants and control littermates were fixed with 4% paraformaldehyde overnight, decalcified for 2 wks in 10% EDTA/2% paraformaldehyde, dehydrated, and embedded in paraffin. Serial 5-µM parasagittal sections from mutants and control littermates were placed on the same slides and processed for histological, histochemical, histomorphometric, and in situ hybridization analyses. Age-matched mutant and wild-type littermates were evaluated at post-natal day 4 (P4, n = 12), P14 (n = 8), 1 mo (n = 12), 2 mos (n = 10), 3 mos (n = 11), and 6 mos (n = 6) of age. Cartilage and bone were stained with Safranin-O/fast green or Masson’s trichrome. Sections from at least 4 control and mutant mice at each stage were hybridized with antisense or sense 35S-labeled probes (Koyama et al., 2007). Images from Safranin-O/fast-green-stained sections and in situ hybridization were analyzed by histomorphometry with ImagePro 4.5 (Leeds Precision Instruments, Minneapolis, MN, USA).
Cell Proliferation and Apoptosis Assays and Statistical Analysis
A single intraperitoneal injection of EdU (5-ethynyl-2′-deoxyuridine) (100 mg/kg) was administered to mice 24 hrs prior to tissue collection. Edu staining was conducted with the Click-iT® EdU imaging kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol, and sections were mounted with Fluoro-Gel II Mounting Medium. Apoptosis detection was carried out on paraffin sections by means of a TUNEL Assay Kit used according to the manufacturer’s instructions (Roche, Mannheim, Germany). Fluorescent images were superimposed onto corresponding bright-field images with Adobe Photoshop software. Data were analyzed by two-sided Student’s t test. p values less than .05 were considered statistically significant (p < .05). All statistical data are presented as means ± SD.
Results
Tissue Hyperplasia and Abnormal Fibrinoid Deposition in Post-natal Prg4–/– TMJ
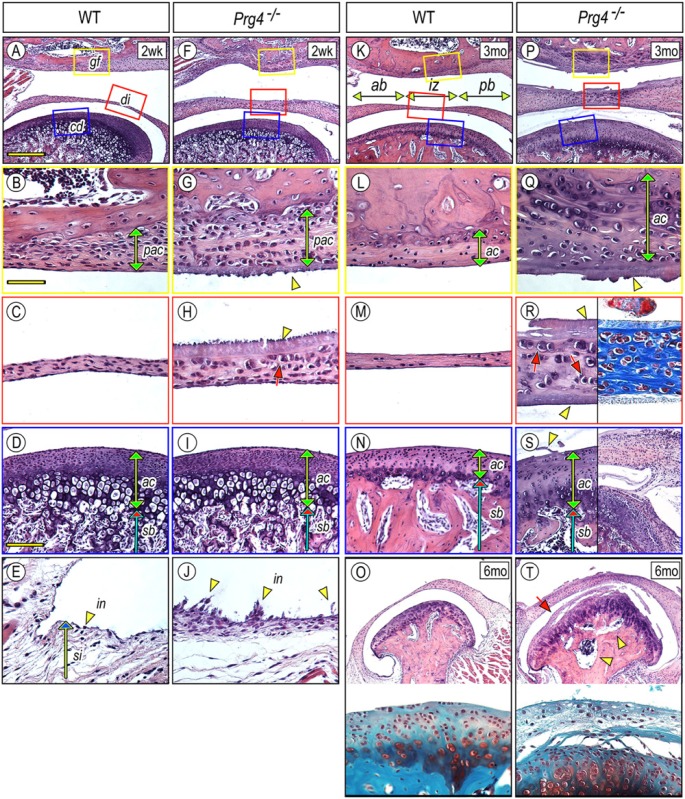
Lubricin roles in TMJ were initially studied by histomorphological comparisons of the mandibular condyle, articular disc, and glenoid fossa in wild-type (control) vs. Prg4–/– mice at different post-natal ages. At post-natal (P) day 2, no apparent differences were observed between mutant and wild types (Appendix Fig. 1). By P14, Prg4 ablation led to several changes in TMJ components facing the upper synovial cavity (Fig. 1), including an increased number of presumptive articular chondrocytes (pac) in the glenoid fossa (Fig. 1G) and thickening of the articular disc (Fig. 1H). The mutant mandibular condylar articular cartilage (ac) facing the lower synovial cavity was relatively normal at P14 and became significantly thicker by 3 mos compared with that of wild-type mice (Figs. 1D, 1I, 1N, 1S, 1S). By 6 mos, the condylar apical zone in Prg4–/– condyles lost integrity and was separated from the underlying cartilage by a polymorphic layer, and the subchondral bone was occupied by large marrow cavities (Figs. 1T, arrow and arrowheads).
Figure 1.
Glenoid fossa, articular disc, synovial membrane, and mandibular condyle are defective in Prg4–/– TMJs. TMJs from 2-week (A-J), 3-month (K-N, P-S), and 6-month-old (O, T) control (A-E, K-O) and Prg4–/– (F-J, P-T) mice were analyzed by histology. Hematoxylin and eosin (H&E) (A-T), Safranin O/Fast green (O, T, lower panel), and Masson’s trichrome (R, right panel) staining. Magnified sagittal views of glenoid fossa (B, G, L, Q), articular disc (C, H, M, R), and mandibular condyle (D, I, N, S, O, T) were obtained from each colored boxed area in the top panel of each line or corresponding sections. Note that H&E- or Masson’s trichrome-stained substance covers the surface of the glenoid fossa, disc, and condyle (G, H, Q, R, S arrowhead). There are no clear signs of inflammatory cell invasion into the synovial membrane (E, J). Scale bars: 250 µm in A for A, F, K, P, O, T; 65 µm in B for B, C, E, G, H, J, L, M, Q, R; and 150 µm in D for D, I, N, S. gf, glenoid fossa; di, disc; cd, condyle; sb, subchondral bone; pac, presumptive articular cartilage; ac, articular cartilage; in, intima; si, subintima; ab, anterior band; iz, intermediate zone; pb, posterior band.
A typical wild-type disc displays a thin intermediate zone (iz) flanked by thicker anterior (ab) and posterior bands (pb), and contains spindle-shaped fibroblastic cells (Figs. 1C, 1K, 1M). The mutant articular disc was considerably hyperplastic by P14 and contained many round chondrocytic cells facing the upper synovial cavity (Fig. 1H, arrow). By 3 mos, chondrocytic cells were also found in the lower half of the disc and were embedded in Masson’s trichrome-stained collagen matrix (Fig. 1R, arrows). Notably, mutant discs occasionally adhered to condylar articular cartilage (Fig. 1S, right panel, n = 3/6 mutant mice).
The synovial membrane is composed of two layers. The intima (in) consists of a sheet of synoviocytes, and the subintima (si) is an areolar tissue (Fig. 1E). Mutant synoviocytes developed long microvilli (mv) projecting from their surfaces (Fig. 1J). Notably, a large number of synoviocytes adhered to the lateral surface of mandibular condyle in Prg4–/– TMJ (Appendix Fig. 2).
In mutant TMJs, linings of synovial cavities were coated with a fibrinoid material of unclear origin (Figs. 1F-1H, 1P-1S, arrowhead). The deposits were initially observed on the surfaces lining the upper synovial cavity, such as the glenoid fossa and upper articular disc in P14 mutants (Figs. 1F-1H, arrowhead). Deposits were later observed on the condylar articular cartilage and lower disc border (Figs. 1P-1S, arrowhead). Synovial surfaces were smooth and free of any deposits in wild-type TMJs of all ages.
Molecular and Cellular Changes in Prg4–/– Mandibular Condylar Cartilage and Subchondral Bone
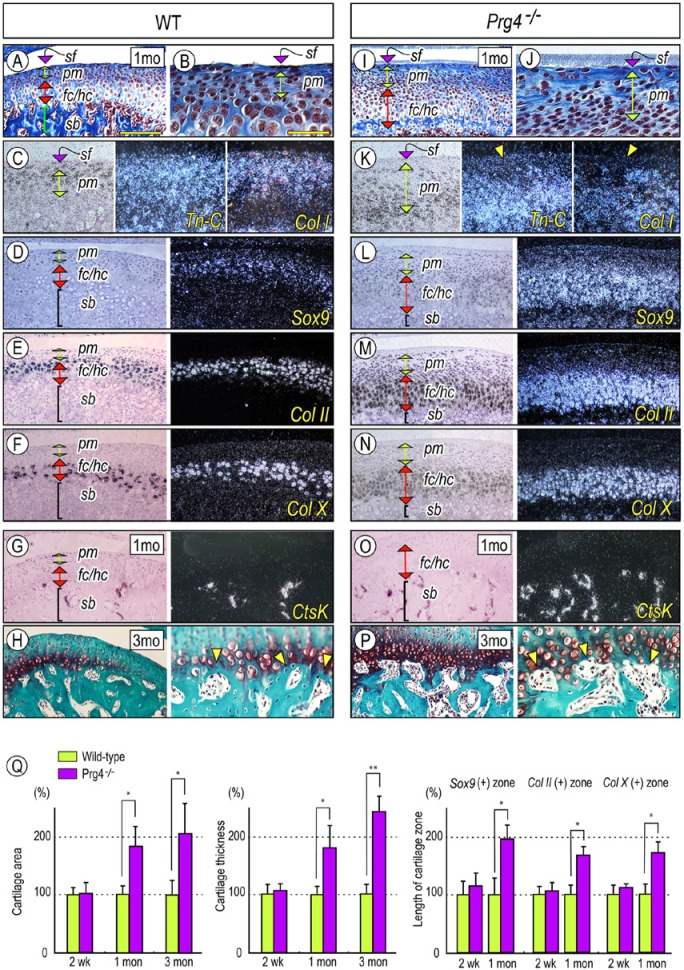
Prg4–/–-induced changes in mandibular condyle were further characterized by in situ hybridization, with several gene markers for chondroprogenitors and differentiated cells. Wild-type condylar articular cartilage displayed characteristic features as described previously (Luder et al., 1988; Shibukawa et al., 2007; Kinumatsu et al., 2011): a superficial layer (sf) composed of 1-3 layers of flattened superficial cells; a polymorphic (pm) cell layer containing chondroprogenitors; and zones of differentiating chondrocytes. Tn-C, an ECM molecule characteristic of permanent articular cartilage, and Type I collagen (Col I) were expressed in superficial/polymorphic cells and in some differentiating chondrocytes in deeper layers. Meanwhile, expression of Sox9, a master regulator for chondrogenesis, was detected in polymorphic cells and Col II–positive chondrocytes (Figs. 2A-2D). Col-X transcripts were detected in hypertrophic chondrocytes, and Catepsin K(CtsK) was expressed in osteoclasts in the chondro-osseous junction and marrow cavities (Figs. 2F, 2G). These patterns were drastically altered in Prg4 mutant condyles. Tn-C- and Col1-expressing cells were markedly reduced in the superficial layer and upper part of the polymorphic zone, and Sox9-, Col II-, and Col X–expression domains were significantly expanded (Figs. 2I-2N). Increased cartilage area and cartilage thickness became apparent by 1 mo of age, and Sox9/Col II–expressing immature chondrocytes and Col X–positive hypertrophic chondrocytes were proportionally increased (Fig. 2Q). Notably, there was an increase in the number of CtsK- and Mmp13-expressing osteoclasts at the Prg4–/– chondro-osseous junction and marrow cavities (Fig. 2O, Appendix Fig. 3). Prg4–/– subchondral bone had more and larger marrow cavities directly in contact with Safranin-O-stained cartilage. Articular bone plate formation was suboptimal (Figs. 2P, 2H, arrowheads).
Figure 2.
Prg4 mutants display defective superficial layer, abnormal subchondral bone, and increased chondrogenesis in the mandibular condyle. Serial parasagittal sections from 1-month (A-G, I-O) and 3-month-old (H, P) control (A-H) and Prg4–/– (I-P) TMJs. Sections were processed for Masson’s trichrome (A, B, I, J) and Safranin O/Fast green (H, P) staining and in situ hybridization (C-G, K-O) with isotope-labeled riboprobes for tenascin-C (Tn-C) (C, K), type I collagen (Col I) (C, K), Sox 9 (D, L), type II collagen (Col II) (E, M), type X collagen (Col X) (F, N), and Catepsin K (CtsK) (G, O). (Q) For quantification of the Safranin O-staining area and the thickness of chondrocyte zones, Sox9-expressing, Col II-expressing, and Col X-expressing zones along the longitudinal axis were measured from randomly selected 3-5 sections per sample (n = 3 for each mouse/each group) and presented as average ± SD. Control data were set as 100%. p values less than .05 were considered as statistically significant (*p < .01, **p < .005). Scale bars: 250 µm in A for A, D-H, I, L-P; 55 µm in B for B, C, H (right panel), J, K, and P (right panel). sf, superficial layer; pm, polymorphic layer; fc, flattened chondrocyte zone; hc, hypertrophic chondrocyte zone; sb, subchondral bone.
Increased Apoptosis in Superficial Layer and Cell Proliferation in Polymorphic Zone of Prg4 –/– TMJ
To identify possible cellular mechanisms underlying superficial cell loss and tissue hyperplasia in mutant TMJs, we analyzed apoptosis and cell proliferation in sections from P14-, 1- and 3-month-old control and mutant mice. TUNEL-positive cells in condylar superficial and polymorphic layers were not apparent at 2 wks of age, but were significantly increased about 3-fold in 1-month- and 3-month-old-Prg4–/– TMJs (Figs. 3B, 3E, 3G; p < .01). Cell proliferation was assessed at various regions as indicated in Fig. 3H. The glenoid fossa (gf), articular disc, and synovial membrane (sy), which surround the upper synovial cavity, exhibited significantly increased EdU incorporation in 2-week-old Prg4–/– mice. In wild-type articular discs, the intermediate zone (im) had fewer mitotic cells compared with the anterior (ant) and posterior (post) regions; however, in mutant discs, mitotic activity was similarly high in all areas (Fig. 3I). The synovial membrane showed increased cell proliferation in 1-month-old Prg4–/– TMJ (Fig. 3L). The increase in cell proliferation was somewhat delayed in mutant condyles and became apparent by 1 mo of age in the polymorphic layer (pm) (Figs. 3C, 3F, 3J). Although the trend toward increased cell proliferation persisted after peaking at 1 mo post-natally (p < .01), it did not show statistical significance in 3-month-old TMJ.
Figure 3.
Prg4 mutants display abnormal mitotic activity and apoptosis in TMJs. Serial parasagittal sections from wild-type (A-C) and Prg4–/– (D-F) mice were processed for TUNEL (B, E, G), Edu (C, F), and staining. TUNEL-positive apoptotic cells in superficial and polymorphic layers of distinct TMJ sections from control and Prg4–/– mice at 2 wks, 1 mo, and 3 mos of age were counted (approximately 100-120 cells in the condyle). TUNEL fluorescence images were merged with the corresponding bright-field image, and the representative image was presented in (B, E). Data were collected from randomly selected 4-6 sections per sample (n = 3 for each mouse/each group) and presented as average ± SD; p values < .05 were considered statistically significant (*p < .01, **p < .005) (G). Edu-incorporated proliferating cells in distinct TMJ sections from control and mutant mice at 2 wks, 1 mo, and 3 mos of age were counted in articular disc (I), condylar superficial/polymorphic layers (J), glenoid fossa (K), and synovial membrane (L), corresponding to the illustrated image (H) (approximately 100-120 cells in the glenoid fossa, synovial membrane, and condyle and 40-70 cells in the disc). Images visualized with DAPI fluorescence for nuclei were merged with Edu-incorporated GFP-positive cells to visualize dividing cells, and the representative image was presented (C, F). Data were corrected and processed for statistical analysis as above. gf, glenoid fossa; ac, articular cartilage; cd, condyle; sy, synovial membrane; sf/pm, superficial/polymorphic layers; ant, anterior band; im, intermediate zone; post, posterior band.
Increased Chondrogenesis and Has2 Expression in Prg4 –/– TMJ
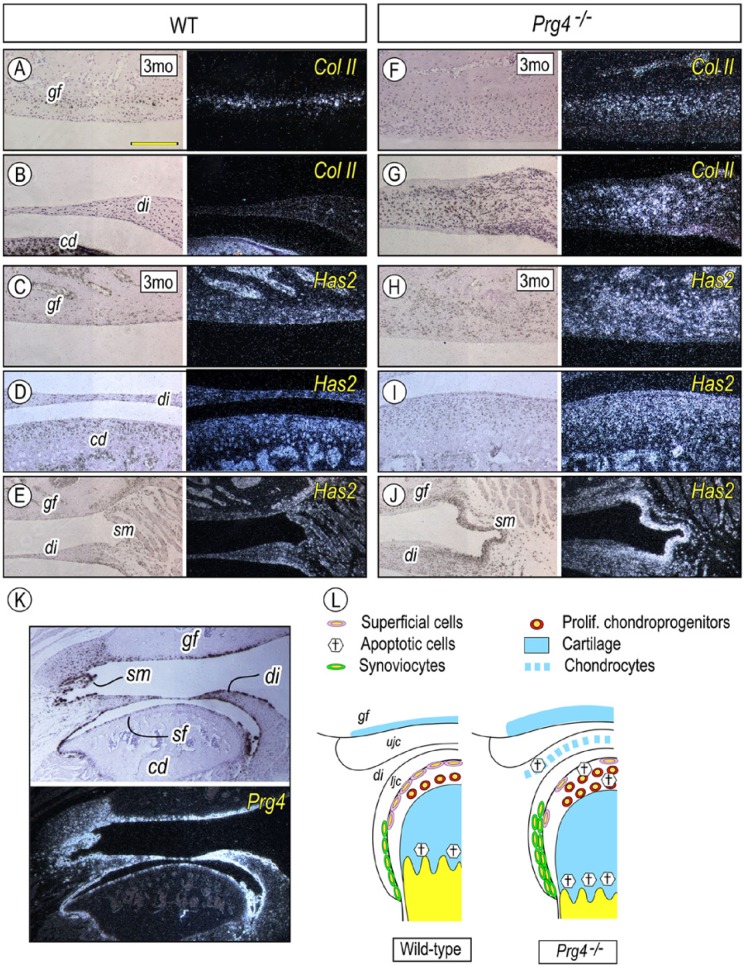
The 3-month-old wild-type mouse glenoid fossa typically contains a thin layer of Col-II-expressing chondrocytes (Fig. 4A). As predicted from histology (Fig. 1Q), the Col-II-positive chondrocyte population was significantly increased in Prg4–/– glenoid fossa compared with that in the wild-type control (Figs. 4A, 4F). Col-II-expressing chondrocytic cells were increased and distributed throughout the Prg4–/– articular disc (Fig. 4G), while Col-II-positive cells were barely detectable in the wild-type articular disc (Fig. 4B).
Figure 4.
Prg4 mutants display excessive chondrogenesis in the glenoid fossa and articular disc and increased Has2 expression. Serial parasagittal sections from 3-month-old control (A-E, K) and Prg4–/– (F-J) TMJs were processed for in situ hybridization with isotope-labeled riboprobes for Col II (A, B, F, G), Hyaluronic acid synthase 2 (Has2) (C-E, H-J), and Prg4 (K). Note the increased chondrogenesis in the Prg4–/–- glenoid fossa (F) and thickened disc (G) and increased Has2 expression in the glenoid fossa (H), disk (J), synovial membrane (J), and mandibular condyle (I). (L) Summary of abnormalities identified in TMJ lacking Prg4. Scale bars: 180 µm in A for A-J; 350 µm in K for K. gf, glenoid fossa; di, articular disc; co, mandibular condyle; sm, synovial membrane; sf, superficial layer.
Hyaluronan (HA) is another major joint lubricant (Nitzan, 2003; Tanaka et al., 2008). We thus asked whether loss of lubricin activity influenced HA production for functional compensation. HA synthases-2 (Has2) plays important roles in skeletal development, including synovial joints (Li et al., 2007; Matsumoto et al., 2009). Compared with that in wild-type mice, Prg4–/– TMJ showed increased Has2 expression in articular chondrocytes of the glenoid fossa and condyle and in the synoviocytes of thickened synovial intima (Figs. 4C-4E, 4H-4J).
Discussion
Our study demonstrates, for the first time, that lubricin plays essential roles in the maintenance of TMJ integrity. Lack of lubricin expression resulted in a multitude of changes in TMJ structure. Some of the changes were similar to what has been reported in Prg4–/– long bone synovial joints. For instance, the mutant TMJ synovial membrane showed thickening of the intima and signs of aggressive cell invasion and deterioration of articular surface associated with increased superficial cell apoptosis. The TMJ and long bone articular cartilage eventually developed osteoarthritic changes. However, Prg4–/– mice also showed a phenotype unique to TMJ. Prg4–/– mandibular condyles displayed thickening of cartilage from increased chondroprogenitor proliferation, an abnormal gene expression pattern consistent with disturbed cellular organization, and defective subchondral bone formation associated with increased osteoclastic activity. The Prg4–/– articular disc was hyperplastic and displayed excessive cell proliferation, ectopic chondrogenic differentiation, and occasional fusion to the articular surface. Given the severity and encompassing nature of these changes, it is quite clear that continuous expression of lubricin is required in TMJ to orchestrate and sustain the basic and fundamental processes and activities needed for post-natal functioning.
Our finding that the Prg4–/–-induced changes in TMJ components facing the upper cavity occurred prior to those seen in the lower cavity may be related to the distinct TMJ motion when in use. The upper TMJ compartment facilitates the condyle’s gliding motion, while the lower compartment allows for mandibular condyle rotation. Thus, our findings raise the interesting possibility that boundary lubrication may be more critical for joint surfaces subjected to gliding motion than rotational movement. Like other TMJ mouse models of OA or malocclusion (Wadhwa et al., 2005; Ishizuka et al., 2014; Schminke et al., 2014), Prg4–/– articular cartilage undergoes osteoarthritic changes accompanied by ECM degenerative changes in periarticular tissue and subchondral bone. Analysis of our data clearly suggests that altered endochondral ossification and abnormal cartilage and bone turnover (indicated by an increased number of osteoblasts and osteoclasts at the chondro-osseous junction) may be at least partly involved in aberrant subchondral bone formation in Prg4–/– TMJ. Further experiments will be necessary to clarify the mechanisms by which lubricin deficiency alters chondrocyte responses and may directly or indirectly affect the activation of osteoclastogenesis.
As noted above, hyaluronan is an important component of synovial fluid and is used as a treatment of osteoarthritis, including affected TMJs. Intra-joint injections are found to improve and mitigate the long-term clinical symptoms and problems associated with TMD (Liu and Steinkeler, 2013). We find that, among the HAS family members, Has2 is preferentially up-regulated in chondrocytes, disc cells, and/or synoviocytes in Prg4 mutant mice, suggesting that it may represent a mechanism to compensate for lubricin loss. Recent studies have indicated that hyaluronan acts synergistically with lubricin to provide friction reduction and greater wear protection in certain experimental conditions (Das et al., 2013). Our study would suggest that increased Has2 expression is not sufficient to prevent the articular cartilage damage and dysfunction seen in Prg4 mutant mice.
The intermediate zone of the TMJ articular disc is a dense fibrous tissue and typically shows low levels of cell proliferation. Conversely, the intermediate zone of the Prg4–/– disc shows increased cell proliferation and chondrogenic differentiation. Importantly, these phenotypic alterations are reminiscent of the early pathology seen in patients with internal derangement (ID). In these patients, the articular disc shows anterior or antero-medial displacement relative to the condyle in a closed-mouth position (de Leeuw et al., 1995; Molinari et al., 2007) and often displays hypertrophic deformation accompanied by increased matrix production and ectopic chondrogenesis (Loreto et al., 2009; Kiga et al., 2010). Though we have not tested whether disc displacement occurred in our mutant mice, it will be interesting to determine whether biomechanical changes in TMJ caused by lubricin deficiency increase the risk for ID and TMD. Additionally, further research could determine if the local administration of lubricin into TMJ cavities can protect articular surface and disc structure and prevent TMJ from disease progression.
Supplementary Material
Acknowledgments
We thank Dr. Matthew Warman for kindly proving the Prg4 mice.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
The study was supported by institutional funds, and additional support was provided by National Institutes of Health (NIH) grant AR062908.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML. (1998). The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum 41:730-735. [DOI] [PubMed] [Google Scholar]
- Das S, Banquy X, Zappone B, Greene GW, Jay GD, Israelachvili JN. (2013). Synergistic interactions between grafted hyaluronic acid and lubricin provide enhanced wear protection and lubrication. Biomacromolecules 14:1669-1677. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Boering G, Stegenga B, de Bont LG. (1995). TMJ articular disc position and configuration 30 years after initial diagnosis of internal derangement. J Oral Maxillofac Surg 53:234-241. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, et al. (1999). Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun 254:535-541. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, et al. (2009). Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum 60:840-847. [DOI] [PubMed] [Google Scholar]
- Ishizuka Y, Shibukawa Y, Nagayama M, Decker R, Kinumatsu T, Saito A, et al. (2014). TMJ degeneration in SAMP8 mice is accompanied by deranged Ihh signaling. J Dent Res 93:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay GD, Harris DA, Cha CJ. (2001). Boundary lubrication by lubricin is mediated by O-linked beta(1-3)Gal-GalNAc oligosaccharides. Glycoconj J 18:807-815. [DOI] [PubMed] [Google Scholar]
- Kiga N, Tojyo I, Matsumoto T, Hiraishi Y, Shinohara Y, Fujita S. (2010). Expression of lumican in the articular disc of the human temporomandibular joint. Eur J Histochem 54:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinumatsu T, Shibukawa Y, Yasuda T, Nagayama M, Yamada S, Serra R, et al. (2011). TMJ development and growth require primary cilia function. J Dent Res 90:988-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, et al. (2007). Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development 134:2159-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto C, Musumeci G, Leonardi R. (2009). Chondrocyte-like apoptosis in temporomandibular joint disc internal derangement as a repair-limiting mechanism. An in vivo study. Histol Histopathol 24:293-298. [DOI] [PubMed] [Google Scholar]
- Li Y, Toole BP, Dealy CN, Kosher RA. (2007). Hyaluronan in limb morphogenesis. Dev Biol 305:411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Steinkeler A. (2013). Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent Clin North Am 57:465-479. [DOI] [PubMed] [Google Scholar]
- Luder HU, Leblond CP, von der Mark K. (1988). Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat 182:197-214. [DOI] [PubMed] [Google Scholar]
- Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, et al. (1999). CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet 23:319-322. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, et al. (2009). Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development 136:2825-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam SB. (2005). Pathogenesis of degenerative temporomandibular joint arthritides. Odontology 93:7-15. [DOI] [PubMed] [Google Scholar]
- Molinari F, Manicone PF, Raffaelli L, Raffaelli R, Pirronti T, Bonomo L. (2007). Temporomandibular joint soft-tissue pathology, I: Disc abnormalities. Semin Ultrasound CT MR 28:192-204. [DOI] [PubMed] [Google Scholar]
- Nitzan DW. (2003). ‘Friction and adhesive forces’—possible underlying causes for temporomandibular joint internal derangement. Cells Tissues Organs 174(1-2):6-16. [DOI] [PubMed] [Google Scholar]
- Novince CM, Entezami P, Wilson CG, Wang J, Oh S, Koh AJ, et al. (2012a). Impact of proteoglycan-4 and parathyroid hormone on articular cartilage. J Orthop Res 31:183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novince CM, Michalski MN, Koh AJ, Sinder BP, Entezami P, Eber MR, et al. (2012b). Proteoglycan 4: a dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism. J Bone Miner Res 27:11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. (2005). The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest 115:622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan MZ, Erez A, Guse K, Dawson B, Bertin T, Chen Y, et al. (2013). Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med 5:176ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schminke B, Muhammad H, Bode C, Sadowski B, Gerter R, Gersdorff N, et al. (2014). A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell Mol Life Sci 71:1081-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, et al. (2007). Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn 236:426-434. [DOI] [PubMed] [Google Scholar]
- Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. (1985). The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J 225:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Detamore MS, Tanimoto K, Kawai N. (2008). Lubrication of the temporomandibular joint. Ann Biomed Eng 36:14-29. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Embree MC, Kilts T, Young MF, Ameye LG. (2005). Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarthritis Cartilage 13:817-827. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Kapila S. (2008). TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ 72:930-947. [PMC free article] [PubMed] [Google Scholar]
- Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. (2013). Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci USA 110:5852-5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.