Abstract
Prenatal hypoxia (HPX) reduces mitochondrial cytochrome c oxidase (CCO and COX) activity in fetal guinea pig (GP) hearts. The aim of this study was to quantify the lasting effects of chronic prenatal HPX on cardiac mitochondrial enzyme activity and protein expression in offspring hearts. Pregnant GPs were exposed to either normoxia (NMX) or HPX (10.5%O2) during the last 14 days of pregnancy. Both NMX and HPX fetuses, delivered vaginally, were housed under NMX conditions until 90 days of age. Total RNA and mitochondrial fractions were isolated from hearts of anesthetized NMX and HPX offspring and showed decreased levels of CCO but not medium-chain acyl dehydrogenase activity, protein levels of nuclear- and mitochondrial-encoded COX4 and COX1, respectively, and messenger RNA expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, COX5b, and 4.1 compared to NMX controls. Prenatal HPX may alter mitochondrial function in the offspring by disrupting protein expression associated with the respiratory chain.
Keywords: cardiac, electron transport chain, programming
Introduction
Chronic intrauterine hypoxia (HPX) induces fetal growth restriction and contributes to fetal programming.1–4 Fetal HPX has been shown to alter signaling mechanisms of multiple fetal organs such as the heart,5–9 brain,10,11 liver,12–16 and skeletal muscle.16 Chronic intrauterine HPX has been shown to contribute to permanent changes in cardiovascular function of the offspring through programming.9,13,17–19 Central to all organ functions is the role of mitochondria in the cell’s ability to maintain adequate energy supply in the presence of low oxygen levels. Yet, the postnatal effect of intrauterine HPX on cardiac mitochondrial function in the offspring remains incompletely understood.
The embryonic heart relies on anaerobic glycolysis, lactate oxidation, and fatty acid metabolism for generating adenosine triphosphate (ATP).20 The immature heart utilizes glycolysis as its predominant energy pathway because of the availability of glucose in utero and the upregulation of enzymes in the glycolytic pathway.21 As the fetal heart matures, fatty acid oxidation is also utilized in cellular respiration for ATP synthesis but to a smaller percentage compared to glycolysis.20 At the time of birth, the immature heart undergoes a metabolic switch, increasing its reliance on fatty acid oxidation as circulating free fatty acids increase and the dependence on the glycolytic pathway is decreased due to downregulation of glycolytic enzymes and negative feedback regulation of glycolysis.20,21 Since the heart has a high metabolic rate, its reliance on mitochondrial function for energy production is critical for normal cardiac function.22
Proper regulation of mitochondrial respiration is important for maintaining an efficient energy supply for the heart. This is met by the synthesis of ATP by F1F0ATPsynthase, which is dependent on the electron flux through the electron transport chain, the resulting H+ gradient across the inner mitochondrial membrane by the electron transport chain, and the adenosine diphosphate levels. Cytochrome c oxidase (CCO) is the major site of O2 consumption in the mitochondria and the terminal site of reduction of O2 to H2O. Altered regulation of the mitochondrial respiratory chain can result in inefficient respiration where ATP synthesis is reduced.23 In adult rat hearts exposed to hypobaric HPX of 11%O2 for 7 days, left ventricles showed a decrease in mitochondrial transcript levels of peroxisome proliferator–activated receptor gamma coactivator 1-alpha (PGC-1α), cytochrome c oxidase subunit II (COXII), and uncoupling proteins, which was associated with a decrease in mitochondrial respiratory activity.22 Similarly, a 21-day exposure to chronic HPX also decreased the activities of complexes I, II, III, and IV in both adult heart ventricles.24 In addition, adult rat hearts exposed to HPX followed by normoxia (NMX) had decreased complex III activity and postischemic contractile dysfunction.25 Further, cardiac mitochondrial energy metabolism was not fully restored after NMX recovery from chronic HPX, suggesting a permanent alteration in mitochondrial respiration to HPX stress.26
The role of prenatal HPX on fetal cardiac mitochondrial function has had limited attention. We have reported that chronic maternal HPX of pregnant guinea pigs generates increases in fetal cardiac HPX-inducible factor (HIF-1α),6 peroxynitrite,7 and malondialdehyde levels,27 indicative of HPX stress. Further, we have shown that chronic HPX generates oxidative and nitrosative stress within fetal heart ventricles,7 concomitant with reduced cardiac CCO activity.27 Therefore, chronic HPX has a negative impact on fetal cardiac mitochondrial function although its lasting consequences on cardiac function in the offspring remain unknown. Only 2 studies have investigated the role of mitochondrial function in programming of offspring hearts, with one showing decreased rat cardiac CCO activity with maternal copper-deficient diet28 and the other increased sheep cardiac mitochondrial H2O2 release with maternal dexamethasone administration.29 Given the impact of intrauterine stress on cardiovascular programming and cardiac contractile function of the offspring,17,18,30 we propose that chronic in utero exposure to HPX alters the expression of mitochondrial proteins that render the offspring hearts susceptible to injury. We hypothesize that the HPX-induced decrease in CCO activity measured in fetal guinea pig hearts27 increases the vulnerability of the offspring heart by compromising mitochondrial function. This was tested by measuring the enzyme activities of medium-chain acyl-coenzyme A dehydrogenase (MCAD) and CCO, messenger RNA (mRNA)/protein expression of CCO subunits, and PGC-1α and NRF-1, key transcription factors important in the regulation of gene expression associated with mitochondrial function31 in guinea pig hearts of offspring exposed to HPX in utero.
Methods
Animal Model
Female Duncan-Hartley guinea pigs (term = 65 days) were purchased from a commercial breeder (Elm Hill Breeding Labs, Chelmsford, Massachusetts) and time mated. Pregnant guinea pig sows were placed in a plexiglass chamber containing 10.5% O2 for the last 14 days of pregnancy and allowed to deliver. Normoxic controls were maintained at room air (21%O2) throughout their pregnancy. In a separate group of pregnant animals, maternal percentage O2 saturation (SpO2) had been previously measured using a STARR MouseOx pulse oximeter (STARR Life Sciences, Inc, Torrington, Connecticut) on the foot pad of animals quantifying a significant decrease in arterial blood oxygenation of HPX versus NMX animals (maternal SpO2 values: 66.1% ± 1.4% vs 97.7% ± 0.2% [P < .05]; HPX [n = 10] vs NMX [n = 10] animals). In addition, this generates HPX fetuses that exhibit increased levels of fetal cardiac HIF-1α protein,6 hepatic hypoxyprobe staining8 as well as growth restriction.27 After birth, both NMX and HPX newborn pups were reared with their mothers, weaned at 30 days of age, and housed in room air with access to ad libitum food and water until 90 days. Male offspring guinea pigs were studied at an age (90 days old) of reproductive maturity and at the plateau phase of the growth curve. Sex differences were not studied. Both NMX and HPX offspring were anesthetized with ketamine (80 mg/kg intraperitoneal [ip]) and xylazine (1 mg/kg ip), and lidocaine was given subcutaneously along the midline of the abdomen prior to dissection. Arterial blood pressures were measured from the right brachial artery cannulated in anesthetized animals. Body weights, heart weights, and heart weight–body weight ratios were measured and left and right ventricles were excised and snap frozen in liquid N2 and stored at −80°C. The methods used were approved by the University of Maryland Animal Care Committee and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Mitochondrial Isolation
Enriched tissue mitochondrial fractions were generated in a similar manner as described previously.32 Briefly, frozen left and right cardiac ventricles of prenatally NMX and HPX offspring hearts were pulverized with mortar and pestle and 30 to 50 mg homogenized in ice-cold mitochondrial isolation buffer (250 mmol/L sucrose, 5 mmol/L HEPES, 1 mmol/L EDTA, pH 7.5) in a Next Advance Bullet Blender (Averill Park, NY) for 5 minutes and centrifuged at 4°C at 500g to remove the cellular debris. The supernatant fractions were centrifuged at high speed (12.5 × 1000g) to generate an enriched mitochondrial pellet fraction. Cardiac mitochondrial fractions were resuspended in homogenizing buffer and protein concentrations were determined by the Bradford Protein Assay (Bio-Rad Laboratories, Hercules, California).
Cytochrome c Oxidase Assay
Cytochrome c oxidase is the terminal complex of the electron transport chain and the main site of O2 consumption. The CCO activity of the left and right ventricles of NMX and HPX offspring hearts was quantified by monitoring the oxidation rate of reduced cytochrome C. Equal amounts of isolated mitochondria (0.5 μg) from NMX and HPX offspring heart ventricles were solubilized with 50 µmol/L n-dodecyl-beta-d-Maltoside and added to a 96-well plate containing the assay buffer (100 mmol/L K+PO4 − pH 7.4 and 220 µmol/L reduced cytochrome c). Samples were read at 550 nm and the activity values expressed as µmol/L of oxidized cytochrome c/min/mg of mitochondria.
Medium-Chain Acyl-CoA Dehydrogenase Assay
Medium-chain acyl-CoA dehydrogenase is a mitochondrial enzyme that oxidizes medium-chain (6-10 carbon) fatty acids and reduces flavin adenine dinucleotide (FAD) to FADH2. The MCAD activity of left and right ventricles of NMX and HPX offspring hearts was quantified by monitoring the rate of ferrocenium reduction to ferrocene. Briefly, equal amounts (20 µg) of solubilized mitochondria of NMX and HPX offspring heart ventricles were added to a buffered solution containing 100 mmol/L K+PO4 − pH 7.4, 1 mmol/L EDTA, 500 µmol/L sodium tetrathionate, and 200 µmol/L ferrocenium hexafluorophosphate in a 96-well plate. The reaction was initiated by the addition of 500 µmol/L octanoyl-CoA, the 8-carbon substrate for MCAD, and read at 300 nm. The MCAD activity is expressed as mmol/L reduced ferrocenium/min/mg of mitochondria.
Mitochondrial Protein Western Blot
Left ventricles were isolated from NMX and HPX offspring hearts and the protein expression of COX4, a nuclear-encoded subunit of CCO, and COX1, a mitochondria-encoded subunit of CCO, was analyzed using Western blot analysis. Briefly, 5 µg of mitochondrial protein extracted from offspring NMX and HPX left ventricles were incubated with Laemmli Buffer at 55°C for 10 minutes, resolved on a 4% to 15% precast gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to an Immun-Blot polyvinyl difluoride membrane (Bio-Rad). The membranes were probed with a primary mouse monoclonal antibody (1:10 000) that recognizes COX4 and COX1 (Mitosciences, Eugene, Oregon) in 5% nonfat dry milk over night at 4°C. Membranes were then incubated for 1 hour in 5% nonfat dry milk containing horseradish peroxidase-conjugated chicken anti-mouse secondary antibody (1:10 000, Santa Cruz Biotechnology, Santa Cruz, California). Bands were detected and visualized using enhanced chemiluminiscence (Western Lightning Plus, Perkin Elmer, Waltham, Massachusetts) and quantified using densitometry in ImageJ (U.S. National Institutes of Health, Bethesda, Maryland; http://imagej.nih.gov/ij/). The intensities of COX4 and COX1 bands were normalized to porin expression (Mitosciences) and expressed as ratios. Porin is a mitochondrial protein located in the outer membrane and used as a loading control. There were no significant differences in porin expression between NMX and HPX samples.
RNA Isolation, Complementary DNA Generation, and Real-Time Reverse Transcriptase–Polymerase Chain Reaction
Total RNA was isolated from left ventricles of NMX and HPX offspring hearts using Rneasy Fibrous Tissue total RNA isolation mini kit from Qiagen (Valencia, California). RNA samples were converted to complementary DNA (cDNA) using ABI High-Capacity cDNA Reverse Transcription kit. Briefly, 10 µL of 2× RT master mix are added to 10 µL of RNA (100 ng total). The reaction is then incubated at 37°C for 2 hours followed by a 5-minute incubation at 85°C to terminate the reaction. Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) reactions were conducted using Fermentas Maxima SYBR Green qPCR (Thermoscientific/Fermentas, Inc, Glen Burnie, Maryland). The primer sequences were obtained from Genebank and targeted COX4.1: (5′-TACACGTAGCGCTTCTCCCAGAT-3′; 5′-AGATGAACAGGGTCAGCAACCAGT-3′), COX4.2 (5′-GCCACCAAATCAGCAAAGCCGTTA-3′, 5′-TTCCGTGAGACCTTCGCAGAGATGAA-3′), COX5b (5′-CACCAGTTTGTAATGGGTTCCGCA-3′, 5′-AGGACAACAGCACTGTCATCTGGT-3′), PGC-1α (5′-AGTTCTGTCCGTGTTGTGTCAGGT-3′, 5′-ATCAGAAAGGGCCAAACAGAGGGA-3′), and NRF-1 (5′-GGCCGTTTCCGTTTCTTTCCTGTT-3′, 5′-TGGCCACTTACACTGAGCATAGCA-3′). The 40-cycle amplification protocol of cDNA samples was initiated by a 10-minute incubation at 95°C followed by 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Data were obtained from cycling time (CT) values (cycle number at which PCR product crosses threshold) quantified by the delta–delta CT (2-DDCt) method.33 The mRNA levels for mitochondrial subunits (COX4.1, 4.2, 5b) and PGC-1α and NRF-1 were normalized to total RNA.
Statistics
Data are expressed as mean ± standard error of the mean. Student t test was used to analyze statistical significance between NMX and HPX groups. A P value of less than .05 was considered statistically significant.
Results
Animal Parameters
There were no differences in body weights (692.9 ± 30.4 vs 662.7 ± 13.3 g, NMX vs HPX, respectively), heart weights (1.95 ± 0.1 vs 1.84 ± 0.1 g), or heart weight–body weight ratios (0.0028 ± 0.001 vs 0.0028 ± 0.0001) between groups at 90 days of age despite previously reported fetal growth restriction of HPX guinea pig fetuses.5–7,34 Offspring guinea pigs exposed to prenatal HPX (n = 9) had significantly (P < .05) greater systolic (59.2 ± 4.0 vs 72.3 ± 2.3 mm Hg, NMX vs HPX, respectively), diastolic (41.9 ± 3.4 vs 51.1 ± 1.8 mm Hg), and mean arterial blood pressures (47.1 ± 3.6 vs 58.2 ± 1.9 mm Hg) than that of NMX controls (n = 6). The arterial blood pressures of NMX are representative of values measured in anesthetized adult guinea pigs.35,36
Effects of Intrauterine HPX on Offspring Cytochrome Oxidase Activity
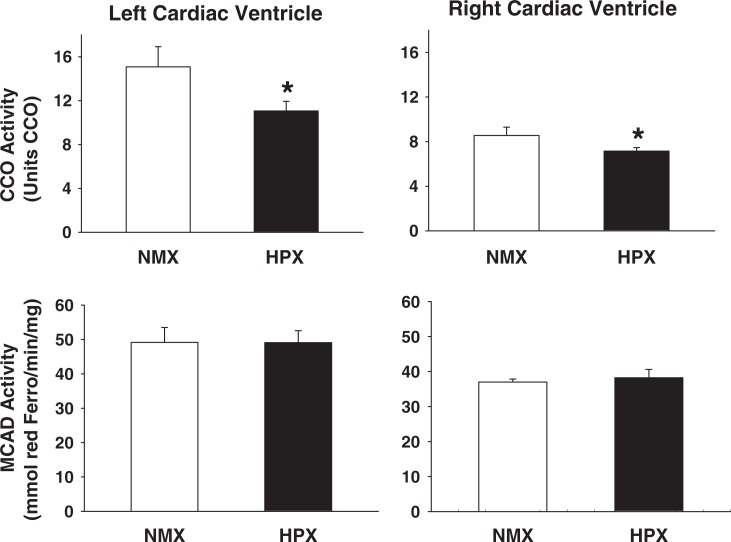
The CCO activity (oxidized cytochrome c/min/mg protein) is measured as an index of complex IV function. Mitochondrial CCO activity of offspring hearts of animals exposed to prenatal HPX and NMX is shown in Figure 1. Prenatal HPX decreased the CCO activity in both the left (26.1%, P < .05) and the right ventricles (16.3%; P < .05) compared to NMX controls.
Figure 1.
Cytochrome oxidase (CCO) and medium-chain acyl-coenzyme A dehydrogenase (MCAD) activity of left and right cardiac ventricles of offspring guinea pigs exposed to prenatal normoxia (NMX; n = 6) or hypoxia (HPX; n = 9). The CCO activity is expressed as units CCO (1 U = 1µmol of oxidized cytochrome c/min/mg protein) and MCAD activity as mmol of reduced ferrocenium/min/mg protein. Data are mean ± standard error of the mean (SEM). * indicates P < .05 versus NMX controls.
Effects of Intrauterine HPX on Offspring Medium-Chain Acyl-Coenzyme A Activity
Medium-chain acyl-coenzyme A dehydrogenase activity (reduced ferrocenium/min/mg protein) is measured as an index of fatty acid β-oxidation capacity. The MCAD activity levels of offspring heart ventricles are shown in Figure 1. The MCAD activity levels in either left or right ventricles were similar between NMX and HPX offspring hearts.
Effects of Chronic Intrauterine HPX on Expression of CCO Subunits 4 and 1
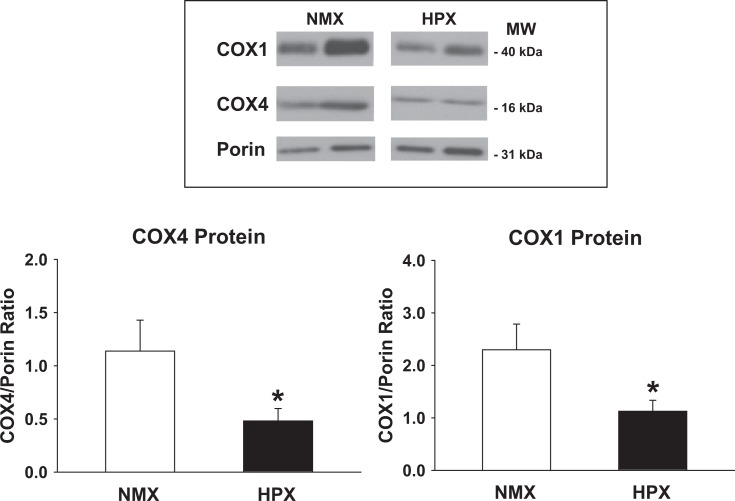
A nuclear-encoded subunit of CCO, COX4, has been demonstrated to play an important role in regulating the stability of the mitochondria-encoded catalytic CCO subunit 1 (COX1).37 Figure 2 illustrates representative Western immunoblots of protein expression of COX4 and COX1 from cardiac left ventricles. Figure 2 presents the ratio of COX4 and COX1 protein expression (normalized to porin). Chronic intrauterine HPX reduces subunit expression of both COX4 and COX1 in cardiac left ventricles (by 57% and 51%, respectively; P < .05) compared to NMX controls.
Figure 2.
Protein levels of cytochrome oxidase subunits 4 and 1 (COX4 and COX1, respectively) of left cardiac ventricles of 90-day-old offspring guinea pigs exposed to prenatal normoxia (NMX; n = 4) or hypoxia (HPX; n = 5). COX4 and COX1 protein expression is measured by Western blot analysis as a ratio of COX4 or COX1/porin. Data are mean ± standard error of the mean (SEM). * indicates P < .05 versus NMX controls.
Effects of Chronic Intrauterine HPX on Expression of CCO Subunit mRNA
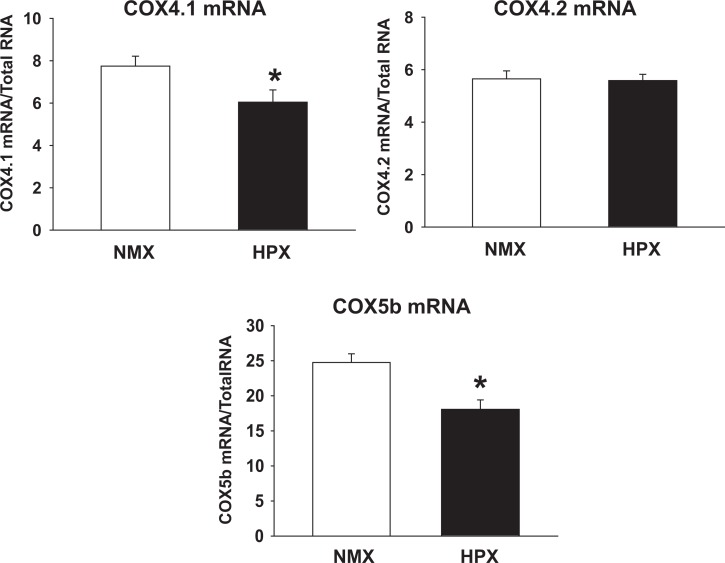
Cytochrome C oxidase subunits 4.1, 4.2 (COX4.1 and COX4.2), and 5b are nuclear-encoded subunits of CCO, which regulate the stability and dimerization of complex IV.37–39 In Figure 3, COX4.1 and 5b (by 22% and 21%, respectively; P < .05) but not COX 4.2 mRNA levels of cardiac left ventricles were decreased in HPX compared to NXM offspring hearts.
Figure 3.
Messenger RNA (mRNA) expression of cytochrome oxidase subunits 4.1, 4.2, and 5b (COX4.1, COX4.2, and COX5b, respectively) in left cardiac ventricles of 90-day-old offspring guinea pigs exposed to prenatal normoxia (NMX) or hypoxia (HPX). COX4.1, 4.2, and COX5b mRNA expression levels are normalized to total RNA. Data are mean ± standard error of the mean (SEM). * indicates P < .05 versus NMX controls. COX4.1 and 4.2 NMX n = 6, HPX n = 9, and COX5b NMX and HPX n = 5.
Effects of Chronic Fetal HPX on Transcriptional Coactivator Gene Expression
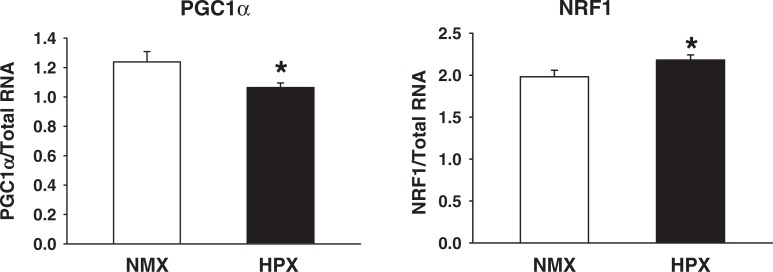
Both PGC-1α and NRF-1 are master regulators of mitochondrial gene expression and biogenesis.38,39 Figure 4 illustrates mRNA expression levels in cardiac left ventricles of offspring hearts from animals exposed to intrauterine HPX. The PGC-1α mRNA levels were reduced (P < 0.05) by 14%, and the NRF-1 levels increased (P < 0.05) by 10% in offspring of HPX cardiac left ventricles.
Figure 4.
Peroxisome proliferator–activated receptor gamma coactivator 1-alpha (PGC-1α) and nuclear respiratory factor 1 (NRF-1) messenger RNA (mRNA) expression of left cardiac ventricles of 90-day-old offspring guinea pigs exposed to prenatal normoxia (NMX; n = 6) or hypoxia (HPX; n = 9). The expression levels of PGC-1α and NRF-1 transcript are normalized to total RNA and presented as a ratio of PGC-1α or NRF-1 levels/total RNA. Data are mean ± standard error of the mean (SEM). * indicates P < .05 versus NMX controls.
Discussion
This study demonstrates that exposure to prenatal HPX reduces mitochondrial CCO activity in both the right and the left ventricles of 90-day-old male offspring. This was accompanied by a concomitant decrease in the cardiac expression of nuclear- and mitochondrial-encoded CCO subunits, COX4 and COX1 proteins, respectively, of age-matched, prenatally exposed, HPX offspring compared to NMX controls. Prenatal HPX caused an isoform-specific reduction in COX5b and COX4.1 but not in COX4.2 mRNA expression and differentially altered mRNA levels of PGC-1α and NRF-1 in offspring hearts, with PGC-1α levels decreased and NRF-1 levels increased compared to controls. Taken together, these results indicate that intrauterine HPX downregulates both expression of mitochondrial CCO subunits and activity of left ventricles of 90-day-old offspring, consistent with decreased transcription of its target genes.
Cytochrome c oxidase is the terminal complex of the electron transport chain that facilitates the oxidation of cytochrome C by O2. The mammalian CCO is made up of 13 subunits, 3 of which are mitochondrial encoded and the remaining 10 are encoded by the nuclear genome.37 The CCO activity is regulated by transcription of both regulatory and catalytic subunits and is tightly coupled to the cell’s energetic demand and metabolic output.40–42 Both COX4, a regulatory subunit, and COX1, a catalytic subunit, are key proteins important in the regulation of CCO activity. The COX4 has been identified as an ATP-binding subunit,43,44 and COX5b provides a binding site for protein kinase A.45 The complex regulation of CCO activity arises in part from the product of the bigenomic contribution of nuclear and mitochondrial DNA, which encodes the protein subunits of CCO41 and their assembly within the inner mitochondrial membrane.40 The structure and activity of CCO is regulated by a variety of mechanisms including subunit assembly as well as allosteric modulation by small molecules such as peroxynitrite and by phosphorylation of specific CCO subunits.37 We proposed that exposure to prenatal HPX would reduce mitochondrial protein expression and function in programmed offspring hearts. The decrease in expression of both COX4 and COX1 proteins measured in the present study is consistent with a decrease in CCO activity of offspring hearts exposed to prenatal HPX. In contrast, prenatal HPX did not alter MCAD activity, suggesting a selective alteration in mitochondrial function. In another study, a copper-deficient diet, fed to pregnant rats, decreased COX1 protein levels and CCO activity in heart ventricles of 21-day-old rat offspring,28 linking mitochondrial protein expression and CCO activity as well as an inhibitory effect of intrauterine stress on cardiac mitochondria. Further, 2 infants who died of hypertrophic cardiomyopathy were identified with a homozygous mutation in C2orf64, an ortholog gene of yeast PET191, important in complex IV assembly.46 Each exhibited impaired complex IV activity associated with decreased levels of fibroblast COX2, COX4, and COX5a subunits. Thus, the reduced expression of specific subunits of cardiac mitochondrial proteins of the electron transport chain may reduce enzyme activity important in mitochondrial respiration.
The molecular mechanisms associated with downregulation of mitochondrial protein expression in the offspring remain unclear. The PGC-1α is a major regulator of mitochondrial biogenesis and oxidative metabolism, inducing the transcription of mitochondrial proteins involved in oxidative phosphorylation and fatty acid oxidation.38,41,42 It is abundantly expressed in heart tissue47 and interacts with NRF-1 and NRF-2 to regulate heart energy metabolism.42 The NRF-1 is a key regulator of nuclear-encoded subunits of CCO as well as all of the 5 respiratory complexes.38,41 The NRF-2 plays a role in the expression of COX 448,49 and COX 5b50,51 subunits along with other nuclear-encoded COX subunits.52 The PGC-1α binds to NRF-1 and NRF-2, which is required for transactivation of NRF target genes.53,54 It is a key regulator between environmental changes in the cell and their effects on gene expression.39 Thus, PGC-1α serves an integrative function in binding to NRF-1 and NRF-2 and facilitating the activation of gene expression of mitochondrial protein subunits. The PGC-1α expression is regulated by HPX,55 oxidative stress,55–57 and hormone receptors,41,58–60 and the loss of function of PGC-1α is associated with reduced mitochondrial ATP synthesis and fatty acid oxidation.61 The downregulation of PGC-1α despite a small increase in NRF-1/2 may be sufficient to contribute to the reduced expression of the downstream targets of NRF-1/2 if there is altered binding of the PGC-1α/NRF complex to the cytochrome subunit promoters. Thus, prenatal HPX exposure resulting in altered expression of NRFs and their coactivators may induce a dysregulation of mitochondrial protein expression that is sustained in the offspring.
There are several mechanisms by which mitochondrial protein expression may be downregulated by prenatal HPX in the offspring. These include epigenetic mechanisms of DNA methylation62 and histone deacetylation as well as posttranslational modification. DNA methylation has been reported to be increased in response to both prenatal HPX in heart ventricles63 and oxidative stress of cancer cells,64 carotid body,65,66 and the heart.67 We have previously shown that prenatal HPX increases HIF-1α protein levels in HPX-exposed fetal hearts,6 indicating local tissue HPX. Further, we have reported that fetal HPX increases fetal cardiac malondialdehyde levels and reduces mitochondrial CCO activity of fetal guinea pig hearts, both of which are reversed by maternal administration of the antioxidant, N-acetylcysteine, suggestive of oxidative stress.27 Although there are no studies linking prenatal HPX and DNA methylation with decreased mitochondrial protein expression, conditions such as aging are associated with increased DNA methylation, decreased PGC-1α and COX7A1 mRNA, a cytochrome c subunit.68 Alternatively, posttranslational modification can occur by direct inhibition of CCO activity by nitric oxide,69,70 peroxynitrite,71 malondialdehyde,72 and acetylation of lysine residues.73 Thus, the elevated nitric oxide, peroxynitrite, and malondialdehyde levels measured in HPX fetal guinea pig hearts in our previous studies6,7 may be the initiating factors in generating a sustained decrease in CCO activity in the offspring. Previous studies have shown that both peroxynitrite and malondialdehyde can form adducts with mitochondrial complex activity in I, II, and V71 and CCO,72 respectively, to inhibit mitochondrial enzyme activity. Therefore, several signaling mechanisms initiated by intrauterine HPX may contribute to the altered mitochondrial protein activity measured in offspring hearts.
Finally, arterial blood pressure was increased in offspring exposed to prenatal HPX—a finding measured in some74 but not all studies.75,76 This indicates that prenatal HPX induces a cardiovascular risk of pressure overload in the offspring, in addition to a secondary risk of downregulation of mitochondrial protein expression in offspring hearts. Other studies have identified a downregulation of PGC-1α mRNA levels and mitochondrial energy metabolism following transverse aortic constriction and ventricular pressure overload in adult mouse hearts,77,78 suggesting that increased blood pressure may have deleterious effects on hearts whose respiratory activity is compromised.
In summary, this study demonstrates that exposure to prenatal HPX alters mitochondrial protein expression and reduces CCO activity in offspring heart ventricles. This was attributed to downregulation of PGC-1α, a master regulator of mitochondrial protein expression, concomitant with a decrease in mRNA and protein levels of COX4 and COX1, key subunits of the CCO protein complex. These data suggest that offspring exposed to prenatal HPX may be at risk of mitochondrial dysfunction if intrauterine HPX induces permanent changes in cardiac mitochondrial protein expression and decreases electron transport chain function. Further study is needed to study the impact of altered mitochondrial protein expression on mitochondrial respiration and cardiac function with cardiovascular programming in the offspring.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported in part by NIH Grants R01HL 49999 (LPT) and T32-HL072757 (YMA). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
References
- 1. Hanson MA, Gluckman PD, Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102(2):90–93 [DOI] [PubMed] [Google Scholar]
- 2. Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan. Prog Biophys Mol Biol. 2011;106(1):323–336 [DOI] [PubMed] [Google Scholar]
- 3. Nuyt AM, Alexander BT. Developmental programming and hypertension. Curr Opin Nephrol Hypertens. 2009;18(2):144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nesterenko TH, Aly H. Fetal and neonatal programming: evidence and clinical implications Am J Perinatol. 2009;26(93):191–198 [DOI] [PubMed] [Google Scholar]
- 5. Thompson LP, Dong Y. Chronic hypoxia decreases endothelial nitric oxide synthase protein expression in fetal guinea pig hearts. J Soc Gynecol Invest. 2005;12(6):388–395 [DOI] [PubMed] [Google Scholar]
- 6. Thompson L, Dong Y, Evans L. Chronic hypoxia increases inducible NOS-derived nitric oxide in fetal guinea pig hearts. Pediatric Res. 2009;65(2):188–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans LSC, Liu H, Pinkas GA, Thompson LP. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatric Res. 2012;71(1):25–31 [DOI] [PubMed] [Google Scholar]
- 8. Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstet Gynecol. 2008;199(1):78.e1–6 [DOI] [PubMed] [Google Scholar]
- 9. Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther. 2009;330(2):624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong Y, Hou W, Wei J., Weiner CP. Chronic hypoxemia absent bacterial infection is one cause of the fetal inflammatory response syndrome (FIRS). Repro Sci. 2009;16(6):650–656 [DOI] [PubMed] [Google Scholar]
- 11. Dong Y, Yu Z, Sun Y, et al. Chronic fetal hypoxia produces selective brain injury associated with altered nitric oxide synthases. Am J Obstet Gynecol. 2011;204(3):254.e16–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashimoto K, Pinkas G, Evans L, Liu H, Al Hasan Y, Thompson LP. Protective effect of N-acetylcysteine on liver damage during chronic intrauterine hypoxia in fetal guinea pig. Repro Sci. 2012;19(9):1001–1009. doi: 10.1177/1933719112440052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Camm EJ, Martin-Gronert MS, Wright NL, Hansell JA, Ozanne SE, Giussani DA. Prenatal hypoxia independent of undernutrition promotes molecular markers of insulin resistance in adult offspring. FASEB J. 2011; 25(1): 420–427 [DOI] [PubMed] [Google Scholar]
- 14. Gentili S, Morrison J, McMillen IC. Transcriptional coregulators in the control of energy homeostasis. Trends in Cell Biol. 2009; 17(6): 292–301 [DOI] [PubMed] [Google Scholar]
- 15. Peterside IE, Selak AM, Simmons Ra. Imparied oxidative phsophorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol Endocrin Metab. 2002; 285: E1258–E1266 [DOI] [PubMed] [Google Scholar]
- 16. Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, et al. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150(7):3021–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12(1):2–13 [DOI] [PubMed] [Google Scholar]
- 18. Rueda Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res. 2011; 90: 285–294 [DOI] [PubMed] [Google Scholar]
- 19. Rueda-Clausen CF, Dolinsky VW, Morton JS, Proctor SD, Dyck JRB, Davidge St. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 2011; 60: 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56(2):130–140 [DOI] [PubMed] [Google Scholar]
- 21. Onay Besikci A. Regulation of cardiac energy metabolism in newborn. Mol Cell Biochem. 2006;287(1-2):1–11 [DOI] [PubMed] [Google Scholar]
- 22. Zungu M, Young ME, Stanley WC, Essop MF. Chronic treatment with the peroxisome proliferator-activated receptor α agonist Wy-14,643 attentuates myocardial respiratory capacity and contractile function. Mol Cell Biochem. 2009;330(1-2):55–62 [DOI] [PubMed] [Google Scholar]
- 23. Diaz Moreno I, Garcia Heredia JM, Diaz Quintana A, De la Rosa MA. Cytochrome c signalosome in mitochondria. Eur Biophys J. 2011;40(12):1301–1315 [DOI] [PubMed] [Google Scholar]
- 24. Nouette Gaulain K, Malgat K, Rocher C, et al. Time course of differential mitochondrial energy metabolism adaptation to chronic hypoxia in right and left ventricles. Cardiovasc Res. 2005;66(1):132–140 [DOI] [PubMed] [Google Scholar]
- 25. Petrosillo G, Ruggiero FM, DiVenosa N, Paradies G. Decreased complex III activity in mitochdonria isolated from rat heart subjected to ischemia and reperfusion: P role of reactive oxygen species and cardiolipin. FASEB J. 2003;17(6):714–716 [DOI] [PubMed] [Google Scholar]
- 26. Nouette Gaulain K, Biais M, Savineau JP, et al. Chronic hypoxia-induced alterations in mitochondrial energy metabolism are not reversible in rat heart ventricles. Can J Physiol Pharmacol. 2011;89(1):58–66 [DOI] [PubMed] [Google Scholar]
- 27. Al Hasan YM, Evans LC, Pinkas GA, Dabkowski ER, Stanley WC, Thompson LP. Chronic hypoxia impairs Cytochrome Oxidase activity via oxidative stress in selected fetal guinea pig organs. Repro Sci. 2013;20(3):299–307. doi: 10.1177/1933719112453509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson WT, Anderson CM. Cardiac cytochrome c oxidase activity and contents of subunits 1 and 4 are altered in offspring by low prenatal copper intake by rat dams. J Nutr. 2008;138(7):1269–1273 [DOI] [PubMed] [Google Scholar]
- 29. Von Bergen NH, Koppenhafer SL, Spitz DR, Volk KA, Patel SS, Roghair RD. Fetal programming alters reactive oxygen species production in sheep cardiac mitochondria. Clin Sci. 2009;116(8):659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10(5):265–274 [DOI] [PubMed] [Google Scholar]
- 31. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116(3):615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gostimskaya I, Galkin A. Preparation of highly coupled rat heart mitochondria. J Vis Exp. 2010;23(43). doi:10.3791/2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 34. Dong Y, Thompson LP. Differential expression of eNOS in coronary and cardiac tissue of hypoxic fetal guinea pig hearts. J Soc Gynecol Invest. 2006;13(7):483–490 [DOI] [PubMed] [Google Scholar]
- 35. Johnson DM, Geys R, Lissens J, Guns PJ. Drug-induced effects on cardiovascular function pentobarbital anesthetized guinea-pigs: invasive LVP measurements versus the QA interval. J Pharm Toxic Meth. 2012;66(2):152–159 [DOI] [PubMed] [Google Scholar]
- 36. Marks L, Borland S, Phip K, et al. The role of the anaesthetised guinea –pig in the preclinical cardiac safety evaluation of drug candidate compounds. Toxic App Pharmacol. 2012;263(2):171–183 [DOI] [PubMed] [Google Scholar]
- 37. Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med. 2012;53(6):1252–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18(4), 357–368 [DOI] [PubMed] [Google Scholar]
- 39. Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci. 2008;1147:321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fontanesi F, Soto IC, Horn D, Barrientos A. Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am J Physiol Cell Physiol. 2006;291(6):C1129–C1147 [DOI] [PubMed] [Google Scholar]
- 41. Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97(4):673–83 [DOI] [PubMed] [Google Scholar]
- 42. Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beauvoit B, Rigoulet M. Regulation of cytochrome c oxidase by adenylic nucleotides. Is oxidative phosphorylation feedback regulated by its end-products? IUBMB Life. 2001;52(3-5):143–152 [DOI] [PubMed] [Google Scholar]
- 44. Napiwotzki J, Kadenbach B. Extramitochondrial ATP/aDP-ratios regulate cytochrome c oxidase activity via binding to the cytosolic domain of subunit IV. Biol Chem. 1998:379(3):335–339 [DOI] [PubMed] [Google Scholar]
- 45. Yang WL, Iacono L, Tang WM, Chin KV. Novel function of the regulatory subunit of protein kinase A: regulation of cytochrome c oxidase activity and cytochrome c release. Biochem. 1998:37(40):14175–14180 [DOI] [PubMed] [Google Scholar]
- 46. Huigsloot M, Nijtmans LG, Szklarczyk R, et al. A mutation in C2orf64 causes impaired cytochrome c oxidase assembly and mitochondrial cardiomyopathy. Am J Human Gen. 2011;88(4):488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29(4):339–345 [DOI] [PubMed] [Google Scholar]
- 48. Virbasius JV, Scarpulla RC. Transcriptional activation through ETS domain binding sites in the cytochrome c oxidase subunit IV gene. Mol Cell Biol. 1991;11(11):5631–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carter RS, Bhat NK, Basu A, Avadhani NG. The basal promoter elements of murine cytochrome c oxidase subunit IV gene consist of tandemly duplicated ets motifs that bind to GABP-related transcription factors. J Biol Chem. 1992;267(32):23418–23426 [PubMed] [Google Scholar]
- 50. Virbasius JV, Virbasius CA, Scarpulla Rc. Identify of GABP with NRF-2, a multisubunit activator of cytochrome oxidase expression, reveals a cellular role for an ETS domain activator of viral promoters. Genes Dev. 1993:7(3):380–392 [DOI] [PubMed] [Google Scholar]
- 51. Sucharov C, Basu A, Carter RS, Avadhani NG. A novel transcriptional initiator activity of the GABP factor binding ets sequence repeat from the murine cytochrome c oxidase Vb gene. Gene Expr. 1995;5(2):93–111 [PMC free article] [PubMed] [Google Scholar]
- 52. Ongwijitwat S, Wong-Riley MT. Is nuclear respiratory factor 2 a master transriptional coordinator for all ten nuclear –endoced cytochrome c oxidase subunits in neurons? Gene. 2005;360(1):65–77 [DOI] [PubMed] [Google Scholar]
- 53. Andersson U, Scarpulla RC. PGC-1 related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription I mammalian cells. Mol Cell Biol. 2001;21(11):3738–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1 M and TFB2 M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25(4):1354–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levett DZ, Radford EJ, Menassa DA, et al. Acclimatization of skeletal muscle mitochondria to high-altitude hypoxia during an ascent of Everest. The FASEB J. 2012;26(4):1431–1441 [DOI] [PubMed] [Google Scholar]
- 56. Zhao J, Li L, Pei Z, et al. Peroxisome proliferator activated receptor (PPAR)-γ co-activator 1-α and hypoxia induced factor-1α mediate neuro- and vascular protection by hypoxic preconditioning in vitro. Brain Res. 2012;1447:1–8 [DOI] [PubMed] [Google Scholar]
- 57. Shoag J, Arany Z. Regulation of hypoxia-inducible genes by PGC-1 alpha. Arterioscler Thromb Vasc Biol. 2010;30(4):662–666 [DOI] [PubMed] [Google Scholar]
- 58. Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17(6):292–301 [DOI] [PubMed] [Google Scholar]
- 59. Glass CK. Going nuclear in metabolic and cardiovascular disease. J Clin Invest. 2006;116 (3):556–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124 [DOI] [PubMed] [Google Scholar]
- 61. Lehman JJ, Boudina S, Banke NH, et al. The transcriptional coactivator PGC-1α is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol. 2008;295(1):H185–H196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–1070 [DOI] [PubMed] [Google Scholar]
- 63. Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKC∊ gene repression in rat hearts. Circ Res. 2010;107 (3):365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266(1):6–11 [DOI] [PubMed] [Google Scholar]
- 65. Nanduri J, Makarenko V, Reddy VD, et al. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci U S A. 2012;109(7):2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol. 2013;591(pt 9):2245–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sanches Roman I, Gomez A, Gomez J, et al. Forty percent methionine restriction lowers DNA methylation, complex I ROS generation, and oxidative damage to mtDNA and mitochondrial proteins in rat heart. J Bioenerg Biomem. 2011;43(6):699–708 [DOI] [PubMed] [Google Scholar]
- 68. Ronn T, Poulsen P, Hansson O, et al. Age influences DNA methylation and gene expression of COX7A1 in human skeletal muscle. Diabetologia. 2008;51(7):1159–1168 [DOI] [PubMed] [Google Scholar]
- 69. Parihar A, Vaccaro P, Ghafourifar P. Nitric oxide irreversibly inhibits cytochrome oxidase at low oxygen concentrations: evidence for inverse oxygen concentration–dependent peroxynitrite formation. Life. 2008;60(1):64–67 [DOI] [PubMed] [Google Scholar]
- 70. Taylor CT, Moncada S. Nitric oxide, cytochrome c oxidase, and the cellular response to hypoxia. Arterioscler Thromb Vasc Biol. 2010;30(4):643–647 [DOI] [PubMed] [Google Scholar]
- 71. Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite. J Biol Chem. 2003;278(39):37223–37230 [DOI] [PubMed] [Google Scholar]
- 72. Chen J, Petersen DR, Schenker S, Henderson GI. Formation of malondialdehyde adducts in livers of rats exposed to ethanol: role in ethanol-mediated inhibition of cytochrome c oxidase. Alcoholism Clin Exp Res. 2000;24(4):544–552 [PubMed] [Google Scholar]
- 73. Zhao S, Xu W, Jiang W, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5958):1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bourque Sl, Gragasin FS, Quon AL, Mansour Y, Morton JS, Davidge ST. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension. 2013;62(4):753–758 [DOI] [PubMed] [Google Scholar]
- 75. Kane AD, Herrera EA, Camm EJ, Giussani DA. Vitamin C prevents intrauterine programming of in vivo cardiovascular dysfunction in the rat. Circ J. 2013;77(10):2604–2611 [DOI] [PubMed] [Google Scholar]
- 76. Rueda Clausen CF, Morton JS, Dolinsky VW, Dyck JR, Davidge ST. Synergistic effects of prenatal hypoxia and postnatal high-fat diet in the development of cardiovascular pathology in young rats. Am J Physiol Regul Integr Comp Physiol. 2012;303(4):R418–R426 [DOI] [PubMed] [Google Scholar]
- 77. Sack MN, Disch DL, Rockman HA, Kell DP. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proc Natl Acad Sci U S A. 1997;94(12):6438–6443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kell DP. Deactivation of peroxisome proliferator-activated receptor-α during cardiac hypertrophic growth. J Clin Invest. 2000;105(12):1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]