Abstract
The aim of this study was to evaluate the effectiveness of improving angiogenesis at graft sites on the survival of follicles in transplanted ovarian tissue. Matrigel containing 5 × 105 of cord blood-derived endothelial progenitor cells (EPCs) or 200 ng of mouse vascular endothelial growth factor (VEGF) was injected subcutaneously into BALB/c-Nu mice. After 1 week, vitrified/warmed ovaries from female B6D2F1 mice were subcutaneously transplanted into the injection sites. After 1, 2, and 4 weeks posttransplantation, the ovaries were recovered and subjected to histological analysis. Oocytes were collected from the transplanted ovaries, and their fertilization, embryonic development, and delivery were also observed. Vitrified/warmed ovaries transplanted into EPC- or VEGF-treated sites developed more blood vessels and showed better follicle survival than those transplanted into sham-injected sites. Normal embryonic development and consequent live births were obtained using oocytes recovered from cryopreserved/transplanted ovaries. Treatment with EPCs or VEGF could prevent the ischemic damage during the early revascularization stage of ovarian transplantation.
Keywords: ovary cryopreservation, heterotopic transplantation, endothelial progenitor cells, blood vessel formation, follicle survival
Introduction
Earlier cancer detection and improvements in chemoradiation therapy have substantially improved the life expectancies of young female patients with cancer. As a result, the focus of cancer therapy has moved from survival to quality of life, particularly in the context of preserving fertility. The fertility of male patients may be effectively and noninvasively retained through the cryopreservation of semen. However, there are currently no routine methods to preserve female fertility in the face of treatment for cancer or severe autoimmune conditions such as rheumatoid arthritis. The current clinical and experimental strategies to preserve fertility in women include cryopreservation of embryos, cryopreservation of oocytes for future in vitro fertilization (IVF), and cryopreservation of ovarian tissue for future transplantation or in vitro growth and in vitro maturation (IVM).
The follicular structure seems to be fairly resilient to cryo-induced damage as indicated by recent advances in whole-ovary cryopreservation and transplantation.1 Moreover, cryopreservation and transplantation of the ovarian cortex has been shown to restore ovarian function, with more than 20 live human births to date.2–12 Although these are promising results, some investigators have reported very low oocyte recovery rates from aspirated follicles, a high incidence of empty follicles, and a limited graft life span in women with transplanted frozen–thawed ovarian tissue. In fact, it seems that only primordial or small preantral follicles survive cryo-induced damage.13,14 It was recently suggested that low follicle survival is not a direct consequence of cryo-induced damage but instead is due to insufficient postgraft vascularization of antral and large preantral follicles. Consistent with this hypothesis, oocytes recovered from antral follicles of cryopreserved murine ovaries were shown to survive the cryopreservation protocol and sustain full developmental competence after IVM and IVF.15 If we can prevent ischemic injury after thawing, transplantation of frozen–thawed ovarian tissue constitutes a feasible strategy to protect patients with cancer from fertility loss in cases where embryo or oocyte cryopreservation is not applicable.
Angiogenesis is an essential process in follicular development and lutenization.16,17 The initiation and maintenance of follicular growth appears to depend on the establishment of an extensive microvascular network that may support the preferential delivery of gonadotropins. Among the many soluble and matrix-derived angiogenic growth factors and regulators involved in the formation of these microvascular networks, vascular endothelial growth factor (VEGF) plays a crucial role by stimulating vessel hyperpermeability, endothelial cell proliferation, and the migration and survival of vascular endothelial cells. Vascular endothelial growth factor is expressed and produced by thecal and/or granulosa cells in the ovary.18,19 Experiments in which angiogenic inhibitors or soluble VEGF receptor 1 inhibitors were locally administered into the preovulatory follicles of primates during the spontaneous menstrual cycle revealed that VEGF antagonists impaired ovulation and attenuated subsequent luteal function.20 Numerous studies have demonstrated that exogenous VEGF expression (at the gene or protein levels) can modulate the development of follicles and vascular networks in the mammalian ovary.21 Although immunohistochemical studies have generally confirmed that follicular VEGF expression increases as follicles mature, several reports have indicated that VEGF is also expressed in preantral follicular compartments.18,22 Furthermore, the VEGF-encoding gene is critically upregulated during the development of primordial follicles in the mouse.23 Administration of VEGF directly to the ovary was shown to dose and time dependently increase the number of preantral follicles. Also, delivery of VEGF on the fibrin-encapsulated follicle promotes twice as many surviving primordial follicles and an increased number of blood vessels than that of the nontreated.24In addition, estrogen was found to be upregulated in both VEGF expression and early follicle growth in the rodent ovary.25 Endothelial progenitor cells (EPCs), which also promote angiogenesis, play a major role in the revascularization of injured tissue and the process of tissue repair.26 Although EPCs may mainly contribute to postnatal angio- and vasculogenesis, they may also be incorporated into sites of active angiogenesis, where they can augment collateral vessel growth to ischemic tissues.27,28 However, it was not well studied whether the promoted angiogensis by introduction of EPCs may have an effect on ovarian graft survival or not.
Accordingly, we hypothesized that VEGF and EPCs could prevent the ischemic damage of follicles during the early revascularization stage of ovarian transplantation. Here, we improved angiogenesis at graft sites by preadministration of human EPCs or mouse VEGF and tested the survival of follicles from transferred mouse ovarian tissues that had undergone vitrification/warming.
Materials and Methods
Animals
Six-week-old female B6D2F1 mice (Samtako Co, Ltd., Seoul, Korea) were used as donors for the frozen ovaries. Six-week-old female BALB/c-Nu mice (Samtako Co, Ltd) were used as ovary recipients. All mice were maintained in a temperature- and humidity-controlled room under a 12-hour light–dark cycle at 22°C ± 2°C. All studies were approved by the Institutional Animal Care and Use Committee at the CHA University, Seoul, South Korea.
Vitrification of Ovarian Tissue
The base medium used for the vitrification and warming was Leibovitz L-15 medium (Gibco-BRL, Franklin, New Jersey) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Hyclone Laboratories, Logan, Utah). Six-week-old B6D2F1 mice were killed by cervical dislocation and their whole ovaries were aseptically removed and kept at room temperature (RT) in 35 × 10 mm Petri dishes containing 1 mL of base medium until use. Each whole ovary was preequilibrated with 10% ethylene glycol (EG; Sigma, St Louis, Missouri) and 10% dimethyl sulfoxide (DMSO; Sigma) for 5 minutes, followed by equilibration with 20% EG, 20% DMSO, and 0.5 mol/L sucrose (Sigma) for 5 to 10 minutes. All processes were performed at RT. Equilibrated ovaries were then mounted on a grid (Gilder, Westchester, Pennsylvania) and plunged into slush nitrogen (SN2) and stored for 14 days until use.29 After storage, the vitrified ovaries were sequentially placed in prewarmed L-15 medium (37°C) containing 0.5, 0.25, 0.125, and 0 mol/L sucrose (5 minutes each) for warming.
Injection of VEGF and Human EPCs into Nude Mice
Aliquots of Matrigel matrix (354230; BD Bioscience, Bedford, Massachusetts) containing mouse VEGF (R&D Systems, Minneapolis, Minnesota) were prepared on ice. The derivation and culture of human cord blood–derived endothelial precursor cells (hEPCs) were performed as described previously.30,31 The hEPCs were isolated using Ficoll reagent (GE Healthcare) from a fresh cord blood sample donated from a healthy volunteer at the CHA Gangnam Medical Center (Seoul, Korea) and cultured on a fibronectin-coated culture dish in EGM-2/MV medium (Lonza, Basel, Switzerland). Briefly, human EPCs were plated on collagen-coated culture dishes and maintained in EBM-2 medium (CC-3156, Lonza, Basel, Switzerland) containing EBM-2 MV SigleQuots (CC-4147, Lonza). After 4 days of culture, nonadherent cells were removed by washing with phosphate-buffered saline (PBS), fresh medium was applied, and the cells were cultured through day 7. Prior to use, the cells were washed twice with PBS and resuspended in EBM-2 medium.
Matrigel (0.5 mL) containing mouse VEGF (100 ng/mL) or human EPCs (5 × 105 cells) was injected into the ventral sides of 6-week-old female BALB/c-Nu mice and allowed to gel for about 10 seconds before removal of the needle. As a control, matrigel alone was injected into the other ventral side (sham-injected site). Seven days later, we observed blood vessel formation on the ventral sides of treated mice.
Transplantation of Ovary onto the Injection Site
Matrigel-injected mice were anesthetized with 10 mg/kg xylazine (Rompun; Bayer, Leverkusen, Germany) and 80 to 100 mg/kg ketamine hydrochloride (Ketamidor 10%; Richter Pharma AG, Wels, Austria). After warming, ovaries were dissected into 2 pieces. One half of the ovary was placed on the injected site (with large number of formed blood vessels) and the other was on the sham-injected site (with small number of blood vessels) randomly.
Histological and Immunohistochemical Analysis of Transferred Ovarian Tissue
The quality of transplanted ovarian tissues was primarily assessed by histological evaluation. After 1, 2, and 4 weeks of ovarian transplantation, tissues (including ovarian fragments, n ≥ 8 ovaries in each group) were recovered and fixed in buffered 10% formaldehyde for 2 to 3 days, then embedded in paraffin wax for serial sectioning (section thickness, 5 μm). Samples were stained with hematoxylin and eosin (H&E) and evaluated for follicular density, general tissue and oocyte morphology, and blood vessel density. Follicles and blood vessels were counted in every 4 sections of whole samples.
For immunohistochemistry for blood vessels, the ovaries were stained overnight at 4°C with primary antibodies PECAM (CD31, 1:100; Millipore, Billerica, Massachusetts), followed by 1 hour incubation at RT with 555-labeled goat antimouse secondary antibodies (A21424; Invitrogen, Carlsbad, CA) diluted to 1:200 with Dulbecco PBS. Sample was counterstained with 1 µg/mL 6-diamidino 2-phenyindiol (DAPI; Sigma Aldrich, St Louis, Missouri) diluted to 1:1000 with DPBS for 15 minutes at RT. Sample image was captured with a Carl Zeiss LSM 510 META conforcal laser-scanning microscope (Carl Zeiss, Jena, Germany). The image of the stained cells was reconstructed using LSM 510 META software.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labelling Assay for the Assessment of Apoptosis
The ovarian sections were stained using a Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labelling (TUNEL) kit. Fixed tissue sections were deparaffinized and incubated in 0.5% Triton X-100 for 1 hour at RT. The presence of apoptotic cells was assessed using an In Situ Cell Death Detection kit (Roche Diagnostics, Roche, Mannheim, Germany), as described by the manufacturer. The slides were washed, transferred to a solution containing 10 µg/mL Hoechst 33342 (Sigma), and incubated for 30 minutes at RT in the dark. After 3 more washes, the slides were mounted and the numbers of apoptotic nuclei and total nuclei were determined using an epifluorescence microscope (Nikon Corp, Tokyo, Japan).
In Vitro Maturation , Intracytoplasmic Sperm Injection, IVF, and In Vitro Culture
Two weeks after transplantation, follicle growth in the transplanted mouse ovarian tissues was hyperstimulated by injection of 5 IU pregnant mare serum gonadotropin (Sigma) followed by 5 IU human chorionic gonadotropin (hCG, Sigma). Cumulus–oocyte complexes (COCs) were retrieved from ovaries at 9 to 10 hours post-hCG treatment and placed in Quinn advantaged medium with HEPES (Quinn’s-HEPES; Sage, In Vitro Fertilization, Trumbull, Connecticut) containing 10% substitute protein serum (Sage). For IVF or intracytoplasmic sperm injection (ICSI), the cumulus-enclosed oocytes were matured in vitro in 50 µL TCM-199 containing 10% fetal bovine serum, 0.0075 IU/mL follicle-stimulating hormone (FSH; Gonal-F; Serono, Modugno Bari, Italy), 0.5 IU/mL hCG (Ovidrel, Serono), and 1 µg/mL E2 (Sigma) in a 5% CO2 atmosphere for 7 to 9 hours. In our study, for the purpose of obtaining a better oocyte quality, we used E2 and FSH that related to maturation of oocyte cytoplasm for in vitro maturation process as in Randall et al.32 For IVF, capacitated spermatozoa (1-2 × 106/mL) were incubated with cumulus-enclosed oocytes in Quinn Advantage Fertilization medium (Sage) for 6 hours, and the cumulus cells were removed by repeated pipetting through a glass pipette. The ICSI was performed using a micromanipulator (Narishige, Tokyo, Japan) mounted on an inverted microscope (Nikon Corp) with a Piezo microinjection system (Prime Tech Ltd, Ibaraki-ken, Japan). Sperm heads were separated from tails by applying a Piezo pulse to the neck region in 6% polyvinylpyrrolidone (PVP; Medicult, Jyllinge, Denmark), and heads were injected into the oocytes. The fertilized oocytes were cultured in KSOM (KSOM, Millipore) media at 37°C in 5% CO2 for 5 days (up to the blastocyst stage).
Embryo Transfer
Pseudopregnant ICR mice (Samtako Co) were mated with vasectomized ICR males and used as recipients. Morula and blastocysts derived from IVF or ICSI were transferred into the upper parts of the left uterus horns of pseudopregnant mice at day 3.5 and normal delivery of offspring was assessed.
Statistical Analysis
Data were analyzed for statistical significance using 1-way analysis of variance (Duncan test). P values <.05 were considered statistically significant.
Results
Blood Vessel Formation at Human EPC and Mouse VEGF-Injection Sites
Microscopic examination showed that the injection of Matrigel containing human EPCs or mouse VEGF into the ventral side of BALB/c-Nu mice accelerated the formation of cords, tubules, and blood-filled channels containing red blood cells (Supplementary Figure 1).
Blood Vessel Formation in Ovaries Transplanted into Human EPC- or Mouse VEGF-Injected Sites
Ovarian grafts (n ≥ 8 ovaries in each group) were surgically collected at 1, 2, and 4 weeks posttransplantation, and the numbers of blood vessels were compared between fresh ovaries and those transplanted into sham-injected, EPC-injected, or VEGF-injected sites (Figure 1A-C). Compared to fresh ovaries, the vitrified/warmed ovaries transplanted into sham-injected sites showed significantly fewer blood vessels at 1, 2, and 4 weeks posttransplantation. However, in the ovaries transplanted into human EPC- or VEGF-injected sites, the number of blood vessels did not significantly differ from that in the fresh sample (Figure 1D and E). Thus, the vitrified/warmed ovaries transplanted into human EPC- or VEGF-injected sites grew significantly more blood vessels than those transplanted into sham-injected sites at 1, 2, and 4 weeks posttransplantation.
Figure 1.
Blood vessel formation in ovaries transferred to sham-, human EPC-, or mouse VEGF-injected sites. A-F and a-f, H&E staining (A-C and a-c) and immunohistochemical staining (D-F and d-f) of blood vessels (black and white arrows) in ovaries transplanted into sham-injected (A and D, a and d), human EPC-injected (B and E, b and e), and mouse VEGF-injected (C and F, c and f) sites. D-F and d-f, Red (PECAM, Blood vessels) and Blue (DAPI, cells) G, Mean number of blood vessels in vitrified/warmed ovaries following heterotopic transplantation into sham-injected or human EPC-injected sites. H, Mean number of blood vessels in vitrified/warmed ovaries following heterotopic transplantation into sham-injected or VEGF-injected sites. Scale bar, 50 µm. Different superscripts indicate statistically significant results (a vs b: P < .05). EPC indicates endothelial progenitor cell; VEGF, vascular endothelial growth factor; DAPI, diamidino 2-phenyindiol; H&E, hematoxylin and eosin.
Follicle Numbers in Ovaries Transferred into Human EPC- or Mouse VEGF-Injected Sites
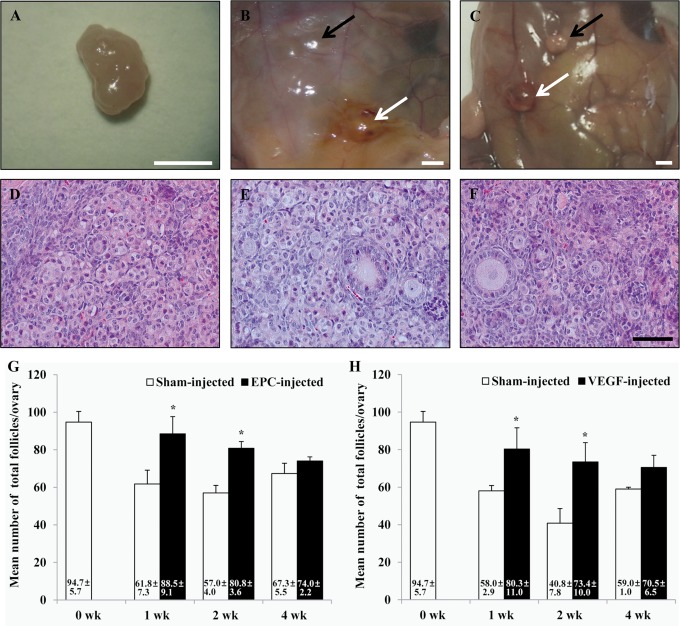
Figure 2 summarizes the number of total follicles in each group. All grafts (n ≥ 8 ovaries in each group) were recovered from all the groups, and the follicles in each graft were counted. Prior to transplantation, the vitrified/warmed ovaries grafted contained 94.7 ± 5.7 follicles. After transplantation into sham-injected sites, the follicle numbers were significantly decreased after 1, 2, and 4 weeks (Figure 2G and H). Vitrified/warmed ovaries transplanted into human EPC-injected sites had significantly more total surviving follicles than those in sham-injected sites at 1 week (88.5 ± 9.1 vs 61.8 ± 7.3; P < .05) and 2 weeks (80.8 ± 3.6 vs 57.0 ± 4.0; P < .05) posttransplantation but not at 4 weeks (74.0 ± 2.2 vs 67.3 ± 5.5; P > .05) after transplantation. Similarly, ovaries transplanted into VEGF-injected sites had significantly more total surviving follicles than those in sham-injected sites at 1 week (80.3 ± 11.0 vs 58.0 ± 2.9; P < .05) and 2 weeks (73.4 ± 10.0 vs 40.8 ± 7.8, P < .05) posttransplantation but not at 4 weeks (70.5 ± 6.5 vs 59.0 ± 1.0; P > .05) after transplantation.
Figure 2.
Morphology of ovarian tissue after cryopreservation and transplantation. A, Nontransferred (fresh) ovary. B, Vitrified/warmed ovaries transplanted into sham-injected (black arrow) and human EPC-injected (white arrow) sites. C, Vitrified/warmed ovaries transplanted into sham-injected (black arrow) and human VEGF-injected (white arrow) sites. D-F, H&E staining of ovaries at 1 week posttransplantation, showing representative images of follicles from nontransferred ovaries (D), ovaries transplanted into human EPC-injected sites (E), and ovaries transplanted into VEGF-injected sites (F). G, Mean number of total follicles in vitrified/warmed ovaries after heterotopic transplantation into sham-injected or human EPC-injected sites. H, Mean number of total follicles in vitrified/warmed ovaries after heterotopic transplantation into sham-injected or VEGF-injected sites. The white scale bar represents 2 mm and the black scale bar represents 50 µm. *P < .05. EPC indicates endothelial progenitor cell; VEGF, vascular endothelial growth factor; DAPI, diamidino 2-phenyindiol; H&E, hematoxylin and eosin.
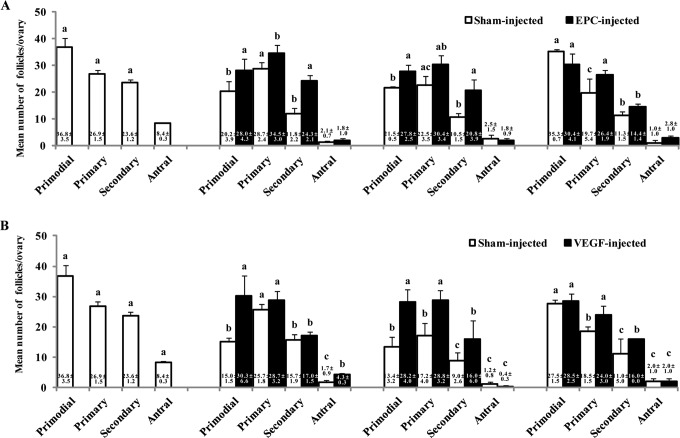
Next, we examined the survival of specific stages of follicles in vitrified/warmed ovaries according to the blood supply at the transplantation site (Figure 3). The number of primordial follicles was significantly higher in vitrified/warmed ovaries transplanted into human EPC-injected sites than those in sham-injected sites at 1 and 2 weeks posttransplantation. The number of primary follicles was significantly higher in ovaries transplanted into human EPC-injected sites compared to those in sham-injected sites at 1 and 4 weeks posttransplantation. The number of secondary follicles was higher in ovaries transplanted into human EPC-injected sites than those in sham-injected sites at 1 and 2 weeks posttransplantation. There was no significant difference in the numbers of antral follicle in ovaries transplanted to EPC-injected sites and sham-injected sites (Figure 3A).
Figure 3.
Mean number of follicles at each stage in vitrified/warmed ovaries following heterotopic transplantation into sham-injected or human EPC-injected sites (A), heterotopic transplantation into sham-injected or VEGF-injected sites (B), or no transplantation. Different superscripts indicate statistically significant results (a vs b, b vs c, a vs c: P < 0.05). EPC indicates endothelial progenitor cell; VEGF, vascular endothelial growth factor.
In vitrified/warmed ovaries transplanted into VEFG-injected sites, the numbers of primordial follicles were significantly higher than those in sham-injected sites at 1 and 2 weeks posttransplantation. The numbers of primary and secondary follicles were higher in ovaries transplanted into VEGF-injected sites than sham-injected sites at 2 and 4 weeks posttransplantation. Finally, the numbers of antral follicles in the VEGF-injected group were higher than those in the sham-injected controls at 1 week posttransplantation (Figure 3B).
Apoptosis in the Grafted Ovary
The apoptosis of follicles in vitrified/warmed ovaries was analyzed by TUNEL assay (Supplementary Figure 2). At 1, 2, and 4 weeks after transplantation, fewer than 4% of the total follicles in the grafted ovaries were apoptotic. There was no difference in the number of apoptotic follicles among the sham-, human EPC-, and mouse VEGF-injected groups.
In Vitro Maturation, In Vitro Fertilization, and In Vitro Culture of Immature Oocytes Derived From Ovaries Grafted into Human EPCs-Injected Sites
As summarized in Table 1, the developmental potential of fresh ovary (control)-derived embryos following IVM, IVF, in vitro culture was compared with those derived from vitrified/warmed ovaries grafted into human EPC-injected sites. When we used conventional IVF for fertilization, embryos derived from ovaries grafted into human EPC-injected sites and control ovaries developed to the 2-cell and blastocyst stages at rates of 43.3% versus 47.1% and 30.1% versus 25.0%, respectively. When we used ICSI as a fertilization method, embryos derived from ovaries grafted into human EPC-injected sites and control ovaries developed to the 2-cell and blastocyst stages at rates of 43.7% versus 50.0% and 28.5% versus 33.3%, respectively (Table 1; P > .05 for all comparisons).
Table 1.
Embryonic Development of Immature Oocytes Obtained From Fresh or Vitrified Ovaries Grafted into Human EPC-Injected Sites After IVF or ICSI.
| Method of Fertilization (No. of Oocytes) | No. of 2-Cell Embryos (%) | No. of Blastocysts (%) | |
|---|---|---|---|
| Fresh ovaries | IVF (30) | 16 (47.1) | 4 (25.0) |
| ICSI (32) | 15 (50.0) | 5 (33.3) | |
| Vitrified/grafted ovaries | IVF (34) | 13 (43.3) | 4 (30.1) |
| ICSI (30) | 14 (43.7) | 4 (28.5) |
Abbreviations: EPC, endothelial progenitor cell; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization.
After fertilization, blastocysts derived from grafted ovaries were transferred to surrogate mothers. In the human EPC-injected group, 53 blastocyst embryos were transferred to 4 recipients; one recipient carried to term and delivered 8 healthy pups (Supplementary Figure 3).
Discussions
Here, we provide evidence for the influence of human EPC- or mouse VEGF-induced blood vessel formation at the graft site on the folliculogenesis of transplanted vitrified/warmed mouse ovaries. Improved angiogenesis at the graft site facilitated the formation of blood vessels in the transferred ovaries, increasing the survival of ovarian follicles. This suggests that the regulation of angiogenesis could be an efficient fertility-preserving strategy for achieving embryonic development and live births after ovarian cryopreservation and transplantation.
The main limitation of ovarian tissue cryopreservation is the potential for ischemic damage to the thawed/grafted tissue; delayed reestablishment of vascular supply may cause significant follicular loss and thus reduce ovarian function.33 Although murine ovary revascularization occurs within 48 hours after transplantation,34 many follicles are lost during the initial ischemia.35 Furthermore, apoptosis of follicle cells can occur shortly after transplantation, decreasing the primordial content of the grafts.33 This, too, maybe due to ischemic–reperfusion injury during the generation of new blood vessels in the grafts.36 In our study, when ovarian tissues were transferred onto hEPC/VEGF-injected sites, the number of survived follicles at 1 and 2 weeks posttransplantation was much higher than those of sham-injected sites, but those numbers between 2 groups have become similar at 4 weeks (Figure 2). However, after 1 to 4 weeks of transplantation, we have observed a rare of apoptosis in either ovarian tissues that transferred into sham- or hEPC/VEGF-injected sites (Supplementary Figure 2). These data may support the results of previous studies that reestablishment of vascular supply has an important role in follicle survival after transplantation but suggested that our TUNEL assay result did not reflect actual apoptosis rate of ovarian follicle. However, the hEPC/VEGF-treated group showed a blood vessel formation after vitrification and warming. We assume that these new blood vessels help in the follicle growth by reducing damages of survived small follicles at the early stage of transplantation which prevented from atresia or arrest.
Extensive follicular damage can occur when the graft is detached from the bloodstream; therefore, rapid neovascularization and reconstruction of vascular support are main goals in transplantation.33 Graft size is also critical as bigger, thicker tissues take longer to be invaded by blood vessels, potentially resulting in follicular loss due to ischemic reperfusion injury.37 Therefore, the transplantation site and its ability to provide sufficient blood flow can critically impact the survival of primordial follicles and their development to the antral stage.38,39 Several clinical reports have examined surgical approaches for the autotransplantation of fresh or frozen–thawed human ovarian tissue to different heterotopic body locations (eg, the pelvic sidewall and anterior abdominal wall) as well as orthotopic transplantation. However, researchers have not yet clearly established which site yields the best results for ovarian tissue transplantation. Soleimani et al reported that the back muscle is a promising site for ovarian allografts in mice,38 providing the first report of live offspring obtained after back muscle grafting using both IVF and ICSI. Here, we conducted a similar experiment and also obtained live offspring.
Within the ovary, angiogenesis is constantly required for the growth and development of follicles and corpus lutea, and the ovary has been shown to synthesize a number of potential angiogenic factors. Several mechanisms have been suggested to regulate the production of angiogenic factors in the grafted ovary. Numerous cytokines and growth factors, including VEGF, transforming growth factor β (TGF-β), stem cell factor (SCF), and growth differentiation factor 9 (GDF-9), have been implicated in oocyte maturation, follicular development, ovulation, and corpus luteum formation.40–42 Cytokines and growth factors, such as interleukin 6 and insulin-like growth factor 1, are known to induce VEGF mRNA expression and protein secretion.43 Indeed, VEGF is the most well-known angiogenic factor found in the ovary44; it has a direct mitogenic effect on endothelial cells but does not affect the growth of fibroblasts or epithelial cells.45,46 Vascular endothelial growth factor is also a potent promoter of vascular permeability, suggesting that it could play a crucial role in neovascularization. Studies have shown that VEGF is directly involved in the physiological regulation of ovarian angiogenesis in a gonadotropin-dependent manner.47 Its effect on the thecal vasculature of antral follicles was shown to appear on day 4 after VEGF gene injection when increased formation of thecal vasculature and decreased follicular atresia were observed.21 The transplantation-induced loss of primordial follicles was reportedly prevented by VEGF and granulocyte colony-stimulating factor (G-CSF) in mice,48 suggesting that these growth factors may play crucial roles in regulating germ cell survival in the ovary. Cotreatment with VEGF and G-CSF significantly improved the number of primordial follicles. Quintana et al further demonstrated that direct injection of VEGF into mouse ovaries enhanced the vascular network, promoted follicular development, and diminished apoptosis.49 However, different isoforms of VEGF appear to have different characteristics during angiogenesis,48,50 and there has been no long-term follow-up or functional assessment of VEGF-injected ovaries subjected to transplantation.
The therapeutic potential of EPCs has been reported in both animal models and humans with ischemic diseases, including myocardial infarction, stroke, and peripheral arterial diseases. Transplantation of EPC could also benefit the treatment of wounds that resist healing, as this is often associated with decreased peripheral blood flow.27,28 Cord blood contains more EPCs than peripheral blood; cord blood EPCs are functional, capable of being expanded in culture,30 and could be used to treat a wide range of diseases, including cardiovascular disease. Moreover, Yi et al showed that EPCs can enhance the survival and quality of transplanted fat tissues, probably through their ability to induce angiogenesis.51 Although several studies have documented the presence of injected EPCs at newly formed capillaries in injured tissues, the actual extent of vascular EPC incorporation varied considerably.
Here, we report for the first time that transplantation of vitrified/warmed ovaries into human EPC- or VEGF-injected sites increased the numbers of blood vessels and surviving follicles. There was no significant difference in follicular apoptosis among the human EPC-treated, VEGF-treated, and nontreated groups. In addition, to analyze the function of vitrified/warmed ovaries transferred into hEPC/VEGF-injected sites, immature oocytes were recovered from those at 2 weeks after transplantation and their developmental potential was compared with those of fresh ovary. The oocytes were well matured and fertilized after ICSI, and normal embryonic development and subsequent live births were obtained using immature oocytes derived from vitrified/warmed ovaries transplanted into human EPC-injected sites. Embryonic development between those groups was not different (Table 1). These results can provide that ovarian tissue grafting could be a useful tool for the production of female gametes needed for the future assisted reproductive technology procedures and fertility preservation. In conclusion, injection of human EPC or mouse VEGF into graft sites prior to transplantation can increase angiogenesis and may prevent or minimize ischemia-induced follicle loss. Therefore, this human EPC- or VEGF-facilitated technique may improve the transplantation efficiency of cryopreserved ovaries. However, further studies on an orthotopic transplantation model will be necessary to further assess the potential use of this technique in assisted human reproduction.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A120080).
Supplemental Material: The online [appendices/data supplements/etc] are available at http://rs.sagepub.com/supplemental.
References
- 1. Wang X, Chen H, Yin H, Kim SS, Lin Tan S, Gosden RG. Fertility after intact ovary transplantation. Nature. 2002;415(6870):385. [DOI] [PubMed] [Google Scholar]
- 2. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410 [DOI] [PubMed] [Google Scholar]
- 3. Meirow D, Levron J, Eldar Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–321 [DOI] [PubMed] [Google Scholar]
- 4. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist. 2007;12(12):1437–1442 [DOI] [PubMed] [Google Scholar]
- 5. Andersen CY, Rosendahl M, Byskov AG, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266–2272 [DOI] [PubMed] [Google Scholar]
- 6. Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94(6):2191–2196 [DOI] [PubMed] [Google Scholar]
- 7. Sanchez-Serrano M, Crespo J, Mirabet V, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93(1):268 e211–263 [DOI] [PubMed] [Google Scholar]
- 8. Donnez J, Squifflet J, Jadoul P, et al. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril. 2011;95(5):1787. e1781–1784 [DOI] [PubMed] [Google Scholar]
- 9. Demeestere I, Simon P, Moffa F, Delbaere A, Englert Y. Birth of a second healthy girl more than 3 years after cryopreserved ovarian graft. Hum Reprod. 2010;25(6):1590–1591 [DOI] [PubMed] [Google Scholar]
- 10. Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25(5):1280–1281 [DOI] [PubMed] [Google Scholar]
- 11. Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–450 [DOI] [PubMed] [Google Scholar]
- 12. Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod. 2011;26(5):1097–1103 [DOI] [PubMed] [Google Scholar]
- 13. Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1, 2-propanediol. Hum Reprod. 1999;14(8):2061–2068 [DOI] [PubMed] [Google Scholar]
- 14. Gook DA, McCully BA, Edgar DH, McBain JC. Development of antral follicles in human cryopreserved ovarian tissue following xenografting. Hum Reprod. 2001;16(3):417–422 [DOI] [PubMed] [Google Scholar]
- 15. Sztein JM, O'Brien MJ, Farley JS, Mobraaten LE, Eppig JJ. Rescue of oocytes from antral follicles of cryopreserved mouse ovaries: competence to undergo maturation, embryogenesis, and development to term. Hum Reprod. 2000;15(3):567–571 [DOI] [PubMed] [Google Scholar]
- 16. Anasti JN, Kalantaridou SN, Kimzey LM, George M, Nelson LM. Human follicle fluid vascular endothelial growth factor concentrations are correlated with luteinizaton in spontanuously developing follicles. Hum Reprod. 1998;13(5):1144–1147 [DOI] [PubMed] [Google Scholar]
- 17. Fraser HM, Lunn SF. Angiogenesis and its control in the female reproductive system. Br Med Bull. 2000;56(3):787–797 [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto S, Konishi I, Tsuruta Y, et al. Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol Endocrinol. 1997;11(6):371–381 [DOI] [PubMed] [Google Scholar]
- 19. Mattioli M, Barboni B, Turriani M, et al. Follicle activation involves vascular endothelial growth factor production and increased blood vessel extension. Biol Reprod. 2001;65(4):1014–1019 [DOI] [PubMed] [Google Scholar]
- 20. Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod. 2002;67(4):1305–1312 [DOI] [PubMed] [Google Scholar]
- 21. Shimizu T, Iijima K, Ogawa Y, Miyazaki H, Sasada H, Sato E. Gene injections of vascular endothelial growth factor and growth differentiation factor-9 stimulate ovarian follicular development in immature female rats. Fertil Steril. 2008;89(5 suppl):1563–1570 [DOI] [PubMed] [Google Scholar]
- 22. Greenaway J, Connor K, Pedersen HG, Coomber BL, LaMarre J, Petrik J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology. 2004;145(6):2896–2905 [DOI] [PubMed] [Google Scholar]
- 23. Kezele PR, Ague JM, Nilsson E, Skinner MK. Alterations in the ovarian transcriptome during primordial follicle assembly and development. Biol Reprod. 2005;72(1):241–255 [DOI] [PubMed] [Google Scholar]
- 24. Shikanov A, Zhang Z, Xu M, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17(23-24):3095–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod. 2003;68(5):1736–1741 [DOI] [PubMed] [Google Scholar]
- 26. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967 [DOI] [PubMed] [Google Scholar]
- 27. Botta R, Gao E, Stassi G, et al. Heart infarct in NOD-SCID mice: therapeutic vasculogenesis by transplantation of human CD34+ cells and low dose CD34+KDR+ cells. FASEB J. 2004;18(12):1392–1394 [DOI] [PubMed] [Google Scholar]
- 28. Madeddu P, Emanueli C, Pelosi E, et al. Transplantation of low dose CD34+KDR+ cells promotes vascular and muscular regeneration in ischemic limbs. FASEB J. 2004;18(14):1737–1739 [DOI] [PubMed] [Google Scholar]
- 29. Lee DR, Yang YH, Eum JH, et al. Effect of using slush nitrogen (SN2) on development of microsurgically manipulated vitrified/warmed mouse embryos. Hum Reprod. 2007;22(9):2509–2514 [DOI] [PubMed] [Google Scholar]
- 30. Lee MJ, Kim J, Lee KI, Shin JM, Chae JI, Chung HM. Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy. 2011;13(2):165–178 [DOI] [PubMed] [Google Scholar]
- 31. Park SJ, Moon SH, Lee HJ, et al. A comparison of human cord blood- and embryonic stem cell-derived endothelial progenitor cells in the treatment of chronic wounds. Biomaterials. 2013;34(4):995–1003 [DOI] [PubMed] [Google Scholar]
- 32. Randall GW, Awadalla SG, Shivers CA. Isolation, in vitro maturation, and fertilization of germinal vesicle oocytes obtained from the intact murine ovary. J In Vitro Fert Embryo Transf. 1990;7(6):314–320 [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Van der Elst J, Van den Broecke R, Dhont M. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod. 2002;17(3):605–611 [DOI] [PubMed] [Google Scholar]
- 34. Israely T, Dafni H, Granot D, Nevo N, Tsafriri A, Neeman M. Vascular remodeling and angiogenesis in ectopic ovarian transplants: a crucial role of pericytes and vascular smooth muscle cells in maintenance of ovarian grafts. Biol Reprod. 2003;68(6):2055–2064 [DOI] [PubMed] [Google Scholar]
- 35. Jeremias E, Bedaiwy MA, Gurunluoglu R, Biscotti CV, Siemionow M, Falcone T. Heterotopic autotransplantation of the ovary with microvascular anastomosis: a novel surgical technique. Fertil Steril. 2002;77(6):1278–1282 [DOI] [PubMed] [Google Scholar]
- 36. Israely T, Nevo N, Harmelin A, Neeman M, Tsafriri A. Reducing ischaemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod. 2006;21(6):1368–1379 [DOI] [PubMed] [Google Scholar]
- 37. Soleimani R, Heytens E, Van den Broecke R, et al. Xenotransplantation of cryopreserved human ovarian tissue into murine back muscle. Hum Reprod. 2010;25(6):1458–1470 [DOI] [PubMed] [Google Scholar]
- 38. Soleimani R, Van der Elst J, Heytens E, et al. Back muscle as a promising site for ovarian tissue transplantation, an animal model. Hum Reprod. 2008;23(3):619–626 [DOI] [PubMed] [Google Scholar]
- 39. Van Eyck AS, Bouzin C, Feron O, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93(5):1676–1685 [DOI] [PubMed] [Google Scholar]
- 40. Phillips HS, Hains J, Leung DW, Ferrara N. Vascular endothelial growth factor is expressed in rat corpus luteum. Endocrinology. 1990;127(2):965–967 [DOI] [PubMed] [Google Scholar]
- 41. Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P. The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol. 1993;157(1):100–109 [DOI] [PubMed] [Google Scholar]
- 42. Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140(9):4262–4271 [DOI] [PubMed] [Google Scholar]
- 43. Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271(2):736–741 [DOI] [PubMed] [Google Scholar]
- 44. Ravindranath N, Little Ihrig L, Phillips HS, Ferrara N, Zeleznik AJ. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology. 1992;131(1):254–260 [DOI] [PubMed] [Google Scholar]
- 45. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309 [DOI] [PubMed] [Google Scholar]
- 46. Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246(4935):1309–1312 [DOI] [PubMed] [Google Scholar]
- 47. Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13(1):18–32 [DOI] [PubMed] [Google Scholar]
- 48. Skaznik Wikiel ME, Sharma RK, Selesniemi K, Lee HJ, Tilly JL, Falcone T. Granulocyte colony-stimulating factor in conjunction with vascular endothelial growth factor maintains primordial follicle numbers in transplanted mouse ovaries. Fertil Steril. 2011;95(4):1405–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quintana R, Kopcow L, Sueldo C, Marconi G, Rueda NG, Baranao RI. Direct injection of vascular endothelial growth factor into the ovary of mice promotes follicular development. Fertil Steril. 2004;82 suppl 3:1101–1105 [DOI] [PubMed] [Google Scholar]
- 50. Yang H, Lee HH, Lee HC, Ko DS, Kim SS. Assessment of vascular endothelial growth factor expression and apoptosis in the ovarian graft: can exogenous gonadotropin promote angiogenesis after ovarian transplantation? Fertil Steril. 2008;90(4 suppl):1550–1558 [DOI] [PubMed] [Google Scholar]
- 51. Yi C, Pan Y, Zhen Y, et al. Enhancement of viability of fat grafts in nude mice by endothelial progenitor cells. Dermatol Surg. 2006;32(12):1437–1443 [DOI] [PubMed] [Google Scholar]