Abstract
The study aimed to investigate the effect of imatinib coadministration on in vitro oocyte acquisition and subsequent embryo development in cyclophosphamide (Cp)-treated mice. Female BDF1 mice were injected with 5 IU equine chorionic gonadotropin (eCG) followed by 5 IU human chorionic gonadotropin 48 hours later and then oocytes were retrieved 14 hours later. Twenty-four hours prior to eCG administration, 25, 50, or 75 mg/kg Cp with or without 7.5 mg/kg imatinib was injected. In the 25 and 50 mg/kg Cp groups, imatinib coadministration significantly enhanced the percentage of mature oocytes (+16.4% and +10.4%) and significantly decreased the percentage of dead oocytes (−25.9% and −15.3%). Imatinib coadministration significantly enhanced the fertilization rate (FR) in the 50 mg/kg Cp group (+12.2%). Intraoocyte spindle integrity was significantly affected by Cp and was rescued by imatinib coadministration. Coadministration of imatinib prior to ovarian stimulation has the benefit of enhancing oocyte maturity and the in vitro FR in Cp-treated mice .
Keywords: cyclophosphamide, imatinib, in vitro fertilization, blastocyst
Alkylating chemotherapeutic agents, particularly cyclophosphamide (Cp), are harmful to gonadal function.1,2 Its administration causes ovarian follicle depletion and eventually ovarian failure.3–6
For the purpose of fertility preservation, in vitro embryo production via in vitro fertilization (IVF) is a well-established option.7 Alternatively, oocyte or ovarian tissue could be preserved although the latter approach requires further research. Chemotherapy must be postponed until mature oocytes are obtained in patients with cancer who wish to cryopreserve their oocytes or embryos for future fertility. Cryopreservation of ovarian tissue can usually be performed without delaying chemotherapy as it does not require ovarian stimulation but patients should undergo surgery to excise her ovary.
Usually, we do not perform ovarian stimulation promptly after chemotherapy, but patients with chemotherapy in their medical history always have concern about the effects of these kinds of treatments on their future reproductive health.
It has been reported that administering chemotherapeutic agents before ovarian stimulation can affect the integrity of the resultant oocytes or embryos. An earlier report in a murine model indicated that Cp, if administered in vivo 1 day before ovarian stimulation, reduces the oocyte number and impairs fertilization and subsequent embryo development.8 A recent report suggested that the oocyte number can be reduced by a single injection of Cp, but embryo development after IVF is dependent on the treatment dose.9
After a single injection of Cp, even oocytes retrieved 6 weeks later showed significantly reduced fertilization and embryo formation rates.10 In vitro Cp treatment also inhibited dissolution of the cumulus and reduced fertilization and early cleavage rates in a dose-dependent manner.11 Indeed, significantly decreased oocyte yield (6.5 vs 14.1) and number of embryos available for cryopreservation (3.9 vs 6.8) were observed in 23 female patients with breast cancer who underwent ovarian stimulation immediately after chemotherapy compared to 144 patients who underwent ovarian stimulation before chemotherapy.12
Although the incidence of aneuploid oocytes was similar between Cp-treated mice and untreated controls,13 a recent report indicated that the number of aneuploid embryos was significantly increased in the Cp treatment group.10 In the offspring of mice treated with Cp 1 to 4 weeks before mating, the malformation rate was increased by at least 10-fold.14
Imatinib is a selective tyrosine kinase inhibitor that has been successfully used to treat chronic myeloid leukemia and gastrointestinal stromal tumors.15 This agent was recently reported to counteract cisplatin-induced oocyte damage in cultured mouse ovaries.16 Here, we investigated the effect of coadministration of imatinib on the quantity and quality of in vitro-produced oocytes and subsequent embryo developmental outcomes in Cp-treated mice. Intra-oocyte spindle integrity was also examined.
Methods
Animals
Female BDF1 mice of 4 to 5 weeks old (Orient Co, Seoul, Korea) were maintained under a 12-hour light–12-hour dark cycle at 23°C and fed ad libitum. Animal care and use were in accordance with the institutional guidelines established by the Animal Care and Use Committee of Seoul National University Bundang Hospital.
Collection of Mature Oocytes and IVF
After 1 week of adaptation, mice were treated with intraperitoneal (ip) injection of 5 IU equine chorionic gonadotropin (eCG; Sigma-Aldrich, St Louis, Missouri) followed by ip injection of 5 IU human chorionic gonadotropin (Sigma-Aldrich) 48 hours later. Mice were sacrificed by cervical dislocation 13 to 14 hours later, and the oviducts were collected. The oviducts were dissected and placed in a Petri dish containing modified mouse tubal fluid (mMTF) medium supplemented with 0.4% (w/v) bovine serum albumin (BSA; Sigma-Aldrich). Cumulus-oocyte complexes were released by tearing the ampulla of the oviducts. The cumulus cells were removed enzymatically using 85 IU/mL hyaluronidase (Sigma-Aldrich) and by mechanical dissociation using a glass pipette. Oocytes were classified as mature, immature, dead, or fragmented and their numbers were recorded. Only morphologically normal mature oocytes, as judged by the presence of a first polar body, were used for IVF.
Epididymal spermatozoa were retrieved from the cauda epididymis of 8- to 10-week-old BDF1 mice, and the sperm suspensions were preincubated for 1.5 hours in capacitation medium (mMTF supplemented with 0.8% BSA). The oocytes were then inseminated at a final dilution of 2 million/mL and incubated at 37°C in humidified air with 5% CO2 in air. Inseminated oocytes were washed by pipetting 6 hours later and then placed in embryo maintenance medium (Global medium supplemented with 10% human serum albumin; Life Global, Guilford, Connecticut). Fertilization was assessed by the formation of 2 cells on day 1 after insemination. The cleaved embryos were transferred to new embryo maintenance medium, and blastocyst development was recorded on day 5 after insemination.
Blastocyst Cell Counts and Terminal Deoxynucleotidyl Transferase-Mediated 2′-Deoxyuridine, 5′-Triphosphate Nick End-Labeling Staining
DNA integrity was measured using the terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine, 5′-triphosphate nick end-labeling method. The blastocysts were fixed with 4% paraformaldehyde for 1 hour at room temperature (RT). After washing with phosphate-buffered saline (PBS), the blastocysts were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate (Sigma-Aldrich) for 10 minutes at RT. A commercial apoptosis detection kit was used (In Situ Cell Death Detection Kit; Roche Diagnostics GmbH, Mannheim, Germany). The remaining procedures were performed as directed by the kit instructions. The blastocysts were air dried on a silane-coated slide (DAKO, Glostrup, Denmark). Counterstaining was performed using a mounting medium with 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, California). The blastomeres with fragmented DNA had green-stained nuclei, whereas the nuclei of the other blastomeres were stained blue and visualized with a fluorescence microscope (Carl Zeiss, Axio Imager A1, Göttingen, Germany) with a Hamamatsu digital camera imaging system (Figure 1). Blastomeres with >50% of the area stained green were considered positive. The total blastomere count and the percentage of positive blastomeres were determined in each blastocyst.
Figure 1.
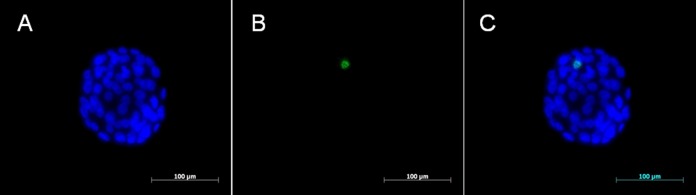
Microphotographs showing fluorescent TUNEL staining of blastocysts derived from mature mouse oocytes. Nuclei are stained blue (A: DAPI) and apoptotic blastomeres appear green (B: TUNEL). Merged capture, 400× (C). TUNEL indicates terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine, 5′-triphosphate nick end labeling; DAPI, 4,6-diamidino-2-phenylindole.
Meiotic Spindle Integrity
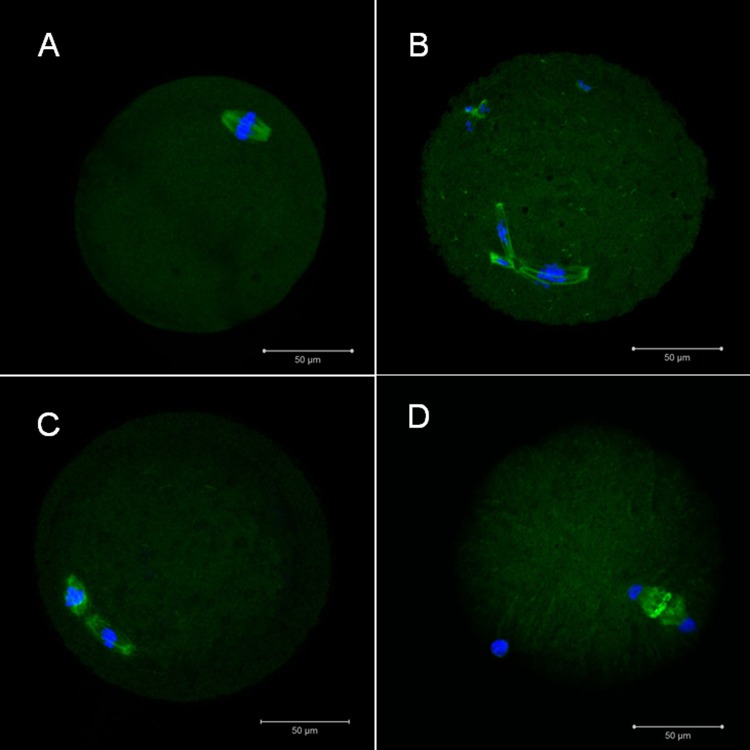
Spindle integrity was assessed using previously described methods.17,18 The mature oocytes were washed 3 times with 1% BSA in PBS for 5 minutes and then fixed with 4% paraformaldehyde for 1 hour at RT. After washing twice with 1% BSA in PBS, permeabilization was performed with 0.25% Triton X-100 in PBS for 10 minutes at RT. After washing twice with 1% BSA in PBS, blocking was performed with 3% BSA in PBS for 1 hour at RT and then washed twice with 1% BSA in PBS. A primary antibody for α-tubulin (Cell Signaling, Danvers, Massachusetts) diluted in 1% BSA (1:100) was added and incubated overnight at 4°C. After washing 3 times with 1% BSA in PBS, a secondary antibody (Alexa fluor 488 goat antirabbit immunoglobulin G; Invitrogen, Carlsbad, California, diluted in 1% BSA [1:100]) was added for 1 hour at RT in the dark. After washing 3 times with 1% BSA in PBS, the oocytes were air dried on a silane-coated slide (DAKO). The slide was counterstained with DAPI and examined using a confocal microscope (Carl Zeiss, LSM 710; Figure 2). A typical barrel-shaped microtubule structure between both poles with centrally aligned chromosomes (metaphase II) was considered normal.
Figure 2.
Representative confocal microphotographs showing the meiotic spindle organization and chromosome alignment in mature mouse oocytes: (A) normal metaphase II, (B and C) abnormal metaphase II, and (D) normal anaphase II (400×).
Experimental Design
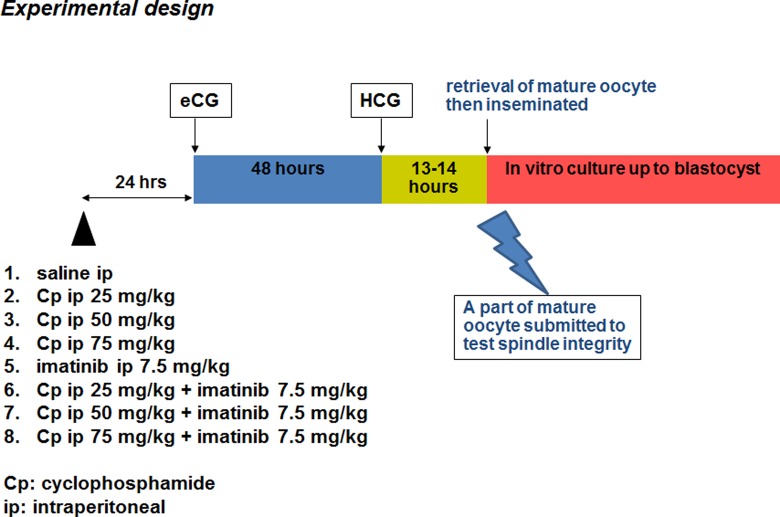
The experimental design is depicted in Figure 3. In the untreated control group, only 0.1 mL of normal saline was injected ip once. In groups 2, 3, and 4, Cp (Cp monohydrate; Sigma-Aldrich; Cat. #29875) 25, 50, or 75 mg/kg body weight was injected ip once into mice at 24 hours prior to eCG administration. In group 5, imatinib (Sigma-Aldrich) 7.5 mg/kg body weight was injected ip once to mice at 24 hours prior to eCG administration. An imatinib stock solution (5 mg/mL) was obtained by dissolving imatinib powder in PBS.19 In groups 6, 7, and 8, Cp (25, 50, or 75 mg/kg body weight) in combination with imatinib (7.5 mg/kg body weight) was injected ip once into mice at 24 hours prior to eCG administration. All final ip injection volumes were adjusted to 0.1 mL mixed with normal saline.
Figure 3.
Experimental design.
Statistical Analysis
Data were analyzed using the SPSS (v 17, SPSS Inc, Chicago, Illinois). From 12 to 15 replicates, each mean number of total, mature, and in vitro fertilized oocytes and the resultant blastocysts was counted in the 8 experimental groups and compared using the Wilcoxon rank sum test. Blastocyst cell numbers were compared using the Student t test. The proportions were compared using the Chi-square test. A P value of <.05 was considered significant.
Results
Oocyte Yield and Maturity and IVF Outcomes
The median numbers of total, mature, and fertilized oocytes and the resultant blastocysts are listed in Table 1. Overall, all treatment groups (except the imatinib-only group) showed decreased numbers of mature and fertilized oocytes or blastocysts relative to untreated controls. Moreover, coadministration of imatinib did not enhance the number of total, mature, and fertilized oocytes or the resultant blastocysts when compared with each corresponding Cp-only group. Insignificant benefits were observed in obtaining more mature oocytes (+1 and +2), fertilized oocytes (+2 and +1), or blastocysts (+2 and +1) after coadministration of imatinib in the 25 and 50 mg/kg Cp-treated groups. In the 75 mg/kg Cp-treated group, the median number of mature and fertilized oocytes or blastocysts was markedly decreased than untreated controls whether imatinib was coadministered or not.
Table 1.
Median Numbers of Total, Mature, and Fertilized Oocytes and Blastocysts in 8 Experimental Groups.a
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Cyclophosphamide | – | 25 | 50 | 75 | – | 25 | 50 | 75 |
| Imatinib | – | – | – | – | 7.5 | 7.5 | 7.5 | 7.5 |
| Mice | 12 | 15 | 15 | 15 | 12 | 14 | 14 | 15 |
| Replicates | 12 | 15 | 15 | 15 | 12 | 14 | 14 | 15 |
| Total oocyte | 23 | 23 | 13 | 12 | 26 | 21 | 19 | 14 |
| P Valueb | – | NS | <.05 | <.05 | NS | NS | NS | <.001 |
| ΔMedian | – | – | – | – | – | -2 | +6 | +2 |
| P Valuec | – | – | – | – | – | NS | NS | NS |
| Mature oocyte | 19 | 10 | 7 | 6 | 14 | 11 | 9 | 5 |
| P Valueb | – | <.001 | <.001 | <.001 | NS | <.05 | <.01 | <.001 |
| ΔMedian | – | – | – | – | – | +1 | +2 | -1 |
| P Valuec | – | – | – | – | – | NS | NS | NS |
| Fertilized oocyte | 17 | 6 | 6 | 5 | 11 | 8 | 7 | 2 |
| P Valueb | – | <.001 | <.001 | <.001 | NS | <.01 | <.01 | <.001 |
| ΔMedian | – | – | – | – | – | +2 | +1 | -3 |
| P Valuec | – | – | – | – | – | NS | NS | NS |
| Blastocyst | 13 | 3 | 4 | 2 | 10 | 5 | 5 | 1 |
| P Valueb | – | <.001 | <.001 | <.001 | NS | <.01 | <.001 | <.001 |
| ΔMedian | – | – | – | – | – | +2 | +1 | -1 |
| P Valuec | – | – | – | – | – | NS | NS | NS |
Abbreviation: NS, not significant.
a Doses indicate mg/kg body weight.
b When compared to untreated controls (group 1) by the Wilcoxon test.
c When compared to imatinib control (ie between groups 2 and 6, between groups 3 and 7, and between groups 4 and 8) by the Wilcoxon test.
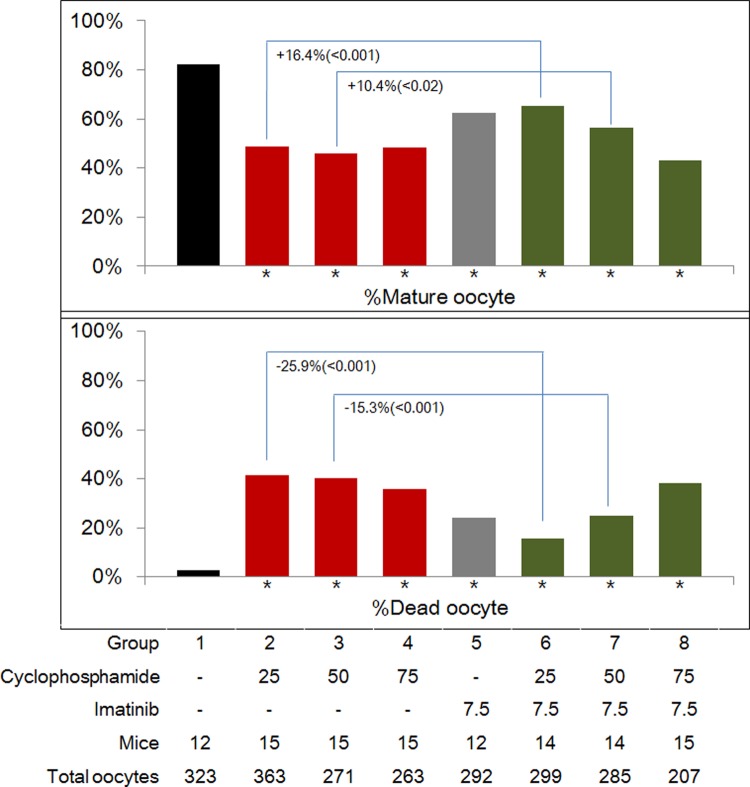
The data were pooled and the percentages of mature and dead oocytes are presented in Figure 4. All treatment groups showed a significantly lower percentage of mature oocytes and significantly higher percentage of dead oocytes compared with untreated controls. However, when compared with the corresponding Cp-only group, the percentage of mature oocytes was significantly enhanced (+16.4%, P < .001 and +10.4%, P < .02), and the percentage of dead oocytes was significantly decreased (−25.9%, P < .001 and −15.3%, P < .001) after coadministration of imatinib; this phenomenon was observed only in the 25 and 50 mg/kg Cp-treated groups.
Figure 4.
Percentages of mature (upper panel) and dead oocytes (lower panel) in the 8 experimental groups. Asterisks (*) indicate a significant difference when compared to untreated controls (group 1).
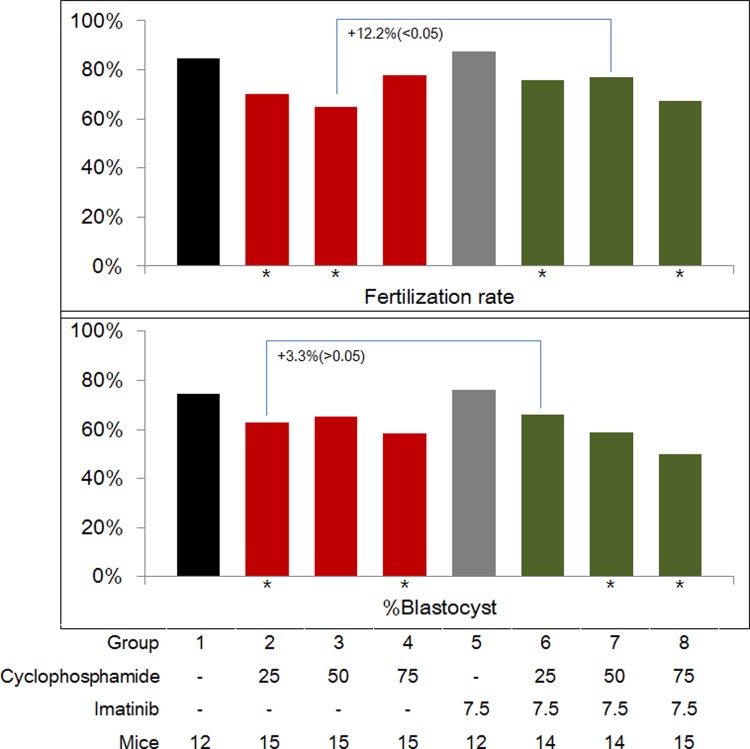
Fertilization rate (FR; per mature oocyte) and percentage of blastocysts (per fertilized oocyte) are depicted in Figure 5. In the imatinib-only group, the FR and the percentage of blastocysts were similar to those of untreated controls. After in vivo treatment of Cp only, the FR of the 25 and 50 mg/kg Cp-treated groups was significantly lower, and after coadministration of imatinib, the FR of the 25 and 75 mg/kg Cp-treated groups was significantly lower than that of the untreated controls. This led to a significantly enhanced FR in the 50 mg/kg Cp group: 64.8% without imatinib and 77% with imatinib (+12.2%, P < .05).
Figure 5.
Fertilization rate (upper panel) and blastocyst formation rate (lower panel) in the 8 experimental groups. Asterisks (*) indicate a significant difference when compared to untreated controls (group 1).
After in vivo treatment of Cp only, the percentage of blastocysts in the 25 and 75 mg/kg Cp-treated groups was significantly lower than that in the untreated controls, and after coadministration of imatinib, the percentage of blastocysts in the 50 and 75 mg/kg Cp-treated groups was significantly lower than that in the untreated controls. This resulted in an enhanced blastocyst percentage in the 25 mg/kg Cp-treated group: 62.9% without imatinib and 66.2% with imatinib, although this difference was not significant (+3.3%, P > .05).
As summarized earlier, coadministration of imatinib significantly enhanced the percentage of mature oocytes and significantly decreased the percentage of dead oocytes in the 25 and 50 mg/kg Cp-treated groups. Coadministration of imatinib significantly enhanced the FR only in the 50 mg/kg Cp-treated group but did not enhance the percentage of blastocysts in any of the 3 Cp-treated groups.
Cell Counts, Apoptosis, and Spindle Integrity
In the 25 and 50 mg/kg Cp-treated groups, the cell number and the percentage of apoptotic cells were similar irrespective of whether imatinib was coadministered (Table 2). In the 75 mg/kg Cp-treated group, the cell number was significantly lower (P < .05) and the percentage of apoptotic cells was significantly higher (P < .001) when imatinib was coadministered.
Table 2.
Cell Numbers and Percentages of Apoptotic Cells of Blastocysts in 8 Experimental Groups.a
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Cyclophosphamide | – | 25 | 50 | 75 | – | 25 | 50 | 75 |
| Imatinib | – | – | – | – | 7.5 | 7.5 | 7.5 | 7.5 |
| Blastocysts examined | 77 | 62 | 53 | 57 | 70 | 73 | 67 | 28 |
| Mean cell number (SD) | 57.8 (23.7) | 75.9 (31.8) | 60.5 (26.3) | 66.2 (24.5) | 73.4 (27.4) | 75.3 (25.9) | 60.2 (28.3) | 57.5 (20.9) |
| P Valueb | – | <.001 | NS | NS | <.01 | <.001 | NS | NS |
| P Valuec | – | – | – | – | – | NS | NS | <.05 |
| Total cell number | 4451 | 4704 | 3206 | 3773 | 5163 | 5695 | 4036 | 1611 |
| Cells with positive signal | 148 | 107 | 122 | 100 | 219 | 99 | 149 | 73 |
| Percent apoptotic cells, % | 3.33 | 2.27 | 3.81 | 2.65 | 4.24 | 1.74 | 3.69 | 4.53 |
| P Valued | – | <.01 | NS | NS | <.05 | <.001 | NS | <.05 |
| P Valuee | – | – | – | – | – | NS | NS | <.001 |
Abbreviations: NS, not significant; SD, standard deviation.
a Doses indicate mg/kg body weight.
b When compared to untreated controls (group 1) by the Student t test.
c When compared to imatinib control (ie, between groups 2 and 6, between groups 3 and 7, and between groups 4 and 8) by the Student t test.
d When compared to untreated controls (group 1) by the chi-square test.
e When compared to imatinib control (ie between groups 2 and 6, between groups 3 and 7, and between groups 4 and 8) by the chi-square test.
Intraoocyte spindle integrity was significantly affected by the Cp treatment in a dose-dependent manner (Table 3). However, this harmful effect disappeared when imatinib was coadministered. The percentage of oocytes with normal metaphase II spindles was higher after coadministration of imatinib in all 3 Cp-treated groups (79.4% vs 97.1% [P = .054], 78.8% vs 100% [P < .05], and 67.7% vs 93.8% [P < .05], respectively).
Table 3.
Percentage of Mature Oocytes With Normal Spindle Integrity in 8 Experimental Groups.a
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Cyclophosphamide | – | 25 | 50 | 75 | – | 25 | 50 | 75 |
| Imatinib | – | – | – | – | 7.5 | 7.5 | 7.5 | 7.5 |
| Oocytes examined | 32 | 34 | 33 | 31 | 32 | 34 | 30 | 32 |
| Normal metaphase II spindle | 32 | 27 | 26 | 21 | 32 | 33 | 30 | 30 |
| Abnormal metaphase II spindle | 0 | 2 | 5 | 8 | 0 | 0 | 0 | 2 |
| Normal anaphase II spindle | 0 | 5 | 2 | 2 | 0 | 1 | 0 | 0 |
| Percentage of oocytes with normal metaphase II spindle, % | 100 | 79.4 | 78.8 | 67.7 | 100 | 97.1 | 100 | 93.8 |
| P Valueb | – | <.01 | <.01 | <.005 | NS | NS | NS | NS |
| P Valuec | – | – | – | – | – | .054 | <.05 | <.05 |
Abbreviation: NS, not significant.
a Doses indicate mg/kg body weight.
b When compared to untreated controls (group 1) by the chi-square test.
c When compared to imatinib control (ie, between groups 2 and 6, between groups 3 and 7, and between groups 4 and 8) by the chi-square test.
Discussion
Our study demonstrated that coadministration of imatinib has a benefit of enhancing oocyte maturity and decreasing the percentage of dead oocytes in relatively low dose Cp-treated mice. Acquisition of mature oocytes is an important first step in fertility preservation. In this context, most clinicians attempt to obtain as many mature and healthy oocytes as possible. It is well known that the number of oocytes obtained in vitro is reduced if patients receive chemotherapy. This was demonstrated in several mouse studies8–10 and 1 human report.12 In the latter study, a significantly lower oocyte yield (6.5 vs 14.1) and fewer embryos available for cryopreservation (3.9 vs. 6.8) were observed in 23 female patients with breast cancer who underwent ovarian stimulation immediately after chemotherapy than in those who did not.12
We also observed a significantly lower mature oocyte yield (10 of 7 vs 19), 2-cell embryo yield (6 of 6 vs 17), and blastocyst yield (3 of 4 vs 13) in 25/50 mg/kg Cp-treated mice relative to untreated controls. After coadministration of imatinib, still fewer mature oocytes (11 of 9), 2-cell embryos (8 of 7), and blastocysts (5 of 5) than those of the untreated controls were obtained. However, under the tested Cp dosages, insignificant benefits were observed with the coadministration of imatinib, as +1/+2 more mature oocytes, +2/+1 more 2-cell embryos, and +2/+1 more blastocysts could be obtained, respectively.
Interestingly, after coadministration of imatinib, the percentage of mature oocytes was significantly increased (+16.4%/+10.4%), and the percentage of dead oocytes was significantly decreased (−25.9%/−15.3%) in 25 of 50 mg/kg Cp-treated mice. This finding indicates that oocyte maturity could be enhanced by the coadministration of imatinib although absolute number of total or mature oocytes was decreased. In addition, we observed that oocyte spindle integrity was rescued by imatinib coadministration.
Although a similar incidence of aneuploid oocytes after in vivo Cp treatment was reported previously,13 we observed that Cp treatment induced spindle abnormalities within oocytes more frequently. Alkylating chemotherapeutic agents work on nonproliferative cells by interfering with cellular DNA function.6 Cyclophosphamide also damages oocytes and surrounding granulosa cells in a dose-dependent manner.7 Our findings clearly indicate that Cp treatment could induce meiotic errors or parthenogenesis (evident by emerging anaphase) in oocytes, and coadministration of imatinib could conserve the spindle integrity and prevent parthenogenesis. This finding is important because in vitro-produced oocytes in the emergency IVF setting will undergo cryopreservation, which can further induce oocyte spindle damage.
A recent report indicates that coinjection of imatinib significantly attenuates the toxic effect of cisplatin on the ovarian follicular reserve and partially rescues female mice from cisplatin-induced infertility.16 The investigators explained that imatinib inhibits c-Abl-TAp63 pathway, which can induce oocyte death following chemotherapy. In a mouse model, the TA isoform of p63 is expressed in the oocytes of primordial follicles,20 and this has been reported to be essential in the process of p53-independent DNA damage-induced oocyte death.21
Gonfloni et al observed an accumulation of p63 and c-Abl protein levels following cisplatin exposure, which eventually leads to cell death of in vitro cultured ovaries.16 However, inhibition of c-Abl, by treatment with c-Abl kinase inhibitor imatinib, could prevent p63 accumulation, thus counteracting the depletion of ovarian follicles induced by cisplatin. This indicates that imatinib cotreatment can preserve oocytes from p63-dependent death.
Inspired by this observation, we explored whether imatinib coinjection has a protective effect against short-term Cp treatment on the quantity and quality of in vitro-produced oocytes, their fertilization potential, and subsequent embryo development up to the blastocyst stage. Although we did not observe any advantage regarding blastocyst formation, coadministration of imatinib has a benefit of enhancing oocyte maturity and decreasing the percentage of dead oocytes in Cp-treated mice. Our observations are in line with the findings of Gonfloni et al, in which oocyte deaths within cultured ovaries could be prevented by imatinib cotreatment.16
In the present study, no benefit of imatinib coadministration was observed in the high-dose Cp-treated group (ie 75 mg/kg). The oocyte yield, FR, the percentage, and cell number of blastocysts were markedly decreased, and blastocyst apoptosis was significantly increased. Nonetheless, spindle integrity was well conserved via coadministration of imatinib in this high Cp dose. This finding suggests that imatinib cotreatment can prevent spindle abnormalities in oocytes but has a negative effect on apoptosis of the resultant blastocyst in this high Cp dose. However, such a high dose of Cp is not currently used in patients with cancer. The usual dose of Cp for patients with breast cancer is 600 mg/m2. If a woman is 161.5 cm in height and 54.7 kg in weight, 942 mg of Cp is needed (body surface area, 1.57); this means a dose of 17.2 mg/kg would be used.
The currently available strategies for preserving female fertility are promising for women undergoing chemotherapy. However, such strategies do not totally guarantee female fertility preservation. Thus, alternative methods should be developed that offer more protection and preservation of ovarian follicles against chemotherapy. We herein suggest that imatinib could prevent chemotherapeutic agent-induced oocyte death and preserve oocyte spindle integrity.
Recently, a protective effect of amifostine against ovarian damage induced by chemotherapy has been reported.22 Amifostine is a prodrug that is dephosphorylated to a free thiol active metabolite in the tissues by alkaline phosphatase, which behaves as a potent scavenger of free radicals induced by chemotherapy protocols and ionizing radiation.23 Barekati et al reported the cytoprotective effects of amifostine (250 mg/kg) against the adverse effects of Cp (75 mg/kg) on preantral follicle survival (harvested 24 hours after treatment) of pubertal mice.22 After 3 weeks of treatment with amifostine and Cp, mice were superovulated and fertilized in vitro. The fertilization potency was significantly improved compared to mice treated with Cp only (88% vs 39%).
Although the offspring of women exposed to cancer treatment do not have a greater risk of chromosomal or congenital abnormalities,24,25 the safety of IVF in patients with cancer who have already undergone chemotherapy is questionable. Therefore, the genetic risk of embryos fertilized from oocytes recovered after Cp treatment should be evaluated. In a mouse model, it has been reported that aneuploidy of in vitro-produced embryos was significantly increased in the Cp treatment group (29.2% vs 8.7%).10 In regard to the adverse effects of Cp on chromosomal integrity, preimplantation genetic screening is needed before transfer of embryos produced from women treated with chemotherapeutic agents.
In the present study, we used a single chemotherapeutic agent (Cp) and a single dose of imatinib (7.5 mg/kg body weight, as in experiment performed by Gonfloni et al)16 with a fixed exposure time (24 hours). Currently, most patients receive a combination of chemotherapeutic agents including platinum-based chemotherapy; therefore, further research is needed to examine the possible protective effects of imatinib against various chemotherapeutic agents. In addition, possible protective effects of various doses of imatinib against a single chemotherapeutic agent should be evaluated. Finally, to confirm its long-term safety, production of normal pups should be assessed in this model.
Acknowledgments
B. C. J and S. Y. M contributed equally to this work as a corresponding author.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by grant no. A120043 from the Korea Health Care Technology R&D Project, Ministry of Health and Welfare, Korea.
References
- 1. Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110(10):2222–2229 [DOI] [PubMed] [Google Scholar]
- 2. Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–543 [DOI] [PubMed] [Google Scholar]
- 4. Gucer F, Balkanli-Kaplan P, Doganay L, et al. Effect of paclitaxel on primordial follicular reserve in mice. Fertil Steril. 2001;76(3):628–629 [DOI] [PubMed] [Google Scholar]
- 5. De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–921 [DOI] [PubMed] [Google Scholar]
- 6. Anchan RM, Ginsburg ES. Fertility concerns and preservation in younger women with breast cancer. Crit Rev Oncol Hematol. 2010;74(3):175–192 [DOI] [PubMed] [Google Scholar]
- 7. Donnez J, Dolmans MM. Preservation of fertility in females with haematological malignancy. Br J Haematol. 2011;154(2):175–184 [DOI] [PubMed] [Google Scholar]
- 8. Pydyn EF, Ataya KM. Effect of cyclophosphamide on mouse oocyte in vitro fertilization and cleavage: recovery. Reprod Toxicol. 1991;5(1):73–78 [DOI] [PubMed] [Google Scholar]
- 9. Koike M, Kumasako Y, Otsu E, Arake Y, Utsunomiya T. The influence of the anti-cancer drug cyclophosphamide on fertilization and embryo growth in a mouse medel. Fertil Steril. 2012;98(suppl 3):S117 [Google Scholar]
- 10. Barekati Z, Gourabi H, Valojerdi MR, Yazdi PE. Previous maternal chemotherapy by cyclophosphamide (Cp) causes numerical chromosome abnormalities in preimplantation mouse embryos. Reprod Toxicol. 2008;26(3-4):278–281 [DOI] [PubMed] [Google Scholar]
- 11. Ataya KM, Pydyn EF, Sacco AG. Effect of “activated” cyclophosphamide on mouse oocyte in vitro fertilization and cleavage. Reprod Toxicol. 1988;2(2):105–109 [DOI] [PubMed] [Google Scholar]
- 12. Jeong K, KArsy M, Oktay K. Impact of chemotherapy exposure on fertility preservation cycle outcomes. Fertil Steril. 2012;98(suppl 3):S95 [Google Scholar]
- 13. Yuan ZP, Mailhes JB. Aneuploidy determination in C-banded mouse metaphase II oocytes following cyclophosphamide treatment in vivo. Mutat Res. 1987;179(2):209–214 [DOI] [PubMed] [Google Scholar]
- 14. Meirow D, Epstein M, Lewis H, Nugent D, Gosden RG. Administration of cyclophosphamide at different stages of follicular maturation in mice: effects on reproductive performance and fetal malformations. Hum Reprod. 2001;16(4):632–637 [DOI] [PubMed] [Google Scholar]
- 15. Visani G, Piccaluga P, Malagola M, Isidori A. Efficacy of dasatinib in conjunction with alpha-interferon for the treatment of imatinib-resistant and dasatinib-resistant Ph+ acute lymphoblastic leukemia. Leukemia. 2009;23(9):1687–1688 [DOI] [PubMed] [Google Scholar]
- 16. Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15(10):1179–1185 [DOI] [PubMed] [Google Scholar]
- 17. Jo JW, Jee BC, Lee JR, Suh CS. Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertil Steril. 2011;96(5):1239–1245 [DOI] [PubMed] [Google Scholar]
- 18. Jo JW, Jee BC, Suh CS, Kim SH. The beneficial effects of antifreeze proteins in the vitrification of immature mouse oocytes. PLoS One. 2012;7(5):e37043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosten B, Abbara C, Cibert M, et al. Interleukin-2 treatment effect on imatinib pharmacokinetic, P-gp and BCRP expression in mice. Anticancer Drugs. 2010;21(2):193–201 [DOI] [PubMed] [Google Scholar]
- 20. Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mech Dev. 2005;122(9):1043–1055 [DOI] [PubMed] [Google Scholar]
- 21. Suh EK, Yang A, Kettenbach A, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444(7119):624–628 [DOI] [PubMed] [Google Scholar]
- 22. Barekati Z, Golkar-Narenji A, Totonchi M, Radpour R, Gourabi H. Effects of amifostine in combination with cyclophosphamide on female reproductive system. Reprod Sci. 2012;19(5):539–546 [DOI] [PubMed] [Google Scholar]
- 23. List AF, Heaton R, Glinsmann-Gibson B, Capizzi RL. Amifostine protects primitive hematopoietic progenitors against chemotherapy cytotoxicity. Semin Oncol. 1996;23(4 suppl 8):58–63 [PubMed] [Google Scholar]
- 24. Sanders JE, Hawley J, Levy W, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87(7):3045–3052 [PubMed] [Google Scholar]
- 25. Green DM, Fiorello A, Zevon MA, Hall B, Seigelstein N. Birth defects and childhood cancer in offspring of survivors of childhood cancer. Arch Pediatr Adolesc Med. 1997;151(4):379–383 [DOI] [PubMed] [Google Scholar]