Abstract
Introduction:
Women with former preeclampsia (exPE) develop chronic hypertension 4 times more often than healthy parous controls. Women, destined to develop remote chronic hypertension, had increased left ventricular mass index (LVMI) and diastolic blood pressure (BP) prior to the onset of hypertension as compared to those remaining normotensive. However, longitudinal data on the progress of this increased LVMI in women destined to develop hypertension are lacking.
Methods:
We included 20 women with exPE and 8 parous controls. At both 1- and 14-year postpartum (pp), we performed cardiac ultrasound and determined circulating levels of the metabolic syndrome variables. Of 14-year pp, 7 (35%) former patients had developed chronic hypertension. We compared these 7 former patients with both the 13 former patients who remained normotensive and the 8 parous controls using the Mann-Whitney U test and Kruskal-Wallis analysis.
Results:
Women with hypertensive exPE differed from their normotensive counterparts by a higher incidence of early-onset preeclampsia (PE) in their index pregnancy and a higher rate of recurrence in next pregnancies. At 1-year pp, they also had high/normal BP and higher fasting insulin levels. At 14 years pp, the relative left ventricular wall thickness was higher, and the E/A ratio was lower, in the hypertensive group relative to those remaining normotensive.
Conclusion:
Women with exPE are at increased risk of developing chronic hypertension, when (1) the PE in the index pregnancy had an early-onset and/or recurred in next pregnancies and (2) the 1-year pp. Blood pressure was high normal. We also noticed that at 14 years pp, the hypertensive group showed signs of concentric left ventricular remodeling along with a decreased E/A ratio.
Keywords: hypertension, preeclampsia, left ventricular mass, concentric hypertrophy
Introduction
Preeclampsia (PE), a hypertensive pregnancy disorder complicating 3% to 5% of all pregnancies in the Western world, is a major cause of perinatal morbidity and mortality.1 Although a link between PE and future chronic hypertension has already been suggested almost 50 years ago,2 it was only recently that PE has been generally accepted not only to predispose to chronic hypertension but also to premature cardiovascular disease (CVD).3 Preeclampsia and CVD share many risk conditions such as diabetes, obesity, and preexistent hypertension. Still, it is unclear whether PE itself, underlying risk conditions, or a combination contributes to future hypertension and CVD.
Compared to parous controls, women with former preeclampsia (exPE) have a 4-fold higher risk to develop chronic hypertension.4 From the available epidemiological data, it is not possible to determine whether this higher risk applies to all former patients or to a subgroup with specific underlying risk factors or latent disorders. This is relevant as these epidemiologic studies do not stratify for different pathogenetic pathways preceding the onset of PE.5 Previously, we found a larger left ventricular mass (LVM) index and a higher incidence of high-normal diastolic blood pressures (BPs) in women with exPE, normotensive at the 1-year postpartum (pp) screening, who eventually developed chronic hypertension.6 However, the design of that study was cross-sectional with follow-up information being obtained by biennial questionnaires. These do not provide information on cardiac remodeling after the onset of hypertension. The latter is important as only half of the patients with chronic hypertension develop left ventricular remodeling, which may be either concentric or eccentric depending on whether the hypertension is triggered by pressure or volume overload, respectively7,8 In addition, it is not known whether left ventricular remodeling develops similarly in women with exPE who are much younger than patients with hypertension in the general population. Moreover, in the previous study we did not include information on the metabolic syndrome (MetS) that is associated with both PE and chronic hypertension.9,10
Therefore, this explorative pilot study was designed to investigate whether seemingly healthy women with exPE destined to develop hypertension in a 14-year follow-up period since the PE pregnancy (1) shows accelerated cardiac remodeling in this time interval and (2) shows increased MetS risk factors, already present at 1-year pp. To this end, we performed cardiac ultrasound and measured circulating levels of the variables contributing to the MetS at 1-year and 14-year pp in 20 women with exPE and in 8 healthy parous controls. The observations at 14-year pp were used to subdivide the 20 former patients into 2 subgroups depending on whether chronic hypertension had developed (hypertensive exPE [HT-exPE]) or not (normotensive exPE [NT-exPE]).
Patients and Methods
Study Population
Before the start of the study, the hospital’s Medical Ethical Committee approved the study protocol (MEC 08-2-130). We defined PE according to the criteria of the International Society for the Study of Hypertension in Pregnancy.11 Preeclampsia was considered “early onset,” when the disorder was diagnosed before 34 weeks of pregnancy. We considered newborns to be small-for-gestational-age (SGA) if their birthweight was below the fifth centile according to the birthweight reference curves of the Perinatal Registry in the Netherlands.12 We sent an invitation to the women, who had participated in a previous study between 1996 and 1999,13 to take part in this follow-up study. The study population in that previous report consisted only of caucasian women, with 10 women being healthy normotensive controls and 39 being primiparous after a preeclamptic pregnancy. At that time, women with a history of PE were recruited at the outpatient clinic at pp follow-up. Most of them experienced a severe form of PE, either early-onset PE or PE complicated by fetal growth restriction or fetal demise. Parous controls were recruited by advertisement. We invited the 10 parous controls and 33 former patients for the 14-year pp follow-up measurement who had neither preexisting renal disease nor persistent pp hypertension. Of these former patients, 3 were lost to follow-up, 1 woman had died of pulmonary embolism, and 1 was undergoing chemotherapy. Furthermore, 8 former patients and 2 controls declined participation because of lack of time and/or emotional reasons. Eventually, 20 former patients and 8 parous controls agreed to participate in this study.
We divided the former patients into 2 subgroups based on whether or not they had developed chronic hypertension at the 14 years pp measurement session. One subgroup consisted of 13 former patients who were still normotensive at the 14-year pp measurement (NT-exPE), whereas the other subgroup consisted of 7 former patients who had developed chronic hypertension by then (HT-exPE), requiring antihypertensive medication. We diagnosed chronic hypertension on the basis of a BP ≥140/90 mm Hg measured by standard criteria (see subsequently). Prehypertension was defined by systolic and diastolic BPs ranging from 120 to 139, and/or from 80 to 89 mm Hg, respectively. Metabolic syndrome was diagnosed according to the National Cholesterol Education Program-Adult Treatment Panel III criteria (NCEP-ATP III).14
Measurements
The participants underwent the same set of measurements at 1- and 14-year pp. We performed both measurement sessions in the mid-follicular phase of the menstrual cycle (days 5 ± 2). Participants used a standard sodium diet (100 mmol sodium day−1) starting 1 week prior to measurement. Antihypertensive drugs were discontinued 2 weeks earlier. From 10 hours prior to measurements, participants refrained from smoking, eating, and drinking caffeine- or alcohol-containing beverages.
The measurement session started at 8:00 am in a temperature-controlled room (±24°C), with as little as possible external disturbances. Participants were lying on their back on a comfortable bed throughout the measurement session. We measured BP on 2 consecutive occasions using an oscillometric device ((Dinamap Vital Signs Monitor 1846; Critikon Company LLC, Tampa, Florida). First, we recorded BP for 30 minutes at 3-minute intervals after 30-minutes acclimatization in these standardized environmental conditions. In all women enrolled in the “hypertensive” subgroup, we confirmed the diagnosis. That is to say, they all had a median systolic and/or diastolic BP in excess of 140 and/or 90 mm Hg, respectively. After the BP recording, we sampled fasting blood for the later measurement of glucose (mmol L−1), insulin (mU·L−1), low-density lipoprotein (LDL, mmol·L−1), high-density lipoprotein (HDL, mmol·L−1), triglycerides (mmol·L−1), and total cholesterol (mmol·L−1) using standard laboratory techniques at the laboratory of the University Medical Center Maastricht. We estimated the degree of insulin resistance using the Homeostatic Model Assessment by the following formula: (glucose [mmolU·L−1] × insulin [mU·L−1])/22.5.15 Body mass index (BMI, kg·m−2) was calculated by dividing body weight in kg by squared length in meters. Overweight was defined as BMI between 25 and 30 kg·m−2. Body surface area (BSA) was calculated as follows: BSA (m2) = 0.007184·height (cm)0.725·weight (kg)0.425.16
We assessed cardiac function with the participant in dorsal recumbence using a phased-array echocardiographic Doppler system (Hewlett-Packard Sonos 2000 and 2500; Hewlett-Packard Company, Palo Alto, California) as detailed previously.13 All data were analyzed offline using specific software (Excelera, Philips, The Netherlands).
By M-mode in the parasternal long-axis view, we measured left ventricular end-diastolic diameter (LVEDD, mm), left ventricular end-systolic diameter (LVESD, mm), and the end-diastolic thickness of both the interventricular septum (IVST, mm) and the posterior wall (PWT, mm). We used the Devereux-formula17 to estimate LVM both as an absolute figure (g) and indexed for height in m2.7.18 We calculated relative wall thickness (RWT) as follows: RWT = [IVST + PWT]/LVEDD. The heart rate (HR, beats·min−1) was obtained by taking the reciprocal of the mean of 5 consecutive RR intervals on the electrocardiogram multiplied by 60. We estimated the mean aortic Velocity Time Integral (VTI) by averaging the outer edge tracings of 5 consecutive Continuous Wave Doppler registrations of the aortic flow at the level of the aortic valve. By taking the product of VTI and the cross-sectional area at the level of the aortic annulus in the parasternal long axis view, we obtained stroke volume (SV, mL). Finally, cardiac output (CO, L·min−1) was obtained by multiplying SV with HR. During cardiac ultrasound, we repeated the BP measurement (in triplicate) using the same semiautomatic oscillometric device as used before. The (median) values of this second set of measurements differed little and inconsistently from the ones obtained earlier but nevertheless were reported in the tables and used for statistical analysis. By measuring the transmitral flow pattern by pulsed-wave (PW) Doppler echocardiography in the apical 4-chamber view, we derived the early diastole (E)/atrial contraction (A) ratio, which provides a crude estimate for diastolic function and corresponds with the ratio of peak mitral flow velocity during early diastole and that during atrial contraction. Doppler-derived indices were averaged over 5 consecutive cardiac cycles. The PW Doppler sample volume (5 mm) was carefully positioned at the tip of the mitral valve leaflets. The sweep rate was set at 50 mm s−1.
Statistical Analysis
We compared the 3 groups by Kruskal-Wallis analysis, and the 2 former patients subgroups using the Mann-Whitney U test. Categorical data were analyzed by the Chi-square test if at least 5 cases were present in each of the 3 groups and by the Fisher exact test if one of the groups contained less than 5 cases. Of the glucose, insulin, and cholesterol data determined at 1-year pp, 4%, 30%, and 48%, respectively, were missing. At 14-year pp, none of the measurements was missing. We used regression to impute missing values as limiting the analysis to complete data sets would have led only to loss of precision or biased results.19 The imputation step and all analyses were performed using SPSS version 17.0. Data are presented as median with interquartile range unless stated otherwise. We considered a P value below .05 (after correction for multiple testing) to be statistically significant.
Results
Of the former patients, 7 (35%) had developed chronic hypertension at 14 years pp compared to only 1 (12.5%) in the control group. Table 1 lists the demography and pregnancy outcomes in our study population. Hypertensive exPE differed from normotensive exPE by having twice as often early-onset PE in their index pregnancy (100% vs 46%, P < .05), giving birth at an earlier gestational age to a child with a lower birthweight. Moreover, HT-exPE women had more often of recurrent hypertensive pregnancy disorders than NT-exPE women (86% vs 22%).
Table 1.
Demography and Obstetrical History of the 2 Subgroups of Former Patients and the Parous Controls.a
| Controls | NT-exPE | HT-exPE | |
|---|---|---|---|
| (n = 8) | (n = 13) | (n = 7) | |
| Age at 1-y pp, years | 33 (32; 34) | 31 (30; 33) | 30 (29; 32) |
| Age at the time of follow-up, years | 45 (44; 47) | 43 (42; 46) | 43 (41; 44) |
| Incidence overweight 1-y pp (n, %) | 2 (25) | 3 (23) | 3 (43) |
| Incidence overweight 14-y pp (n, %) | 2 (25) | 4 (31) | 5 (71) |
| Incidence prehypertension (n, %) | 2 (25) | 3 (23) | 4 (57) |
| Parity at 14-y pp (median, range) | 3 (2-4) | 2 (1-3) | 2 (2-4) |
| Smoking 1-y pp (n, %) | 1 (13) | 0 (0) | 2 (29) |
| Smoking 14-y pp (n, %) | 4 (50) | 0 | 0 |
| Index pregnancy | |||
| Gestational age at birth, wk | 39.6 (38.0; 41.7) | 34.9 (29.6; 37.0)b | 28.7 (27.0; 31.1) b,c |
| Birthweight, g | 3360 (2800; 3645) | 2220 (1007; 2796)b | 920 (670; 1630)b,c |
| Centiles (n, %) | |||
| p < 5 (SGA) | 0/8 | 2/13 (15) | 3/7 (43) |
| p = 5-90 | 8/8 (100) | 10/13 (77) | 4/7 (57) |
| p > 90 | 0/8 (0) | 1/13 (8) | 0/7 (0) |
| Early-onset preeclampsia (n, %) | – | 6 (46%) | 7 (100%)c |
| Subsequent pregnancy | |||
| Number of patients (n, %) | 7 (88) | 10 (77) | 7 (100) |
| Uneventful course/outcome (n, %) | 7 (100) | 8 (80) | 1 (14)c |
| Hypertensive complication (n, %) | – | 2 (20) | 6 (86)c |
Abbreviations: Overweight, body mass index > 25 kg·m2; PE, preeclampsia; NT, normotensive; HT, hypertensive; SGA, small for gestational age; IQR, interquartile range; y, year; wk, week; pp, postpartum; exPE, former preeclampsia.
a Data are Presented as Median With IQR, Unless Stated Otherwise.
b P < .05 compared with controls.
c P < .05 compared with NT-exPE.
Table 2 lists BPs and various metabolic variables in both subgroups of former patients and the controls at 1 and 14 years pp. Already at 1-year pp, HT-exPE differed from NT-exPE by a higher systolic, diastolic, and mean arterial BP, and from the control group by higher diastolic and mean arterial BP. We observed neither at 1-year nor at 14-year pp consistent differences in glucose and insulin between the NT-exPE group and the control group. However, insulin was significantly higher in the HT-exPE group than in the NT-exPE group at both 1 and 14 years pp. The concomitantly measured circulating levels of total- and LDL-cholesterol did not differ appreciably between the 2 former patient subgroups (Table 2). However, only in the HT-exPE subgroup, we noticed an increase in circulating LDL levels during the intermeasurement interval with a trend toward significance. In the HT-exPE subgroup, we also observed a trend to lower HDL-cholesterol at 14-year pp (P = .06) and significantly higher circulating levels of fasting triglycerides at the 14-year pp measurement. The change in most other variables between 1- and 14-year pp in the 3 subgroups did not differ appreciably. The rise in systolic BP in the intermeasurement interval was largest in the HT-exPE subgroup and was accompanied by an increase in pulse pressure.
Table 2.
Blood Pressure and Metabolic Variables in the 2 Subgroups of Former Patients and in the Parous Controls at 1-Year and 14-Year Postpartum With the Absolute Change Accumulated in the Intermeasurement Interval.a
| Controls | NT-exPE | HT-exPE | Overall P Value | P Value NT-exPE Vs HT-exPE | ||
|---|---|---|---|---|---|---|
| (n = 8) | (n = 13) | (n = 7) | ||||
| BMI, kg/m2 | 1-Year pp | 20.8 (19.4; 24.9) | 21.8 (20.4; 24.7) | 24.1 (18.9; 28.7) | .59 | .49 |
| 14-Year pp | 22.5 (20.3; 26.5) | 23.6 (22.5; 25.8) | 27.9 (23.0; 29.2) | .12 | .08 | |
| Diff | 1.4 (−0.1; 3.0) | 2.1 (0.8; 3.3) | 2.7 (1.5; 5.9) | .34 | .31 | |
| Weight, kg | 1-Year pp | 59 (54; 64) | 62 (59; 67) | 69 (55; 74) | .33 | .59 |
| 14-Year pp | 62 (57; 70) | 68 (61; 76) | 76 (69; 87) | .06 | .16 | |
| Diff | 3 (0; 6) | 5 (2; 8) | 7 (5; 13) | .22 | .21 | |
| Systolic BP, mm Hg | 1-Year pp | 117 (108; 126) | 115 (110; 121) | 128 (120; 140)b | <.05 | <.01 |
| 14-Year pp | 128 (110; 146) | 114 (112; 129) | 152 (138; 159)b | <.01 | <.01 | |
| Diff | 11 (−1; 30) | −2 (−8; 6) | 16 (−2; 29)b | .08 | <.01 | |
| Diastolic BP, mm Hg | 1-Year pp | 75 (69; 78) | 69 (64; 74) | 85 (75; 91)b,c | <.01 | <.01 |
| 14-Year pp | 78 (68; 81) | 72 (63; 79) | 92 (90; 99)b,c | <.01 | <.01 | |
| Diff | 3 (−3; 11) | −2 (−7; 6) | −1 (−3; 10) | .49 | .35 | |
| MAP, mm Hg | 1-Year pp | 91 (79; 95) | 84 (79; 89) | 98 (88; 112)b,c | <.05 | <.01 |
| 14-Year pp | 97 (85; 104) | 88 (80; 98) | 114 (107; 121)b,c | <.01 | <.01 | |
| Diff | 10 (3; 18) | 1 (−5; 8) | 5 (0; 20) | .31 | .24 | |
| Pulse pressure, mm Hg | 1-Year pp | 42 (40; 50) | 46 (44; 50) | 45 (39; 49) | .27 | .39 |
| 14-Year pp | 48 (40; 68) | 48 (43; 55) | 57 (54; 61)b,c | .09 | <.05 | |
| Diff | 8 (−2; 22) | 2 (−6; 6) | 8 (6; 21)b | <.05 | <.01 | |
| Glucose, mmol·L−1 | 1-Year pp | 4.8 (4.5; 5.4) | 4.8 (4.0; 5.7) | 4.9 (4.2; 5.8) | .94 | .70 |
| 14-Year pp | 5.2 (4.9; 5.5) | 5.1 (5.0; 5.4) | 5.3 (5.3; 5.7) | .27 | .11 | |
| Diff | 0.6 (0.0; 0.7) | 0.3 (0.0; 0.8) | 0.5 (0.2; 1.1) | .84 | .64 | |
| Insulin, mU·L−1 | 1-Year pp | 6.1 (3.8; 10.2) | 6.8 (4.2; 8.1) | 8.7 (7.9; 13.9)b | .10 | <.01 |
| 14-Year pp | 5.5 (2.0; 8.9) | 5.5 (3.0; 8.3) | 8.9 (6.2; 14.0) | .14 | <.05 | |
| Diff | −2.1 (−3.4; 0.3) | −0.6 (−3.1; 1.7) | 0.2 (−7.2; 6.1) | .67 | .76 | |
| Cholesterol, mmol·L−1 | 1-Year pp | 4.7 (4.5; 6.0) | 5.2 (4.6; 5.8) | 4.5 (4.1; 6.1) | .65 | .59 |
| 14-Year pp | 5.0 (4,2; 5.7) | 4.8 (4.3; 5.9) | 5.0 (4.6; 5.5) | .96 | .76 | |
| Diff | 0.3 (−0.7; 1.1) | −0.2 (−0.6; 0.7) | 0.7 (−0.7; 1.0) | .72 | .44 | |
| HDL-cholesterol, mmol·L−1 | 1-Year pp | 1.6 (1.2; 1.9) | 1.6 (1.2; 1.7) | 1.2 (0.8; 1.3) | .17 | .10 |
| 14-Year pp | 1.1 (1.0; 1.6) | 1.3 (1.2; 1.5) | 0.8 (0.7; 1.3) | .14 | .06 | |
| Diff | −0.3 (−0.7; 0.2) | −0.2 (−0.4; 0.1) | −0.1 (−0.5; 0.3) | .64 | .88 | |
| LDL-cholesterol, mmol·L−1 | 1-Year pp | 2.4 (2.3; 2.9) | 3.8 (2.8; 4.4)c | 2.8 (2.4; 3.0) | <.05 | .08 |
| 14-Year pp | 3.2 (2.4; 3.8) | 3.0 (2.7; 3.9) | 3.0 (3.0; 3.7) | .91 | 1.00 | |
| Diff | 0.8 (−0.1; 1.3) | 0.0 (−0.9; 0.2) | 0.4 (0.1; 0.7) | .06 | .06 | |
| Triglycerides, mmol·L−1 | 1-Year pp | 0.9 (0.6; 1.0) | 1.2 (0.8; 1.3) | 1.2 (0.8; 1.7) | .13 | .49 |
| 14-Year pp | 1.1 (0.8; 1.7) | 0.7 (0.5; 0.8)c | 1.6 (1.3; 2.1)b | <.01 | <.05 | |
| Diff | 0.1 (−0.1; 0.9) | −0.3 (−0.7; 0.1)c | 0.5 (0.0; 0.9) | <.05 | .08 | |
Abbreviations: BP, blood pressure; BMI, body mass index; PE, preeclampsia; NT, normotensive; HT, hypertensive; BMI, body mass index; HOMA, Homeostatic Model Assessment; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Diff, difference between 1-year and 14-year postpartum; MAP, mean arterial pressure; exPE, former preeclampsia; IQR, interquartile range; pp, postpartum; N, number.
a Data are presented as median with IQR or N with %.
b P < .05 compared with NT-exPE.
c P < .05 compared with controls.
Although the cardiac indices in the 3 groups did not differ at 1-year pp (Table 3), HT-exPE had deviated from NT-exPE by 14 years pp (Table 3) indicated by a larger IVST, PWT, RWT and a higher CO, and a trend toward a larger LVM relative to NT-exPE. The relative changes in these variables are shown in Figure 1. Also the E/A ratio had decreased in the HT-exPE group relative to NT-exPE, without concomitant change in LVEDD and LVESD, suggesting concentric remodeling accompanied by a decline in cardiac diastolic function.
Table 3.
Cardiac Indices in the 2 Subgroups of exPE Women and in the Control Group Observed at 1-Year and 14-Year Postpartum With the Absolute Change Accumulated in the Intermeasurement Interval.a
| Controls | NT-exPE | HT-exPE | P Value NT-exPE | |||
|---|---|---|---|---|---|---|
| (n = 8) | (n = 13) | (n = 7) | Overall P Value | Vs HT-exPE | ||
| Cardiac geometry | ||||||
| LAD, mm | 1-Year pp | 34 (32; 36) | 36 (32; 37) | 36 (34; 36) | .67 | .88 |
| 14-Year | 35 (31; 41) | 34 (33; 37) | 37 (34; 40) | .47 | .24 | |
| Diff | 1 (−3; 4) | 0 (−3; 2) | 0 (−2; 3) | .83 | .58 | |
| IVST, mm | 1-Year pp | 7.0 (7.0; 8.0) | 7.0 (7.0; 8.0) | 7.0 (7.0; 8.0) | .92 | .92 |
| 14-Year pp | 8.0 (7.6; 8.8) | 7.4 (7.1; 7.8)a | 8.3 (7.8; 9.0)b | <.01 | <.01 | |
| Diff | 1.0 (0.6; 1.2) | 0.1 (−0.3; 0.5)a | 1.0 (0.5; 1.3)b | <.01 | <.01 | |
| PWT, mm | 1-Year pp | 8.0 (7.0; 8.0) | 8.0 (7.0; 8.0) | 8.0 (7.0; 8.0) | .77 | .70 |
| 14-Year pp | 7.7 (7.0; 7.9) | 7.1 (6.8; 7.9) | 8.3 (8.0; 9.1)a,b | <.01 | <.01 | |
| Diff | −0.2 (−0.9; 0.3) | −0.3 (−1.0; −0.1) | 0.9 (0.2; 1.1)a,b | <.01 | <.01 | |
| LV-mass, g | 1-Year pp | 122 (114; 144) | 134 (127; 146) | 134 (106; 168) | .83 | .88 |
| 14-Year pp | 110 (105; 139) | 115 (95; 140) | 126 (117; 178) | .18 | .08 | |
| Diff | −13 (−16; −1) | −15 (−27; −5) | 11 (−17; 21) | .23 | .08 | |
| LV-mass index, g·m−2.7 | 1-Year pp | 31 (29; 38) | 32 (28; 38) | 33 (25; 38) | 1.00 | 1.00 |
| 14-Year pp | 29 (26; 35) | 28 (24; 34) | 29 (28; 44) | .18 | .12 | |
| Diff | −3 (−4; −1) | −5 (−8; 0) | 4 (−5; 6)b | .13 | <.05 | |
| RWT, ∼ | 1-Year pp | 0.32 (0.30; 0.37) | 0.33 (0.31; 0.34) | 0.33 (0.32; 0,38) | .62 | .54 |
| 14-Year pp | 0.35 (0.32; 0.36) | 0.31 (0.30; 0.32)a | 0.34 (0.33; 0.40)b | <.01 | <.05 | |
| Diff | 0.01 (−0.04; 0.04) | −0.01 (−0.03; −0.00) | 0.02 (−0.03; 0.05) | .13 | .08 | |
| LVEDD, mm | 1-Year pp | 45 (42; 47) | 46 (44; 48) | 46 (40; 48) | .88 | .88 |
| 14-Year pp | 44 (42; 49) | 46 (45; 50) | 47 (45; 53) | .24 | .94 | |
| Diff | 1 (−2; 3) | 1 (−1; 4) | 3 (−2; 7) | .48 | .88 | |
| LVESD, mm | 1-Year pp | 30 (27; 30) | 30 (28; 32) | 27 (26; 32) | .32 | .27 |
| 14-Year pp | 30 (28; 33) | 32 (30; 33) | 30 (29; 34) | .52 | .64 | |
| Diff | 2 (0; 3) | 1 (0; 4) | 2 (1; 6) | .59 | .35 | |
| Hemodynamic variables | ||||||
| EF, % | 1-Year pp | 67 (64; 69) | 62 (61; 65) | 68 (62; 72) | .06 | .08 |
| 14-Year pp | 61 (58; 65) | 62 (58; 65) | 65 (59; 66) | .46 | .27 | |
| Diff | −5 (−8; −2) | −1 (−6; 1) | −5 (−8; 2) | .26 | .35 | |
| EA ratio | 1-Year pp | 1.7 (1.5; 2.0) | 1.6 (1.3; 2.1) | 1.4 (1.3; 1.7) | .36 | .31 |
| 14-Year pp | 1.2 (1.1; 1.3) | 1.4 (1.3; 1.5) | 1.0 (1.0; 1.2)b | <.05 | <.05 | |
| Diff | −0.3 (−0.6; −0.2) | −0.4 (−0.7; 0.07) | −0.15 (−0.7; 0.0) | .77 | .94 | |
| SV, mL | 1-Year pp | 69 (62; 74) | 75 (71; 81) | 73 (65; 75) | .08 | .16 |
| 14-Year pp | 70 (65; 83) | 75 (69; 83) | 77 (66; 104) | .47 | .44 | |
| Diff | 3 (−2; 9) | −5 (−9; 3) | 11 (1; 31) | .06 | .06 | |
| CO, L·min−1 | 1-Year pp | 4.7 (4.4; 5.1) | 5.4 (4.7; 5.7) | 5.4 (4.8; 6.2) | .16 | .49 |
| 14-Year pp | 4.4 (4.0; 5.0) | 4.3 (4.2; 4.9) | 5.8 (4.5; 7.0)a,b | <.05 | <.05 | |
| Diff | −0.2 (−0.8; 0.2) | −0.6 (−1.4; −0.3) | 0.7 (−1.1; 2.2) | .12 | .12 | |
| CI, L·min−1·m−2 | 1-Year pp | 2.9 (2.7; 3.2) | 3.3 (2.7; 3.4) | 3.0 (2.9; 3.5) | .48 | .76 |
| 14-Year pp | 2.6 (2.3; 3.2) | 2.5 (2.4; 2.8) | 3.1 (2.6; 3.3) | .17 | <.05 | |
| Diff | −0.2 (−0.6; 0.0) | −0.4 (−0.9; −0.3) | 0.2 (−0.7; 0.9) | .33 | .18 | |
Abbreviations: PE, preeclampsia; NT, normotensive; HT, hypertensive; LAD, left atrium diameter; IVST, interventricular septum thickness; PWT, posterior wall thickness; LV-mass, left ventricular mass; LV-mass index; RWT, relative wall thickness; EF, ejection fraction; SV, stroke volume; CO, cardiac output; CI, cardiac index; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; Diff, difference between 1-year and 14-year pp; IQR, interquartile range; exPE, former preeclampsia; pp, postpartum.
a Data are presented as median with IQR.
b P < .05 compared with controls.
c P < .05 compared with NT-exPE.
Figure 1.
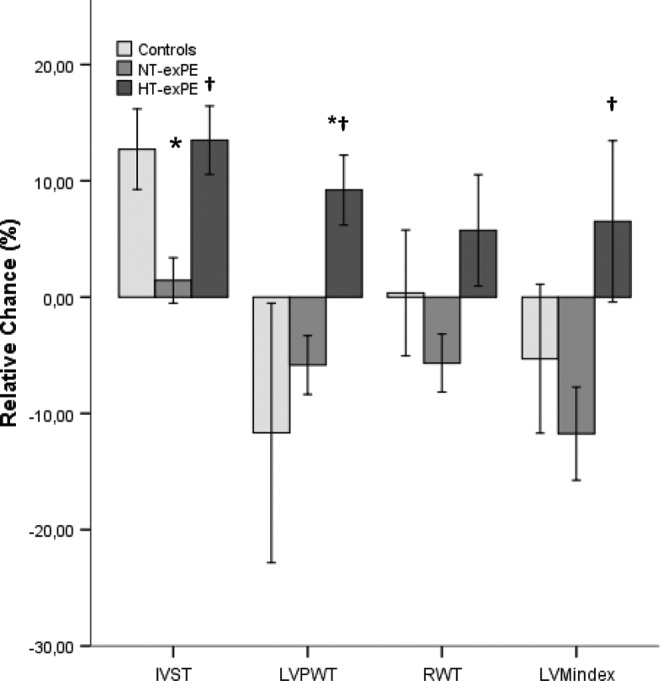
Relative change (±SEM) between 1-year and 14-year postpartum in the control group, normotensive former preeclampsia (NT-exPE) and hypertensive former preeclampsia (HT-exPE) groups. IVS indicates interventricular septum thickness; PWT, posterior wall thickness; LVMindex, left ventricular mass index; RWT, relative wall thickness. *P < .05 compared with controls; †P < .05 compared with NT-exPE; SEM, standard error of mean.
Discussion
This longitudinal pilot study in women with exPE confirms our hypothesis that former patients, destined to develop chronic hypertension, show accelerated left ventricular concentric remodeling relative to their counterparts that remain normotensive. Moreover, this study supports the observation in our earlier cross-sectional study6 that prehypertension is more prevalent in women destined to develop chronic hypertension. We also noticed in the current study that a history of early-onset PE and recurrence of a hypertensive complication in next pregnancies relates to the development of chronic hypertension. This is in line with previous findings,4 but in contrast with a previous study reporting no significant difference in either gestational age of onset or recurrence of hypertensive pregnancy disorder between women with NT-exPE and HT-exPE.6 A possible explanation for this difference may be differences in demography and study design. In the current study, most women had experienced early-onset PE or PE with concomitant SGA. Early-onset PE appears to be a proxy for underlying cardiovascular dysfunction, thus indicating a higher risk developing chronic hypertension.20 Second, in the previous study, we used self-report to determine hypertension in women. This method could have led to underestimation and concomitant bias of the prevalence of patients with hypertension who do not regularly check their BP. In the current study, we used 2 well-defined moments in which we clinically measured the BP in women and diagnosed chronic hypertension based on standard criteria. Finally, this current study provided indirect evidence for lifestyle-related factors, such as overweight, prehypertension, and raised fasting insulin levels, to add to the risk of developing chronic hypertension. Conversely, in this cohort of former patients, LVM and RWT at 1-year pp were not significantly larger in women destined to develop hypertension compared to their counterparts remaining normotensive. These findings are in line with some reports,21 but in contrast to other reports.6,20 It is conceivable that the discrepancy with our previously reported cross-sectional findings6 is related to a difference in the composition of the study populations, whereas also the modest group sizes in this study may have contributed.
Prehypertension, defined as systolic and diastolic BPs ranging from 120 to 139 and from 80 to 89 mm Hg, respectively, is an important risk factor for later hypertension and is associated with cardiovascular morbidity.22 In our study, 57% of the HT-exPE group fulfilled the criteria of prehypertension at the 1-year pp check-up as opposed to only 23% in NT-exPE. However, this difference did not reach statistical significance, probably due to the modest group sizes. Interestingly, 5 of 7 patients with exPE with prehypertension had developed a recurrent hypertensive disorder in their next pregnancy, suggesting prehypertension to predispose to recurrent PE, a finding requiring confirmation in a larger study population.
The higher incidence of overweight in HT-exPE at both measurement sessions, the higher starving insulin levels at 1-year pp, the trend toward a lower HDL-cholesterol, and the raised circulating levels of triglycerides at 14 years may have expedited prehypertension to evolve to chronic hypertension in the HT-exPE subgroup. The prehypertension observed at 1-year pp may already have been present during the index pregnancy contributing to its adverse outcome and to recurrent PE in next pregnancies.
The mechanism that eventually leads to chronic hypertension in patients with NT-exPE is probably multifactorial. A proposed etiology by our group23 and others, at least in part supported by the results of this study, refers to the role of the MetS.9,10,24 Women with a history of PE frequently exhibit features of MetS, which is in line with our findings. PE and CVD share several common risk factors (such as obesity, insulin resistance, and hypertension). It is often suggested that both PE and premature CVD may be manifestations of the MetS.23 Therefore, it is important to screen patients with exPE for underlying additional risk factors to enable the timely institution of preventive measures, thus at least delaying the development of premature CVD.
This study provides unique data on the effect of aging and the superimposed effect of hypertension on cardiac geometry in a relatively young group of women. The observation that 35% of this young, seemingly healthy population develops chronic hypertension even before the onset of the postmenopause, along with a concentrically remodeling left ventricle is to be considered a serious health problem. This observation may in fact be one of the pathways that relate this exPE population to premature CVD.
The subpopulation of women with exPE offers an opportunity to identify women at risk of developing chronic hypertension and premature CVD. This study provides evidence for former patients with prehypertension, a history of early-onset and/or recurrent PE, and/or signs of the MetS to be at extra risk to develop chronic hypertension and its unfavorable impact on cardiac structure and function. The obstetrician counseling a former patient after her complicated pregnancy plays a key role in identifying the risk patient, motivating her to adopt relevant lifestyle changes, and referring her for follow-up visits and fine-tuning of preventive measures to a general practitioner, cardiologist, or vascular medicine specialist.10
Obviously, the size of our study population and with it, the statistical power of this study was modest. However, studying a population during a 14-year follow-up period is unique and enabled us to evaluate a wide range of risk factors potentially relevant in the identification of women at risk for later hypertension. This pilot study provides evidence for prehypertension probably being an important “background” risk factor for both PE and chronic hypertension. It is not surprising that external factors such as lifestyle, eating habits, weight gain, and their unfavorable metabolic effects magnify the adverse impact of prehypertension, both on pregnancy outcome and later cardiovascular health. These pilot data support the need and the potential of the cardiovascular risk assessment at 1-year pp in women with NT-exPE to identify those at risk for later chronic hypertension.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Chahinda Ghossein-Doha is a recipient of a Kootstra Talent Fellowship Grant and a Mosaic NWO Grant.
References
- 1. Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams EM, Macgillivray I. Long-term effect of preeclampsia on blood-pressure. Lancet. 1961;2(7217):1373–1375 [DOI] [PubMed] [Google Scholar]
- 3. Carty DM, Delles C, Dominiczak AF. Preeclampsia and future maternal health. J Hypertens. 2010;28(7):1349–1355 [DOI] [PubMed] [Google Scholar]
- 4. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7(3):375–384 [DOI] [PubMed] [Google Scholar]
- 6. Ghossein-Doha C, Peeters L, van Heijster S, et al. Hypertension after preeclampsia is preceded by changes in cardiac structure and function. Hypertension. 2013;62(2):382–390 [DOI] [PubMed] [Google Scholar]
- 7. Davila DF, Donis JH, Odreman R, Gonzalez M, Landaeta A. Patterns of left ventricular hypertrophy in essential hypertension: should echocardiography guide the pharmacological treatment? Int J Cardiol. 2008;124(2):134–138 [DOI] [PubMed] [Google Scholar]
- 8. Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19(7):1550–1558 [DOI] [PubMed] [Google Scholar]
- 9. Barden AE, Beilin LJ, Ritchie J, Walters BN, Michael C. Does a predisposition to the metabolic syndrome sensitize women to develop pre-eclampsia? J Hypertens. 1999;17(9):1307–1315 [DOI] [PubMed] [Google Scholar]
- 10. Harskamp RE, Zeeman GG. Preeclampsia: at risk for remote cardiovascular disease. Am J Med Sci. 2007;334(4):291–295 [DOI] [PubMed] [Google Scholar]
- 11. Perry IJ, Beevers DG. The definition of pre-eclampsia. Br J Obstet Gynaecol. 1994;101(7):587–591 [DOI] [PubMed] [Google Scholar]
- 12. Visser GH, Eilers PH, Elferink-Stinkens PM, Merkus HM, Wit JM. New Dutch reference curves for birthweight by gestational age. Early Hum Dev. 2009;85(12):737–744 [DOI] [PubMed] [Google Scholar]
- 13. Spaanderman ME, Van Beek E, Ekhart TH, et al. Changes in hemodynamic parameters and volume homeostasis with the menstrual cycle among women with a history of preeclampsia. Am J Obstet Gynecol. 2000;182(5):1127–1134 [DOI] [PubMed] [Google Scholar]
- 14. Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497 [DOI] [PubMed] [Google Scholar]
- 15. Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63 [DOI] [PubMed] [Google Scholar]
- 16. DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med. 1916;17:863–871 [Google Scholar]
- 17. Devereux RB, Casale PN, Kligfield P, et al. Performance of primary and derived M-mode echocardiographic measurements for detection of left ventricular hypertrophy in necropsied subjects and in patients with systemic hypertension, mitral regurgitation and dilated cardiomyopathy. Am J Cardiol. 1986;57(15):1388–1393 [DOI] [PubMed] [Google Scholar]
- 18. de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20(5):1251–1260 [DOI] [PubMed] [Google Scholar]
- 19. Allison PD. Missing data techniques for structural equation modeling. J Abnorm Psychol. 2003;112(4):545–557 [DOI] [PubMed] [Google Scholar]
- 20. Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–715 [DOI] [PubMed] [Google Scholar]
- 21. Evans CS, Gooch L, Flotta D, et al. Cardiovascular system during the postpartum state in women with a history of preeclampsia. Hypertension. 2011;58(1):57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572 [DOI] [PubMed] [Google Scholar]
- 23. Spaan JJ, Sep SJ, van Balen VL, Spaanderman ME, Peeters LL. Metabolic syndrome as a risk factor for hypertension after preeclampsia. Obstet Gynecol. 2012;120(2 pt 1):311–317 [DOI] [PubMed] [Google Scholar]
- 24. Solomon CG, Seely EW. Brief review: hypertension in pregnancy: a manifestation of the insulin resistance syndrome? Hypertension. 2001;37(2):232–239 [DOI] [PubMed] [Google Scholar]


