Abstract
Objective:
The mammalian cytoskeleton is composed in part from keratin filaments which form a complex, highly dynamic intracellular network. We investigate the expression of cytokeratin 15 (CK15) in human endometrium and its regulation by HOXA10 in the human endometrial cell lines.
Methods:
Endometrial biopsies from throughout the menstrual cycle (N = 32) were evaluated for CK15 protein expression by immunohistochemistry using a mouse monoclonal antibody. The human endometrial epithelial cell line (Ishikawa) was transfected with pcDNA/HOXA10. Total RNA was isolated and quantitative real-time polymerase chain reaction was performed to determine expression levels of CK15.
Results:
In the peri-implantation window (days 16 through 23) CK15 protein expression in glandular epithelium of human endometrium decreased to 50% of proliferative phase expression levels. Expression of CK15 messenger RNA decreased by 99% (P < .05) after pcDNA/HOXA10 transfection of Ishikawa cells. The CK15 expression corresponded to the time of maximal secretory epithelial remodeling.
Conclusion:
Gene expression of CK15 is decreased in a HOXA10-dependent fashion in human endometrial epithelial cells. Expression decreases in the peri-implantation period concurrent with maximal HOXA10 expression. Dramatic changes in cellular architecture are necessary to achieve the secretory changes in the endometrial epithelium that bring about the implantation window. Alterations in CK15 likely facilitate these cytoskeletal changes, ultimately promoting endometrial receptivity.
Keywords: HOXA10, cytokeratin 15, endometrium
Introduction
Homeobox genes, or HOX genes, encode transcription factors involved in embryologic and adult development. One HOX gene, in particular, HOXA10, is expressed in the developing uterus as well as during the adult menstrual cycle and varies throughout the cycle. Appropriate HOXA10 expression is essential for reproductive success. An increase in HOXA10 expression as the menstrual cycle progresses leads to alterations in downstream target genes, facilitating the change from proliferative phase endometrium to the secretory endometrium. The resulting glycogen-rich environment maximizes uterine receptivity.1 Targeted disruption of HOXA10 or a reduction in HOXA10 expression in mice prevents development of a receptive endometrium.2,3
We previously conducted a microarray screen of murine endometrium after transfection with pcDNA/HOXA10 or antisense-HOXA10 in order to identify molecular markers regulated by HOXA10 expression.4 We identified cytokeratin 15 (CK15), a cytoskeletal protein, as a putative target: overexpression of HOXA10 resulted in a 77-fold downregulation of CK15. Expression of CK15 has been documented in esophagus and other nonkeratinizing epithelia but has not previously been documented in the uterus.5
The uterine epithelial cytoskeleton is the first site of contact between the blastocyst and the maternal tissue. Specific keratin pairs are coexpressed in a tissue- and differentiation-specific manner and coordinated expression of keratins is essential for intermediate filament assembly and normal epithelial integrity.6 Alterations in the cytoskeleton are necessary to allow endometrial epithelial cells to separate and implantation to occur.7,8 However, there is limited data available on the role and significance of CKs in this process. In this study, we provide the first report of CK15 expression in the human endometrium and establish a relationship between HOXA10 expression and CK15 downregulation.
Materials and Methods
Plasmids, Cell Culture, and Transient Transfection
Complementary DNA (cDNA) of HOXA10 was cloned into the EcoRI site of pcDNA3.1 (+) obtained from Invitrogen (California). pcDNA without a HOXA10 insert was used as a control (Invitrogen). Cells from the human endometrial stromal cell line (HESC) and human endometrial adenocarcinoma cell line (Ishikawa) were maintained in phenol red-free Dulbecco Modified Eagle Medium (DMEM) and minimal essential medium, respectively. Each was supplemented with 10% charcoal-stripped calf serum, 1% penicillin/streptomycin, and 1% sodium pyruvate. The HESCs, grown to 60% to 70% confluence, were transfected using TransIT-LT1 (Mirus; Madison, Wisconsin) with pcDNA3.1(+)/HOXA10 (0.4 μg/well in 6-well plates). Empty pcDNA3.1(+)was used as a control. Ishikawa cells, grown to 60% to 70% confluence, were transfected using Lipofectamine 2000 (Invitrogen) with pcDNA3.1(+)/HOXA10 (0.4 μg/well in 6-well plates), using empty pcDNA3.1(+) as a controls. Total RNA was isolated 72 hours posttransfection. All transfection experiments were conducted in triplicate and performed twice.
Endometrial biopsy samples from 3 patients undergoing laparoscopy without evidence of uterine disease were dissected into minced 3- to 5-mm pieces. After washing with Hank balanced salt solution, the endometrial tissues were placed in serum-free media containing 100 U/L penicillin, 0.1 g/L streptomycin, and 1.25 mg/L fungizone. They were incubated at 37 C for 1 hour. The tubes were then gently mixed and the supernatant was discarded to remove the endometrial epithelial cell aggregates. The partially digested tissues were washed twice in phosphate-buffered saline (PBS) and then placed in serum-free media containing 0.5 g/L collagenase. After incubation for 1 hour at 37°C, the tubes were vortexed for 10 to 12 seconds until the supernatant became turbid with dispersed endometrial stromal cells (ESCs). The contents of the tube were then passed through a 70-mm gauze filter (Millipore, Billerica, Massachusetts). Cells were then maintained in phenol red-free DMEM and supplemented with 10% charcoal-stripped calf serum and 1% penicillin/streptomycin. After reaching 60% to 70% confluence, the primary endometrial epithelial cells were transfected with either pcDNA3.1(+)/HOXA10 (0.4 μg for 6-well plates) or HOXA10 small interfering RNA (siRNA; 20 μmol/L for 6-well plates) using Opti-Mem (Invitrogen) and 3% Lipofectamine 2000 (Invitrogen). Empty pcDNA3.1(+) and nonspecific siRNA were used as controls. The total RNA was isolated 72 hours posttransfection. Transfection experiments were conducted in triplicate and performed twice.
RNA Extraction and Reverse Transcriptase Qualitative Polymerase Chain Reaction
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions and reverse transcribed using the iScript cDNA synthesis kit (BioRad, Hercules, CA). RNA of 1 μg RNA was combined with 19 μL of reaction mixture containing 5× iScript reaction mix, nuclease-free water, and iScript reverse transcriptase. The reaction was carried out for 10 minutes at 25°C, for 30 minutes at 48°C, and for 5 minutes at 95°C, with a final hold step at 4°C. Qualitative polymerase chain reaction (qPCR) was performed using the Lightcycle SYBR Green RT-PCR kit (Roche, Pleasanton, CA). The CK15 intron-spanning primers were selected using the primer selection program PerlPrimer (Sourceforge.net) and subsequent dissociation curve analysis was performed to confirm that each pair of primers produced a single product. Primer sequences were as follows:
HOXA10:
Sense: 5′-GCCCTTCCGAGAGCAGCAAAG-3′
Antisense: 5′-AGGTGGACGCTGCGGCTAATCTCTA-3′
CK15:
Sense: 5′-CTGAGAACTCACGGGCTCCA-3′
Antisense: 5′-TACCAGACAGTCATTGCCTGG-3′
Melting curve analysis was conducted to determine the specificity of the amplified products and to ensure the absence of primer–dimer formation. All products obtained yielded the predicted melting temperature. The qPCR was prepared using a total reaction volume of 20 μL, including 7 μL of SYBR green PCR Master Mix, containing SYBR Green 1 Dye, AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster city, CA; concentrations of Mg++, dNTP, and polymerase not disclosed), 1 μL each of forward and reverse primer (100 nmol/L), and a standard input of 10 ng cDNA. All qPCRs were performed using the Roche Light Cycler (Roche). The qPCR amplifying HOXA10 was performed for 45 cycles of 95°C for 2 seconds; 65°C for 5 seconds; and 72°C for 18 seconds. The PCR amplifying β-actin as a reference gene was performed for 45 cycles of 95°C for 2 seconds; 61°C for 5 seconds; and 72°C for 18 seconds. The PCR amplifying CK15 was performed for 45 cycles of 95°C for 2 seconds; 61°C for 5 seconds; and 72°C for 18 seconds. The Roche Light Cycler monitored the increasing fluorescence of PCR products created during amplification. A quantitative standard curve was then created. Quantification of samples were determined with the Roche Light Cycler and adjusted to the quantitative expression of β-actin from these same samples. Analysis of the relative gene expression was performed using the 2−ΔΔCt method. The Ct values were converted to 2−Ct. Group means were compared using the nonpaired t test.
Western Blot Analysis
Whole protein was extracted from cultured human endometrial cells using Nuclear Extract Kit (Activemotif, Carlsbad, CA) according to the manufacturer’s protocol. Equal amounts of protein (60 μg for both HOXA10 and CK15) were electrophoresed through 4% to 15% polyacrylamide gels (Bio-Rad) at 160 V for 70 minutes and then transferred onto Immun-Blot polyvinylidene difluoride membranes (Bio-Rad) in transfer buffer (25 mmol/L Tris, 192 mmol/L glycine, and 20% methanol) at 100 V for 1 hour. After incubation in blocking buffer (×1 PBS, 0.2% Tween 20, and 5% milk), the membrane was incubated individually with either goat polyclonal HOXA10 antibody (sc-17159) diluted 1:200 or monoclonal CK15 antibody diluted 1:200 overnight at 4°C. After washing, the membranes were incubated with biotinylated horse anti-goat secondary antibody or goat anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA) diluted in the blocking buffer (3.5 μg/mL) at room temperature for 1 hour. The membranes were incubated in ABC Elite (Vector Laboratories, Burlingame, CA) and then stained by diaminobenzidine.
Immunohistochemistry
Thirty-two endometrial biopsy specimens were obtained from fertile control women (aged 23-41) throughout the menstrual cycle. Institutional review board approval with informed consent was obtained prior to collection of primary tissues. Specimens were fixed in formalin and embedded in paraffin. The CK 15 expression was evaluated by immunohistochemistry using a mouse monoclonal antibody to CK15 (200 μg/mL; Santa Cruz Biotechnology, Inc). The sections were deparaffinized in xylene and ethanol. Endogenous peroxidase was blocked with 3% H2O2. After a 60-minute incubation with 1.5% normal goat blocking serum, the sections were incubated overnight at 4°C with primary antibody (anti-CK15, 1:500). The sections were then incubated with biotinylated goat anti-mouse secondary antisera for 60 minutes, avidin and biotinylated peroxidase (Vectastain; Vector Laboratories, Burlingame, California) for 45 minutes, and diaminobenzidine (400 mg/mL) for 45 seconds. Hematoxylin was used for counterstaining. Staining was evaluated in 100 consecutive cells in 2 nonadjacent microscopic fields by a blinded observer to quantify the expression of CK15. Immunohistochemistry specimens were assigned an H-score based on the following formula: H-score = (% at 0) × 0 + (% at 1+) × 1 + (% at 2+) × 2 + (% at 3+) × 3.
Statistical Analysis
Expression of RNA was compared using the nonpaired t test. H-score from immunohistochemistry specimens was quantitatively compared using the Mann-Whitney U test. Differences of P ≤ .05 were considered to be significant.
Results
Cytokeratin 15 Is Expressed in Normal Human Endometrium
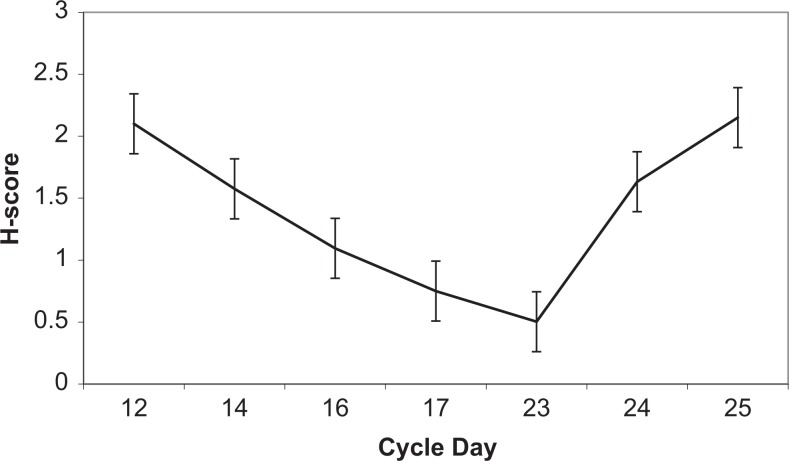
As described previously, HOXA10 expression peaks in the mid-secretory phase at the time of maximal uterine receptivity. In order to also characterize the expression of CK15 in normal human endometrium throughout the menstrual cycle, endometrial biopsy specimens at different phases of the menstrual cycle were stained using a mouse monoclonal antibody for CK15. Immunohistochemistry demonstrated that CK15 was expressed in endometrial glandular epithelial cells and varied throughout the menstrual cycle (Figure 1). During the proliferative and early secretory phases (days 1 through 15), CK15 protein expression was high (H-score 180; max 300; Figure 2). Between cycle days 16 through 23, during which time an increase in HOXA10 expression leads to maximal secretory epithelial remodeling and endometrial receptivity is at its peak, CK15 protein expression decreased to 56% of early proliferative phase expression levels (H-score 78, P < .05; Figure 2). Expression of CK15 returned to higher levels in the late secretory phase with the onset of menstruation (days 24 through 28; H-score 180). No CK15 expression was observed in stromal endometrial cells throughout the menstrual cycle.
Figure 1.
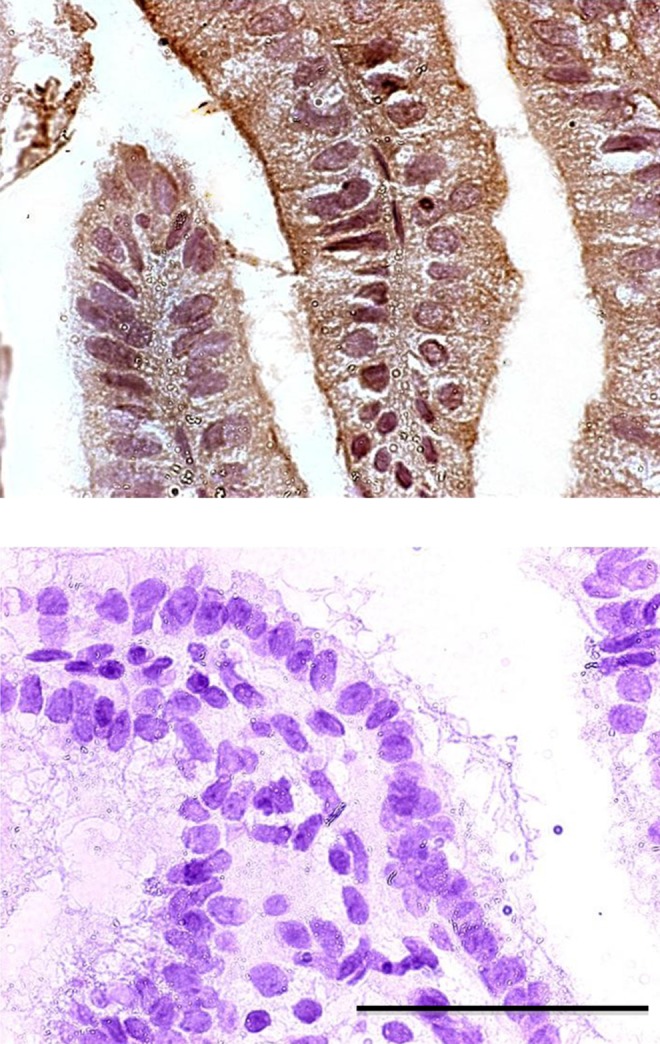
Proliferative and secretory phase CK15 protein expression. On cycle day 3 (above) CK15 expression is high as evidenced by diffuse staining with a mouse monoclonal antibody for CK15. The CK15 protein expression during cycle day 18 (below) has decreased to nearly undetectable levels concurrent with increasing HOXA10 expression. Scale is ×400.
Figure 2.
The CK15 protein expression throughout the menstrual cycle. Human endometrial tissue slides were stained with a mouse monoclonal antibody for CK15 (n = 33), and protein expression was quantified using the H-score method.
Cytokeratin 15 Is Regulated by HOXA10 in Ishikawa and Primary Human Endometrial Cells
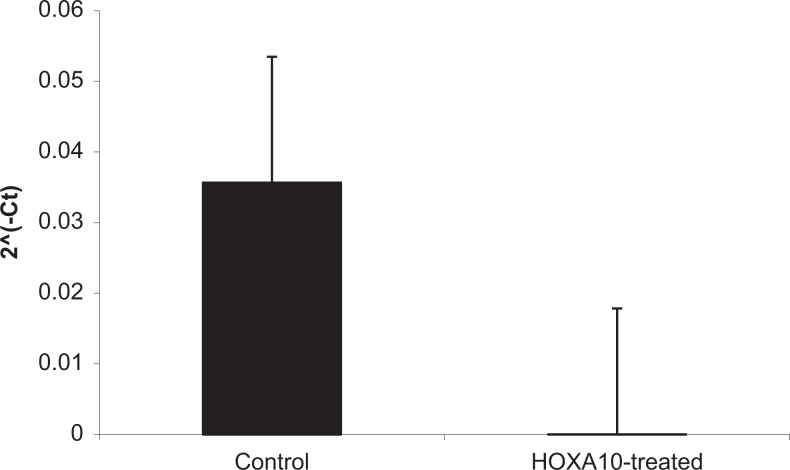
To further characterize the relationship between HOXA10 and CK15, the Ishikawa cell line was transfected with an HOXA10 expression construct. Successful transfection and increased HOXA10 expression was confirmed using reverse transcriptase (RT) qPCR. Subsequent RT-qPCR experiments confirmed that pcDNA/HOXA10 overexpression led to decreased CK15 expression by 99% (P < .05) as compared to transfection with the empty pcDNA vector (Figure 3). Transfection did not alter CK15 expression in HESCs, suggesting that CK15 is not regulated by HOXA10 in ESCs. HOXA10 knockdown did not result in significant alterations in CK15 expression, likely because the cell line has low HOXA10 expression and further decreasing does not affect the CK15 expression.9
Figure 3.
The CK15 gene expression in Ishikawa cells. After transfection (n = 3) with a HOXA10 expression construct, expression of CK15 decreases by nearly 100% as compared to transfection with empty vector. P < .5 in treated compared to control cells. Error bar indicates standard deviation.
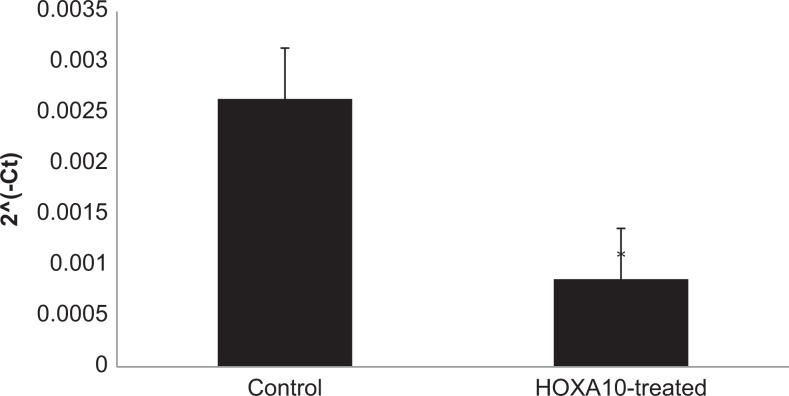
To confirm results obtained in Ishikawa cells, primary human endometrial cells were transfected with a HOXA10 expression construct; successful transfection and increased HOXA10 expression were confirmed using RT-qPCR. Subsequent RT-qPCR again confirmed that pcDNA/HOXA10 transfection led to decreased CK15 expression, this time by 41% (P < .05), as compared to empty pcDNA (Figure 4). Western blot results confirmed that CK15 expression decreased after transfection with the pcDNA-HOXA10 vector (Figure 5).
Figure 4.
The CK15 gene expression in primary human endometrial cells. After transfection (n = 3) with a HOXA10 expression construct, expression of CK15 decreases by over 40% as compared to transfection with empty vector. P < .5 in treated compared to control cells. Error bar indicates standard deviation.
Figure 5.
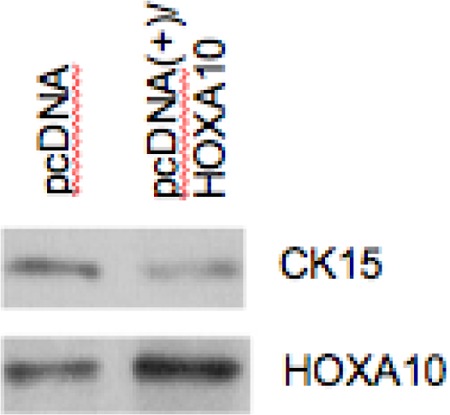
The HOXA10 protein expression in primary human endometrial cells. Western blot results show that pcDNA/HOXA10 transfection induced HOXA10 protein expression in primary human endometrial cells. A reduction in CK15 expression was observed after transfection with pcDNA/HOXA10.
Discussion
Our results demonstrate that CK15 is expressed in human endometrial epithelial cells. This is the first published report documenting CK15 in the endometrium. Additionally, we demonstrate that CK15 is regulated by HOXA10, decreasing in the mid-secretory phase endometrial epithelium concurrent with maximal HOXA10 expression. The CK15 is thus part of the HOXA10-regulated cascade that is necessary for implantation.
The mammalian cellular cytoskeleton forms a complex intracellular network extending from the nuclear surface to the plasma membrane. This static network typically provides nuclear support and tensile strength to the cell but can also be highly dynamic, changing length in response to cell cycle changes and cellular movement and differentiation.10,11 The cellular cytoskeleton is composed in part of intermediate filaments which in epithelial cells consist of keratin proteins. At least 20 keratin proteins have been identified. Specific keratin protein pairs are coexpressed in a tissue- and differentiation-specific manner and coordinated expression of keratins is essential for intermediate filament assembly and normal epithelial integrity.6 Regulation of keratins by HOX genes has been demonstrated in other tissues. For example, the pancreatic HOX transcription factor PDX-1 negatively regulates the ductal-specific keratin 19.12 Additionally, depletion of HOX13 in skin fibroblasts reduces the ability of these cells to induce transcription of keratin 9, which plays a role in site-specific epidermal differentiation.13 However, this is the first report of CK15 regulation by the HOX gene HOXA10.
Cytokeratin 15, found on chromosome 17, has been previously reported in basal keratinocytes of the epidermis, on the surface of the human eye, and as a marker of stem cells in the hair follicle bulge.10 In endometrial epithelial cells, CK15 is regulated by HOXA10 and may modulate the necessary role of HOXA10 in implantation. A reduction in epithelial cell CK content at the time of implantation could lead to a loss of mechanical rigidity and epithelial cell adhesive properties (Steinert and Liem, 1990).11 The resulting dramatic changes in cellular architecture could ultimately promote endometrial receptivity by providing a nascent trophoblast with the ability to invade. Notably, we did not observe a difference in CK15 expression in HESCs after HOXA10 overexpression, the reason for which is unclear. However, tissue-specific HOXA10 regulation has been observed in other genes as well; for example, expression of the PGE2 receptor subtype EP4 is considerably downregulated in ESCs while epithelial expression is unchanged in Hoxa-10 (−/−) mice.14 Taken together with the fact that no CK15 expression was seen in stromal endometrial cells via immunohistochemistry, it seems likely that CK15 is important for endometrial epithelial cell proliferation and differentiation but that its role in stromal cell function is limited or absent.
Other CKs have been identified in endometrial epithelial cells. Cytokeratins 8, 18, and 19 are present in all simple epithelial cells and become undetectable in goat epithelial cells adherent to trophoblasts as implantation proceeds (Guilomot, 1999).18 Conversely, CK13 is upregulated in secretory-phase endometrial biopsies of rabbit uteri, with development of a polarized expression pattern during implantation.15 Alterations in levels of these cytoskeletal proteins (either through upregulation or suppression) likely leads to a major change in cellular architecture and likely promotes successful implantation.
Cytokeratins may play an important role in fertility and contraception. Expression of CK8, 18, and 19 is reduced in endometrial biopsies from women using Norplant (a progestin-containing contraceptive).16 Conversely, HOXA10 expression is also induced by progesterone in endometrial cells.17 Perhaps short-term cytoskeletal remodeling in the mid-secretory phase leads to enhanced implantation but long-term downregulation of CKs causes disruption of epithelial integrity and endometrial atrophy to an extent that ultimately impairs fertility. In this case, CK 15 would play a role in modulating the effects in modulating the effects of HOXA10 on endometrial receptivity in both normal fertility and contraception. It will be interesting to determine whether CK15 expression is also altered in women with endometrial disease.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: NIH U54 HD052668 (Funding source).
References
- 1. Eun Kwon H, Taylor H. The role of HOX genes in human implantation. Ann N Y Acad Sci. 2004;1034:1–18 [DOI] [PubMed] [Google Scholar]
- 2. Bagot CH, Troy P, Taylor H. Alteration of maternal HOXA10 expression by in vivo gene transfection affects implantation. Gene Ther. 2000;7(16):1378–1384 [DOI] [PubMed] [Google Scholar]
- 3. Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in HoxA10 deficient mice. Nature. 1995;374(6521):460–463 [DOI] [PubMed] [Google Scholar]
- 4. Vitiello D, Pinard R, Taylor H. Gene expression profiling reveals putative HOXA10 downstream targets in the periimplantation mouse uterus. Reprod Sci. 2008;15(5):529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whitbread L, Powell B. Expression of the intermediate filament keratin gene, K15, in the basal cell layers of epithelia and the hair follicle. Exp Cell Res. 1998;244(2):448–459 [DOI] [PubMed] [Google Scholar]
- 6. Sato H, Koide T, Sagai T, et al. The genomic organization of type i keratin genes in mice. Genomics. 1999;56(3):303–309 [DOI] [PubMed] [Google Scholar]
- 7. Murphy C, Shaw T. The Cytoskeleton of Uterine Epithelial and Stromal Cells. London: Taylor ad Francis; 1994 [Google Scholar]
- 8. Murphy C. The cytoskeleton of uterine epithelial cells: a new player in uterine receptivity and the plasma membrane transformation. Hum Reprod Update. 1995;1(6):567–580 [DOI] [PubMed] [Google Scholar]
- 9. Sakkas D, Lu C, Zulfikaroglu E, Neuber E, Taylor H. A soluble molecule secreted by human blastocyts modulates regulation of HOXA10 expression in an epithelial endometrial cell line. Fertil Steril. 2003;80(5):1169–1174 [DOI] [PubMed] [Google Scholar]
- 10. Chu PG, Weiss L. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40(5):403–439 [DOI] [PubMed] [Google Scholar]
- 11. Steinert P, Liem R. Intermediate filament dynamics. Cell. 1990;60(4):521–523 [DOI] [PubMed] [Google Scholar]
- 12. Von Burstin J, Reichert M, Wescott M, Rustgi A. The pancreatic and duodenal homeobox protein PDX-1 regulates the ductal specific keratin 19 through the degradation of MEIS1 and DNA binding. PLoS One. 2010;5(8):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rinn J, Wang J, Allen N, et al. A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev. 2008;22(3):303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim H, Ma L, Ma W, Maas R, Dey S. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13(9):1005–1017 [DOI] [PubMed] [Google Scholar]
- 15. Olson G, Winfrey V, Blaeuer G, Palisano J, NagDas S. Stage specific expression of the intermediate filament protein cytokeratin 1 in luminal epithelial cells of secretory phase human endometrium and periimplantation stage rabbit endometrium. Biol Reprod. 2002;66(4):1006–1015 [DOI] [PubMed] [Google Scholar]
- 16. Wonodiresko S, Au C, Hadisputra W, Affandi B, Rogers P. Cytokeratins 8, 18 and 19 in endometrial epithelial cells during the normal menstrual cycle and in women receiving Norplant. Contraception. 1993;48(5):481–493 [DOI] [PubMed] [Google Scholar]
- 17. Taylor H, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7):1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guillomot M. Changes in extracellular matrix components and cytokeratins in the endometrium during goat implantation. Placenta. 1999;20(4):339–345 [DOI] [PubMed] [Google Scholar]