Abstract
The aim of this study was to assess the effects of bacterial lipopolysaccharide (LPS) and Lactobacillus rhamnosus GR-1 supernatant (GR-1SN) on secretion profiles of cytokines, chemokines, and growth factors from primary cultures of human decidual cells. Lipopolysaccharide significantly increased the output of proinflammatory cytokines (interleukin [IL]-1B, IL-2, IL-6, IL-12p70, IL-15, IL-17A, interferon gamma [IFN-γ], and tumor necrosis factor [TNF]); anti-inflammatory cytokines (IL-1RN, IL-4, IL-9, and IL-10); chemokines (IL-8, eotaxin, IFN-inducible protein 10 [IP-10], monocyte chemoattractant protein 1 [MCP-1], macrophage inflammatory protein-1α [MIP-1α], macrophage inflammatory protein-1β [MIP-1β], and regulated on activation normal T cell expressed and secreted [RANTES]); and growth factors (granulocyte colony-stimulating factor [CSF] 3, CSF-2, and vascular endothelial growth factor A [VEGFA]). Lactobacillus rhamnosus GR-1SN alone significantly increased CSF-3, MIP-1α MIP-1β, and RANTES but decreased IL-15 and IP-10 output. The GR-1SN also significantly or partially reduced LPS-induced proinflammatory cytokines TNF, IFN-γ, IL-1β, IL-2 IL-6, IL-12p70, IL-15, IL-17, and IP-10; partially reduced LPS-induced anti-inflammatory cytokines IL-1RN, IL-4 and IL-10, and LPS-induced VEGFA output but did not affect CSF-3, MIP-1α, MIP-1β, MCP-1, IL-8, and IL-9. Our results demonstrate that GR-1SN attenuates the inflammatory responses to LPS by human decidual cells, suggesting its potential role in ameliorating intrauterine infection.
Keywords: lipopolysaccharide, Lactobacillus rhamnosus GR-1, cytokine, chemokine, growth factor, decidual cells
Introduction
Prevention of preterm birth (PTB) remains a major challenge in obstetrics. The condition occurs globally in 9% to 13% of all pregnancies.1 A strong body of evidence suggests that infection and inflammation are important mechanisms that might account for 25% to 40% of PTBs.2,3 Bacterial vaginosis (BV), an alteration in the vaginal microbiota, is associated with a 40% increase in the risk of PTB.4 The consequences of intrauterine infection are not limited to the complication of PTB but are among the leading causes of inflammation-induced neurologic injury resulting in intra- or periventricular hemorrhage and cerebral palsy of the newborn.5 Antibiotics have been used in an attempt to prevent infection-induced PTB; however, there is little benefit in terms of a reduction in the rate of PTB from antimicrobial administration alone. Indeed, there may be even an increased risk of PTB with metronidazole treatments.6,7
Research conducted during the last decade has opened up the possibility that cellular immune effectors in inflammation induced by infection underlie preterm delivery. Dysregulation of the cytokine milieu has been suggested to contribute to preterm labor, and therefore a better understanding of the link between cytokines and PTB may lead to the development of effective interventions to prevent infection- or inflammation-related preterm labor. Traditionally and functionally, cytokines are subgrouped according to the paradigm of the T-helper type 1 (Th1):Th2 cell/response dichotomy. The Th1 cells produce proinflammatory cytokines (interleukin [IL]-1, 2, 6, 12, 15, 17, interferon [IFN-γ], and tumor necrosis factor [TNF]) which activate cytotoxic T cells and macrophages, thereby stimulating cellular immunity and inflammation. On the other hand, Th2 cells produce anti-inflammatory cytokines (such as IL-4, 5, 9, 10, and 13) that stimulate antibody production by B cells and act antagonistically with Th1-type cytokines to promote humoral immunity.8–10 It has been proposed that recurrent spontaneous abortion occurs with Th1 bias, successful term pregnancy is associated with Th2 bias, and an appropriate ratio of Th1-Th2 cytokines is key for successful term pregnancy.11–15 Recently, a number of studies suggest that regulatory T cell and natural killer (NK) cells are also important for the establishment and maintenance of pregnancy.16,17 Thus, the balance of proinflammatory cytokines to anti-inflammatory cytokines is necessary for successful maintenance of pregnancy. Consequently, an overexaggerated maternal immune response to microorganisms through pattern-recognition receptors, mainly toll-like receptors (TLRs) has been suggested as one of the underlying causes of PTB with infection.18,19 Previous studies demonstrated that pregnancies that display signs of infection are characterized by increased proinflammatory cytokines (IL-1β, IL-6, IL-8, and TNF) in the amniotic fluid, myometrium, decidua, fetal membranes, and maternal serum, demonstrating Th1 bias.20 Furthermore, a disproportionate increase in IL-1β over IL-1RN in the cervicovaginal secretion of pregnant women with an altered vaginal microflora correlates with PTB.21 However, the profiles of inflammatory-related cytokine, chemokine, and growth factor production, and especially the balance between proinflammatory and anti-inflammatory cytokines in infection-induced PTB, are less well characterized.
Probiotics are potential alternatives to antibiotics or anti-inflammatory drugs to prevent PTB. Lactobacillus strains can act as competent immune modulators, enhancing intestinal and systemic immune functions.22–26 Lactobacillus rhamnosus GR-1 in combination with Lactobacillus reuteri RC-14 can help return homeostasis to the vagina, suggesting a potential therapeutic option to protect against infection-induced PTB.27–30 Previously, we showed that L rhamnosus GR-1 supernatant (GR-1SN) downregulates the bacterial lipopolysacchride (LPS)-induced TNF output but upregulates IL-10 and CSF-3 output by human placental trophoblast cells in vitro.31,32 However, the mode of action of L rhamnosus GR-1 on a broader profile of cytokine production and on LPS-induced cytokine production profiles in human decidual cells is unknown.
Decidua is the functional layer of the endometrium of pregnancy, containing a high number of immune cells, such as natural killer cells, macrophages, dendritic cells, and T lymphocytes, and the first immunological barrier to microbial infection. Its activation has been suggested to play an important role in labor at term and preterm. Previous studies have demonstrated that LPS induces IL-1RN, IL-6, IL-8, IL-10, IFN-γ, and TNF-α output from human choriodecidual tissues or cells in vitro, using a traditional enzyme-linked immunosorbent assay (ELISA).33–36 Obviously, studies focused on the actions of individual cytokines may not be sufficient for understanding the mechanism of PTB related to infection or inflammation. Because many cytokines that accompany inflammation have closely related or overlapping biological effects and may be involved in different aspects of immune responses, quantitation of single cytokines may be of limited value. However, the more extensive profiles of cytokine, chemokine as well as growth factor production in response to LPS by human decidual cells have not been reported.
In this study, we employed a high-sensitivity multiplex bead immunoassay technique (Luminex assay), which has been shown to have good correlation to ELISA platform assays.37 It allows for the simultaneous detection of cytokine multiplex panels and low expressed cytokines not previously recognized in small-volume samples. This allows simultaneous assessment of a large panel of cytokines and growth factors to obtain a “fingerprint” of innate and adaptive immune responses in inflammation. Using such a tool, we examined the secretion of a broad range of cytokines, chemokines, and growth factors from cultured human primary decidual cells challenged with LPS or/and GR-1SN to characterize the features of inflammatory-related cytokine, chemokine, and growth factor production induced by microorganism products in human decidua.
Methods and Materials
Placenta Collection
Placentas with attached fetal membranes were collected from normal term (>37 weeks of gestation) pregnancies after elective cesarean delivery in the absence of labor (n = 13). Informed consent was obtained before tissue collection. The study was approved by the Review Board for Human Subject Research (IRB no. 04-0018-U) at Mount Sinai Hospital (Toronto, Ontario, Canada) and the University of Toronto in accordance with the Canadian Tri-Council Policy Statements on Human Ethics Reviews. Patients who had multifetal gestations, preterm rupture of membranes, chorioamnionitis, chromosomal abnormalities, and/or preeclampsia were excluded. None of the patients had received prostaglandins, corticosteroids, or oxytocin. The indications for elective cesarean section included breech presentation, previous cesarean delivery, and cephalopelvic disproportion. None of the patients had experienced premature uterine activity during their pregnancy.
Lactobacillus Preparation
Lactobacillus rhamnosus GR-1 supernatant (GR-1SN) was prepared as described previously.25 Briefly, the organism was grown anaerobically for 48 hours to reach the stationary growth phase in de Man, Rogosa, and Sharpe media (MRS). The culture medium was then collected and centrifuged at 6000g for 10 minutes at 4°C. Residual bacteria were removed by filtration of the supernatant through a 0.22-μm pore size filter. The supernatant was divided into aliquots and frozen at −80°C.
Decidua Collection and Cell Culture
Decidua, scraped from fetal membranes, was washed with phosphate-buffered saline (PBS) to remove red blood cells, chopped, and digested with DMEM containing collagenase type H (6 mg/g tissue; Roche, Basel, Switzerland) and DNAse type I (200 μg/mL; Sigma, St. Louis) for 1 hour at 37°C. Following centrifugation (650g, 10 minutes), the cells were passed through a 40-μm filter. The viability of the cells was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.38 After 8 hours of treatment with LPS and/or GR-1SN, the MTT reagent was added to a final concentration of 0.5 mg/mL and the cells were incubated for 2 to 4 hours. After incubation, the culture medium with MTT reagent was removed and the formazan dye generated was dissolved in dimethyl sulfoxide and the absorbance was measured at 570 nm by a plate reader (TECAN infinite M200; TECAN Group Ltd, Mannedorf, Switzerland. Figure 1 shows that LPS in the presence or absence of GR-1SN did not have any detrimental effect on cell viability.
Figure 1.
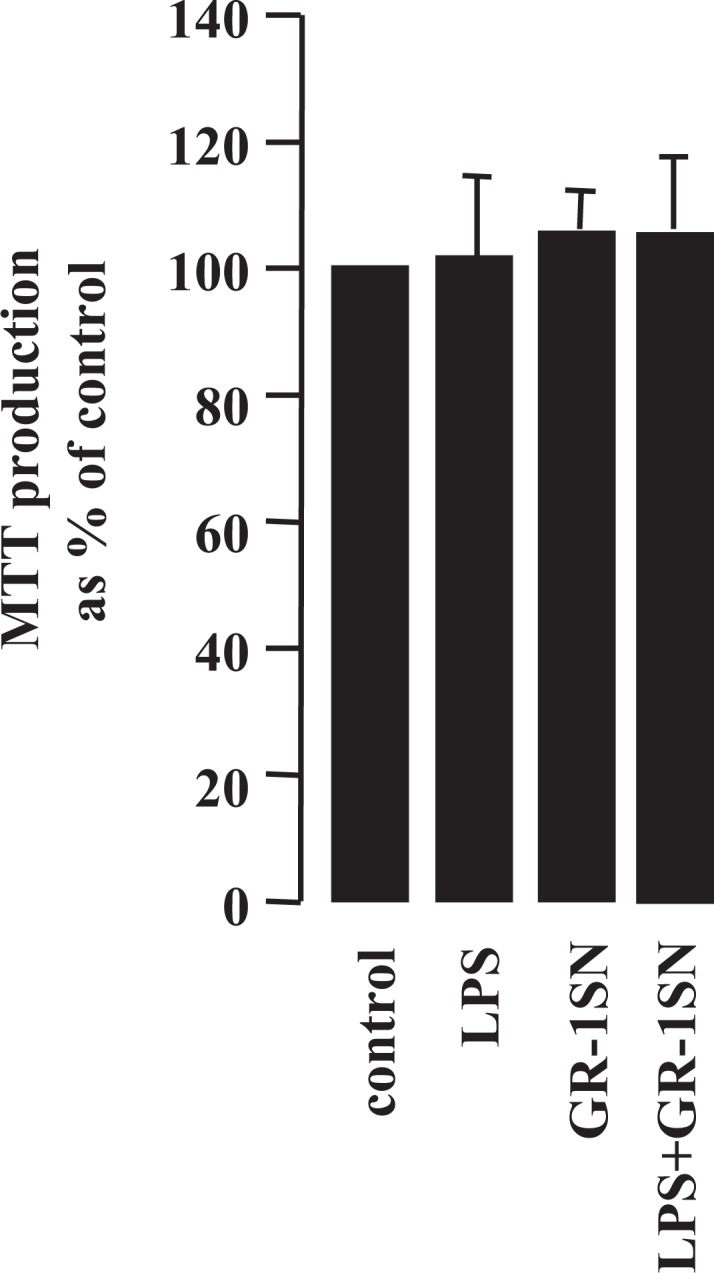
The effect of treatments on decidual cell viability. Cells were treated with lipopolysaccharide (LPS; 10 ng/mL) and/or GR-1SN (1:20) for 8 hours, then the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed as described in the Materials and Methods section. Each bar represents the change relative to control; data are presented as mean ± standard error of the mean.
Treatments
Decidual cells were plated in 24-well plates (0.5 × 106 cells/well) and cultured in DMEM/F-12 (GIBCO Invitrogen, Burlington) supplemented with 10% heat-inactivated fetal bovine serum (Wisent Inc, Montreal) and antibiotics (1000 U/mL of penicillin, 0.1 mg/mL of streptomycin, and 0.23 μg/mL of amphotericin; GIBCO Invitrogen) and estradiol (E2, 10−9 mol/L) at 37°C under 5% CO2/95% ambient air for 48 hours. The culture medium was replaced with DMEM/F-12 as mentioned earlier, with added progesterone (P4, 10−9 mol/L) for further 48 hours of incubation. Cells were washed with PBS and cultured in serum-free DMEM/F-12 with E2 (10−9 mol/L) + P4 (10−9 mol/L). In preliminary experiments, we found that after LPS treatment of cells cultured in serum-free or serum- or charcoal-stripped serum medium cytokine outputs were similar, and modestly, although not significantly higher in the presence of serum than in the absence of serum (data not shown). For consistency between studies, we used serum-free media at the time described throughout these experiments. After 12 hours of starvation and/or preincubation with GR-1SN at a dilution of 1:20,28 further treatments of LPS (10 ng/mL, Sigma) or GR-1SN or LPS plus GR-1SN were added, and the cells were incubated for 8 and 24 hours. Vehicle-treated wells (controls) were present in each experiment. After incubation, the medium was harvested, centrifuged to remove cells and debris, and stored at −80°C until further assay. Original samples were used for multiplex assay and for TNF and IL-10 ELISA. Samples were diluted from 60 to 150 times for IL-6, IL-8, and monocyte chemoattractant protein 1 (MCP-1) ELISA.
Cytokine and Chemokine Measurements
ELISA cytokine assays
In preliminary experiments, concentrations of IL-6, 8, 10, TNF, and MCP-1 in conditioned media were determined by ELISA using a commercial kit according to the manufacturer’s instructions (eBioscience, Inc, California). Plate reading and curve fitting were performed on plate reader (TECAN infinite M200) using Magellan6 software (TECAN Group Ltd).
Bio-plex cytokine assay
Subsequent cytokine/chemokine measurements were made using the Bio-Plex 200 system (Bio-Rad, Hercules, California) and Bio-Plex Human Cytokine 27-plex assay according to the manufacturer’s instructions. The 27-plex assay kit contains beads conjugated with monoclonal antibodies specific for IL-1β (IL-1β), IL-1RN, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17A, eotaxin, fibroblast growth factor (FGF-2), granulocyte colony-stimulating factor (CSF) 3, granulocyte–macrophage-CSF (CSF-2), interferon gamma (IFN-γ), IFN-inducible protein 10 (IP-10), MCP-1, macrophage inflammatory protein 1α (MIP-1α), macrophage inflammatory protein 1β (MIP-1β), platelet-derived growth factor-BB (PDGFB), regulated on activation normal T cell expressed and secreted (RANTES), TNF, and vascular endothelial growth factor A (VEGFA). Standard curves and the concentration of cytokines within samples were generated with the Bio-Plex Manager 4.1 software.
Statistical Analysis
Data were presented as average values ± standard error of the mean (SEM). Original data were log transformed for statistical analysis, and statistical significance between groups was determined using a 2-way analysis of variance followed by Holm-Sidak method post hoc analysis using SigmaPlot version 9.01 statistical software (Jandel Scientific Software, San Rafael, California). The criteria for statistical significance were set at P < .05.
Results
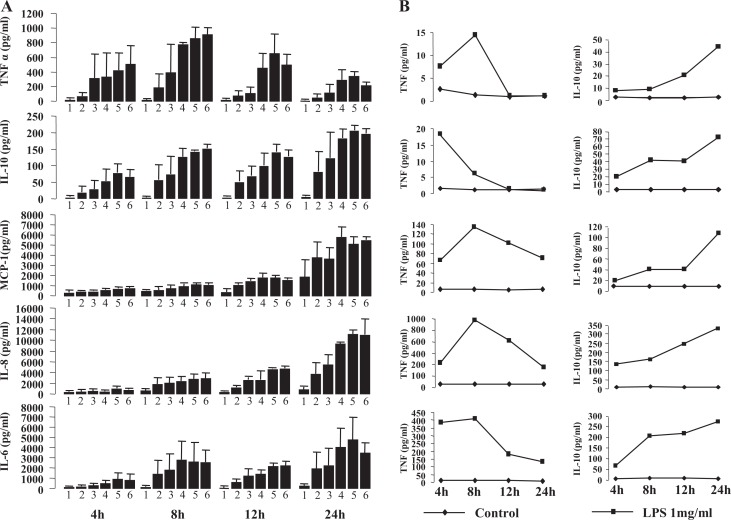
In preliminary experiments using ELISA, we showed that LPS (0.1-1000 ng/mL) stimulated the output of IL-6, IL-8, IL-10, MCP-1, and TNF from decidual cells in a dose- and time-dependent manner (Figure 2A). The TNF response peak reached earlier (8 hour) than that of IL-6, IL-8, IL-10, and MCP-1 (24 hours; Figure 2A). Results from 5 individual experiments also demonstrated that TNF increased to peak levels at 8 hours, which was much earlier than IL-10 at 24 hours (Figure 2B).
Figure 2.
Dose and time curves of tumor necrosis factor (TNF), interleukin (IL) 10, monocyte chemoattractant protein 1 (MCP-1), IL-8, and IL-6 secretion from decidual cells stimulated with lipopolysaccharide (LPS). A, Cells were treated with LPS with different dosages from 0 ng/mL (1); 0.1 ng/mL (2); 1 ng/mL (3); 10 ng/mL (4); 100 ng/mL (5); 1000 ng/mL; (6) and at different times as indicated (n = 2). Each bar represents the concentration of cytokines in medium (pg/mL); data are presented as mean ± standard error of the mean (SEM). B, Cells were treated with vehicle (control) or 1 mg/mL LPS at 4, 8, 12, and 24 hours as indicated. Results are presented as 5 individual experiments.
Based on the preliminary results, we chose LPS at a concentration of 10 ng/mL and incubation duration of 8 hours for multiplex assay in subsequent experiments. Out of 27 cytokines assayed, 24 cytokines were detectable under our experimental conditions; exceptions were IL-5, IL-7, and IL-13. The concentrations of cytokines, chemokines, and growth factors in the control group demonstrated a large range from 1.2 pg/1 × 106 cells (IL-4) to 15 686.5 pg/1 × 106 cells (CSF-3).
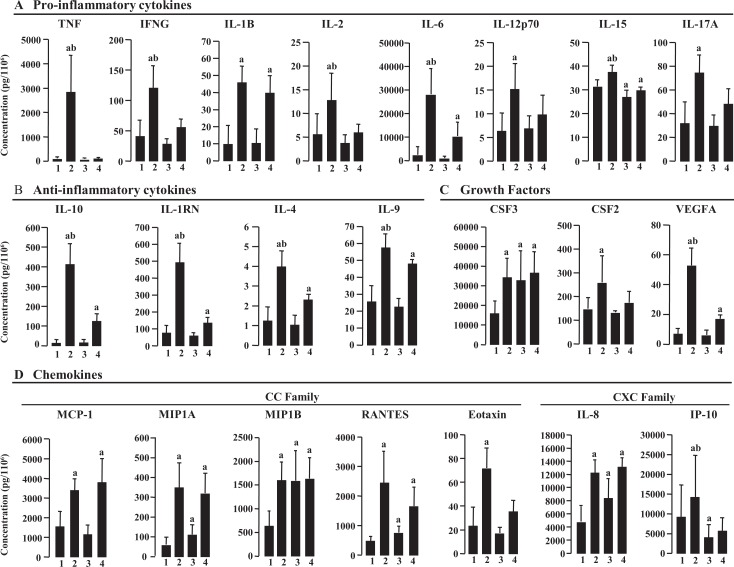
As the concentration of individual cytokines showed large variations between individual experiments (Figure 3), we converted the results to log data for statistical analysis and comparison between treatment groups. After stimulation with LPS, 22 of the 24 cytokines, chemokines, and growth factors were significantly increased, including cytokines with proinflammatory properties (TNF, IFN-γ, IL-1β, IL-2, IL-6, IL-12p70, and IL-17A; Figure 3A); cytokines with anti-inflammatory properties (IL-1RN, IL-4, IL-9, and IL-10; Figure 3B); growth factors(CSF-3, CSF-2, and VEGFA; Figure 3C); and chemokines (IL-8, MCP-1, MIP-1α, MIP-1β, RANTES, IP-10, and eotaxin; Figure 3D). Of these, TNF, IL-6, and IL-10 were the most highly upregulated compared with the control group. There were no significant changes in PGDFbb and FGF-2 (data not shown).
Figure 3.
Effect of lipopolysaccharide (LPS) and GR-1SN on secretion of cytokines, chemokines, and growth factors from decidual cells (n = 6). A, Proinflammatory cytokines. B, Anti-inflammatory cytokines. C, Growth factors. D, Chemokines. Cells were treated with LPS (10 ng/mL) and/or GR-1SN (preincubation with GR-1SN (1:20) for 12 hours) for 8 hours. Each bar represents the concentration normalized to cell number; data are presented as mean ± SEM. a, P < .05 compared with control; b, P < .05 LPS versus LPS + GR-1SN. Control (1); LPS (2); GR-1 (3); and LPS + GR-1SN (4).
The GR-1SN significantly increased the output of the chemokines, IL-8, MIP-1α, MIP-1β, RANTES, and growth factors CSF-3 and significantly decreased the output of proinflammatory cytokine IL-15 and chemokine IP-10 in conditioned medium, compared to the control group (Figure 3A-D).
The combination of LPS and GR-1SN treatment (preincubation) significantly decreased the output of LPS-induced proinflammatory cytokines, TNF, IFN-γ, IL-2, IL-6, and IL-15. The TNF output was attenuated more than 30-fold, and the outputs of IL-1β, IL-12p70, and IL-17A were reduced partially in conditioned medium, compared with LPS treatment alone (Figure 3A). The outputs of LPS-induced anti-inflammatory cytokines, IL-1RN, IL-4, IL-9, IL-10 were significantly reduced by GR-1SN compared with LPS alone but were still significantly higher than the control group (Figure 3B). The GR-1SN had no significant effect on the output of LPS-increased growth factors CSF-3 and chemokine MCP-1, MIP-1α, MIP-1β, RANTES, and IL-8 but significantly decreased LPS-induced IP-10 (Figure 3C and D).
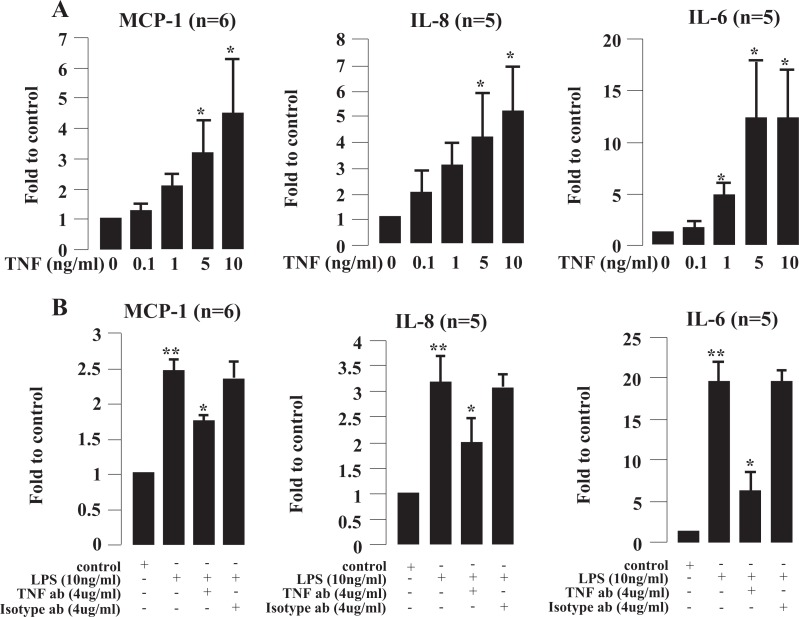
In order to examine the possible intermediary role of TNF in the stimulatory effects of LPS, we cultured cells in the presence of LPS plus TNF antibody. As shown in Figure 4, TNF significantly stimulated IL-6, IL-8, and MCP-1 secretion from decidual cells in a dose-dependent manner (using ELISA; Figure 4A) while addition of TNF-neutralizing antibody significantly reduced TNF-induced IL-6, IL-8, and MCP-1 secretion (Figure 4B).
Figure 4.
Effect of tumor necrosis factor (TNF) and TNF-neutralizing antibody (TNF ab) in lipopolysaccharide (LPS)-induced monocyte chemoattractant protein 1 (MCP-1), interleukin (IL) 8, and IL-6 secretion. A, Cells were treated with TNF for 24 hours. B, Cells were treated with LPS plus TNF ab or isotype antibody for 24 hours. Each bar represents the concentration fold increase relative to control. Data are presented as mean ± standard error of the mean (SEM). * P < .05 versus control (A) or versus LPS (B); **P < .01 versus control.
Discussion
It is well known that cytokines, chemokines, and growth factors play an important role in inflammatory responses through in situ activation and proliferation of innate and adaptive immune cells as well as recruitment of these immunocompetent cells out of the circulation. In this study, we showed a similar increase in IL-1RN, IL-6, IL-8, IL-10, IFN-γ, and TNF production induced by LPS, consistent with previous studies.33–36 We extended those observations to show that LPS also increased the secretion of other inflammatory-related cytokines, including proinflammatory cytokines IL-1B, IL-2, IL-12p70, IL-15, and IL-17A and anti-inflammatory cytokines IL-4, IL-9; chemokines MCP-1, MIP-1α, MIP-1β, RANTES, IP-10, eotaxin, and growth factors, CSF-3, CSF-2, and VEGFA. To our knowledge, this is the first description of secretion of these cytokines, chemokines, and growth factors in human decidual cells in response to LPS in vitro. The present results do not allow us to delineate the cell type responsible for cytokine secretion in mixed decidual cell preparations. In preliminary studies, we have shown that depletion of CD45+ cells from the mixed decidual preparations abolished LPS responsiveness (unpublished results) consistent with these immune cells being the primary source of different cytokines, but the possibility of cell–cell interaction has not yet been explored and cannot be excluded at this time. Indeed, we deliberately utilized an unpurified decidual cell population in culture, in an attempt to allow the cell–cell interactions that might occur in vivo. Given the results we obtained, it will be informative to evaluate the contributions of different cell types, in subsequent, more extensive studies than we have presented herein.
Taken together, our results suggest that LPS is able to stimulate mixed decidual cells to release a broad range of cytokines, chemokines, and growth factors. Therefore, these effectors may play an important role in inflammatory responses and in the further activation and regulation of the innate immune system and subsequent antigen uptake (TNF, IL-1B, IL-12p70, IL-17A, IFN-γ, IL-10, IL-1RN, and CSF-3) as chemoattractants for various immune cells including monocytes, NK cells, and T cells (MIP-1α, MIP-1β, IL-8, MCP-1 RANTES, IP-10, eotaxin, and VEGFA) and stimulating activation and proliferation of T cells (IL-2, IL-4, IL-12p70, IL-6, IL-9, IL-15, CSF-3, and CSF-2). Notably, IL-15 is now recognized as a cytokine with potent survival and immunomodulatory effects on cells of both the innate and the adaptive immune systems, including activating and expanding NK cells, NKT cells, CD8+ effector memory, and central memory T lymphocytes, which play a central role in defense mechanisms against pathogens, and it is elevated in preterm labor. 39,40 It has also been demonstrated that IL-17A is involved in the development of inflammation by inducing the expression of proinflammatory cytokines, chemokines, antimicrobial peptides, and matrix metalloproteinases from different cells, and it is also the signature cytokine of the recently identified Th17 cell subset.41 A recent study showed that Th17 cell number is increased in the chorioamniotic membrane of preterm delivery cases with choroamnionitis (CAM) and IL-17 levels in severe CAM preterm delivery cases were significantly higher than those in CAM-negative preterm delivery cases.42 In this study, we found that LPS-induced IL-15 and IL-17A production in human decidual cells, suggesting that the IL-15 and the Th17 cell subset may be involved in infection-induced preterm labor. Overall, these findings suggest that there may be an overexaggerated maternal inflammatory response to microorganism products and/or microorganisms through proinflammatory cytokines/chemokines and activated innate and adaptive immune systems.
Lipopolysaccharide-induced IL-6, IL-8, and MCP-1 outputs were partially blocked by TNF-neutralizing antibody and stimulation of TNF by LPS, indicating that TNF may be an initial regulator and enhancer with positive feedback in LPS-stimulated cytokine production. The different time courses of TNF and IL-10 responses to LPS indicate subtle temporal differences that need consideration in the design of future studies as well as in interpretation of the underlying biology. For example, it has been suggested that the later rise in IL-10 downregulates TNF output by negative feedback, a response that is abrogated by administration of IL-10 neutralizing antibody.43 At this time, one can only speculate on the importance that different cell–cell interactions may have in determining these responses
Proinflammatory cytokine bias in pregnancy disorders has led us to consider the therapeutic possibility of manipulating/redirecting the cytokine balance in order to downregulate proinflammatory and/or upregulate anti-inflammatory cytokines, thereby creating a milieu that is favorable toward successful pregnancy maintenance. The L rhamnosus GR-1 strain is one of a family of immunoregulatory probiotics. Our data indicate that the stimulated levels of proinflammatory cytokines TNF, IFN-γ, IL-2, IL-15, and VEGFA induced by LPS were significantly attenuated by GR-1SN in human decidual cells. In particular, LPS-induced TNF was substantially downregulated by GR-1SN, consistent with our previous studies on human placental trophoblast cells31 and amniotic epithelial cells.44 Conversely, levels of the anti-inflammatory cytokines IL-1RN, IL-4, and IL-10 in decidual cells were significantly higher after exposure to LPS, and LPS-induction of these cytokines was partially reduced by GR-1SN but was still significantly higher than the control group by GR-1SN. The GR-1SN did not significantly impact LPS-induced CSF-3, although it did stimulate secretion of CSF-3, consistent with our previous studies on placental trophoblast cells.32 Studies have already shown that IL-4, IL-10, CSF-3, and CSF-2 are not only regulators of hematopoiesis with Th2-inducing capacity but are also potent inhibitors of the production of inflammatory mediators such as proinflammatory cytokines and prostaglandin.45–50 This indicates that GR-1SN can manipulate the LPS-induced proinflammatory cytokine bias, accounting for its anti-inflammatory activity and suggesting that L rhamnosus GR-1 may induce a tolerogenic milieu within the fetomaternal interface. Excessive activation of Th2 is harmful for fetal survival, indicating that an appropriate ratio of Th1-Th2 cytokines is key for successful term pregnancy.15 That GR-1SN partly suppresses LPS-stimulated production of anti-inflammatory cytokine may be favorable to keep an appropriate ratio of proinflammatory to anti-inflammatory cytokine. Moreover, that GR-1SN alone inhibited IL-15 secretion from decidual cells suggests that GR-1SN may play a role in defense mechanisms against pathogens through immunomodulatory effects of IL-15 on cells of both innate and adaptive immune systems.
Chemokines are a group of low-molecular-weight peptides that induce chemotaxis of different leukocyte subtypes to inflammation sites.51 Here, GR-1SN stimulated secretion of MIP-1α, MIP-1β, RANTES, and IL-8. However, it decreased secretion of IP-10. The GR-1SN did not affect LPS-induced secretion of MCP-1, MIP-1α, MIP-1β, RANTES, and IL-8 but reduced LPS-induced secretion of IP-10 and eotaxin. These results suggest that GR-1SN is able to promote recruitment of phagocytes (including macrophages and neutrophils) and other immune cells by certain chemokines with or without infection. Whether GR-1SN is able to stimulate the activity of phagocytes remains to be investigated.
It has been reported that RANTES, MIP-1α, and MIP-1β are the most potent chemokines with anti-HSV-1 activity 52 and also that they have anti-HIV activity.53 The IFN-inducible protein 10, a strong Th1 cell chemokine that is positively associated with bacterial and viral-induced tissue damage,54 was inhibited by GR-1SN, indicating an additional anti-inflammatory property of GR-1SN. We speculate that patients with bacterial or viral infection may benefit from the ability of GR-1SN to promote the production of these chemokines and to inhibit IP-10 production.
In conclusion, bacteria through endotoxin production can induce the production of a broad range of inflammatory-related cytokines, chemokines, and growth factors in human deciduas. This then leads to an overexaggerated inflammatory response potentially resulting in preterm labor. The ability of lactobacilli to produce substances that directly and/or indirectly reduce production of inflammatory mediators, through altering microorganism-activated decidual cell (including immune cells and nonimmune cells) responses or recruiting and activating phagocytes at the decidual interface, suggests a potential mechanism for preventing some cases of PTB.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CIHR (Canadian Institutes of Health Research, #MOP-82799).
References
- 1. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–584 [DOI] [PubMed] [Google Scholar]
- 3. Martius J, Eschenbach DA. The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity-a review. Arch Gynecol Obstet. 1990;247(1):1–13 [DOI] [PubMed] [Google Scholar]
- 4. Hiller SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333(26):1737–1742 [DOI] [PubMed] [Google Scholar]
- 5. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy. Its roles in reproductive physiology, obstetrical complications and fetal injury. Nutr Rev. 2007;2(12 pt 2):5194–5202 [DOI] [PubMed] [Google Scholar]
- 6. Romero R, Espinoza J, Chaiworapongsa T, Kalche K. Infection and prematurity and role of preventive strategies. Semin Neonatol. 2002;7(4):259–274 [DOI] [PubMed] [Google Scholar]
- 7. Shennan A, Crawshaw S, Briley A, et al. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET study. BJOG. 2006;113(1):65–74 [DOI] [PubMed] [Google Scholar]
- 8. Mosmann TR, Sad S. The expanding universe of T-cell subsets. Immunol Today. 1996;17(3):138–146 [DOI] [PubMed] [Google Scholar]
- 9. Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine. 2008;42(3):277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173 [DOI] [PubMed] [Google Scholar]
- 11. Wegmann TG, Lin H, Guilbert I, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14(7):353–356 [DOI] [PubMed] [Google Scholar]
- 12. Hill JA, Anderson DJ, Polgar K. T helper 1-type cellular immunity to trophoblast in women with recurrent spontaneous abortions. J Am Med Assoc. 1955;273(24):1933–1958 [PubMed] [Google Scholar]
- 13. Orsi NM. Cytokine network in the establishment and maintenance of pregnancy. Hum Fertil. 2008;11(4):222–230 [DOI] [PubMed] [Google Scholar]
- 14. Piccinni MP, Beloni L, Live C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4(9):1020–1024 [DOI] [PubMed] [Google Scholar]
- 15. Hayakawa S, Fujikawa R, Fukuoka H, et al. Murine fetal resorption and experimental pre-eclampsia are induced by both excessive Th1 and Th2 activation. J Reprod Immunol. 2000;47(2):121–138 [DOI] [PubMed] [Google Scholar]
- 16. Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007;29(2):95–113 [DOI] [PubMed] [Google Scholar]
- 17. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610 [DOI] [PubMed] [Google Scholar]
- 18. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, and Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–215 [DOI] [PubMed] [Google Scholar]
- 19. Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J. Med 2000;342(20):1500–1507 [DOI] [PubMed] [Google Scholar]
- 21. Genc MR, Witkin SS, Delaney ML, et al. A disproportionate increase in IL-1beta over IL-1ra in the cervicovaginal secretions of pregnant women with altered vaginal microflora correlates with preterm birth. Am J Obstet Gynecol. 2004;190(5):1191–1197 [DOI] [PubMed] [Google Scholar]
- 22. Bloksma N, Heer E, Dihk H, Willers JM. Adjuvanticity of lactobacilli, 1.Differential effects of viable and killed bacteria. Clin Exp Immunol. 1979;37(2):367–375 [PMC free article] [PubMed] [Google Scholar]
- 23. Forsythe P, Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol Invest. 2010;39(4-5):429–448 [DOI] [PubMed] [Google Scholar]
- 24. Gill HS, Rutherfurd KJ, Prasad J., Gopal PK. Enhancement of natural and acquired immunity by Lactobacillus rhammosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr. 2000;83(2):167–176 [DOI] [PubMed] [Google Scholar]
- 25. Kim SO, Sheikh JI, Ha SD, Martins A, Reid G. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol. 2006;8(12):1958–1971 [DOI] [PubMed] [Google Scholar]
- 26. Harikrishnan R, Balasundaram C, Heo MS. Lactobacillus sakei BK19 enriched diet enhances the immunity status and disease resistance to streptococcosis infection in kelp grouper, Epinephelus Bruneus. Fish Shellfish Immunol. 2010;29(6):1037–1043 [DOI] [PubMed] [Google Scholar]
- 27. Reid G, Charbonneau D, Erb J, et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol Med Microbiol. 2003;35(2):131–134 [DOI] [PubMed] [Google Scholar]
- 28. Reid G, Burton J, Hammond JA, Bruce AW. Nucleic acid-based diagnosis of bacterial vaginosis and improved management using probiotic lactobacilli. J Med Food. 2004;7(2):223–228 [DOI] [PubMed] [Google Scholar]
- 29. Reid G, Bocking A. The potential for probiotics to prevent bacterial vaginosis and preterm labor. Am J Obstet Gynecol. 2003;189(4):1202–1208 [DOI] [PubMed] [Google Scholar]
- 30. Myhre R, Brantsaeter AL, Myking S, et al. Intake of probiotic food and risk of spontaneous preterm delivery. Am J Clin Nutr. 2011;93(1):151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeganegi M, Watson CS, Martins A, et al. Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: inplications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol. 2009;200(5):532–538 [DOI] [PubMed] [Google Scholar]
- 32. Yeganegi M, Leung CG, Martins A, et al. Lactobacillus rhamnosus GR-1 stimulates colony-stimulating factor 3 (Granulocyte) (CSF3) output in placental trophoblast cells in a fetal sex-dependent manner. Biol Reprod. 2011;84(1):18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fidel PL, Jr, Romero R, Ramirez M, et al. Interleukin-1 receptor antagonist (IL-1ra) production by human amnion, chorion, and deciduas. Am J Reprod Immunol. 1994;32(1):1–7 [DOI] [PubMed] [Google Scholar]
- 34. Dudley DJ, Edwin SS, Dangerfield A, Jackson K, Trautman MS. Regulation of decidual cell and chorion cell production of interleukin-10 by purified bacterial products. Am J Reprod Immunol. 1997;38(4):246–251, [DOI] [PubMed] [Google Scholar]
- 35. Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear Factor Kappa B Regulation of proinflammatory cytokines in human gestational tissues in vitro . Biol Reprod. 2002;67(2):668–673 [DOI] [PubMed] [Google Scholar]
- 36. Negishi M, Izumi Y, Aleemuzzaman S, Inaba N, Hayakawa S. Lipopolysaccharide (LPS)-induced Interferon (IFN)-gamma production by decidual mononuclear cells (DMNC) is interleukin (IL)-2 and IL-12P70 dependent. Am J Reprod Immunol. 2011;65(1):20–27 [DOI] [PubMed] [Google Scholar]
- 37. Butterfield LH, Potter DM, Kirkwood JM. Multiplex serum biomarker assessments: technical and biostatistical issues. J Transl Med. 2011;9:173–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved senstitivity and reliability. J Immunol Methods. 1986;89(2):271–277 [DOI] [PubMed] [Google Scholar]
- 39. Perera PY, Lichy JH, Waldmann TA, Perera LP. The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes Infect. 2012;14(3):247–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fortunato SJ, Menon R, Lombardi SJ. IL-15, a Novel Cytokine Produced by Human Fetal Membranes, Is Elevated in Peterm Labor. Am J Reprod Immunol. 1998;39(1):16–23 [DOI] [PubMed] [Google Scholar]
- 41. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162 [DOI] [PubMed] [Google Scholar]
- 42. Ito M, Nakashima A, Hidaka T, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84(1):75–85 [DOI] [PubMed] [Google Scholar]
- 43. Sato TA, Keelan JA, Mitchell MD. Critical paracrine interactions between TNFα and IL-10 regulate lipopolysacchride-stimulated human choriodecidual cytokine and prostaglandin E2 production. J Immunol. 2003;170(1):158–166 [DOI] [PubMed] [Google Scholar]
- 44. Koscik RJE, Li W, Martins A, et al. The effect of Lactobacillus rhamnosus GR-1 on amnion cytokine and chemokine production. Reprod Sci. 2011;18(4 suppl):F–236 [Google Scholar]
- 45. Simhan HN, Chura JC, Rauk PN. The effect of the anti-inflammatory cytokines interleukin-4 and interleukin-10 on lipopolysaccharide-stimulated production of prostaglandin E2 by cultured human decidual cells. J Reprod Immunol. 2004;64(1-2):1–7 [DOI] [PubMed] [Google Scholar]
- 46. Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765 [DOI] [PubMed] [Google Scholar]
- 47. Hartung T, Volk HD, Wendel A. G-CSF– an anti-inflammatory cytokine. Innate Immunity. 1995;2(3):195–201 [Google Scholar]
- 48. Sugita K, Hayakawa S, Karasaki-Suzuki M, et al. Granulocyte colony stimulation factor (G-CSF) suppresses interleukin (IL)-12 and/or IL-2 induced interferon (IFN)-γ production and cytotoxicity of decidual mononuclear cells. Am J Reprod Immunol. 2003;50(1):83–89 [DOI] [PubMed] [Google Scholar]
- 49. Solaroglu I, Cahill J, Tsubokawa T, Beskonakli E, Zhang JH. Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol Res. 2009;31(2):167–172 [DOI] [PubMed] [Google Scholar]
- 50. Clark DA, Chaouat G, Mogil R, Wegmann TG. Prevention of spontaneous abortion in DBA/2-matedCBA/J mice by GM-CSF involves CD8+ T cell-dependent suppression of natural effector cell cytotoxicity against trophoblast target cells. Cell Immunol. 1994;154(1):143–152 [DOI] [PubMed] [Google Scholar]
- 51. Laing KJ, Secombes CJ. Chemokines. Dev Comp Immunol. 2004;28(5):443–460 [DOI] [PubMed] [Google Scholar]
- 52. Nakayama T, Shirane J, Hieshima K, et al. Novel antiviral activity of chemokines. Virology. 2006;250(2):484–492 [DOI] [PubMed] [Google Scholar]
- 53. Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–1815 [DOI] [PubMed] [Google Scholar]
- 54. Liu M, Guo S, Hibbert JM, Jain V Singh N, Wilson NO, Stiles JK. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2001;22(3):121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]