Abstract
Nucleolar channel systems (NCSs), micron-sized organelles specific to nuclei of human endometrial epithelial cells (EECs), are robust markers of the midluteal phase under the apparent control of progesterone. To gain further insight into the role of progesterone in NCS formation, we quantitatively assessed their sensitivity to oral contraceptive pills (OCPs) using immunofluorescence-based detection of NCSs. Comparison of endometrial biopsies and serum progesterone levels on cycle day (CD) 10 and 20 (LH +6/7) of 6 naturally cycling women and 6 OCP users demonstrated that OCPs interfered with NCS formation on CD20, their natural peak presence. Although this confirmed prior observation based on electron microscopic sampling, OCPs unexpectedly induced limited but distinct amounts of NCSs already on CD10, when they are never present in natural cycles. Thus, OCPs can cause secretory changes in the endometrium during the proliferative phase. In a novel finding, robust NCS formation on CD20 was dependent on a 4 ng/mL progesterone threshold but did not correlate linearly with serum progesterone levels. Given the threshold being close to that serving as evidence for ovulation, NCSs can serve as ovulation markers.
Keywords: nucleolar channel system, endometrium, oral contraceptive pills, progesterone, endometrial receptivity
Introduction
Nucleolar channel systems (NCSs) are specific to healthy human endometrial epithelial cells (EECs) appearing transiently during the midluteal, receptive phase.1 They are unique spherical subcellular organelles of 1-μm diameter that, although consisting of membrane tubules, reside in the normally membrane-free cell nucleus.
Since their discovery in 1960 and for nearly 50 years since, NCS detection was limited to electron microscopy.2–4 Their small size, transient appearance, the lack of an animal model, and variable success to study NCSs in vitro5,6 led their investigation to fall by the wayside. Nevertheless, over the years, several studies braved the challenges of electron microscopic NCS detection describing occasionally inconsistent results, likely due to undersampling.7–14 Having identified molecular markers for NCSs allows their quantitative detection on a light microscopic level.1,15 In particular, using indirect immunofluorescence with monoclonal antibodies directed against a subset of nuclear pore complex proteins that are enriched in NCSs, we determined that the midluteal presence of NCSs is robust (about half of EECs harbor an NCS), their appearance is independent of fertility status, they are uniformly distributed throughout the upper uterine cavity, and they prefer the functional luminal layers of the endometrium.16,17
This apparently imperturbable though transient midluteal formation of NCSs prompted us to reevaluate their progesterone responsiveness. Several electron microscopy-based studies suggested a progesterone dependence of NCSs.5,18,19 Here, we used our light microscopic NCS detection method to test the hypothesis that oral contraceptive pills (OCPs), by lowering serum progesterone levels, would interfere with NCS formation and to report 2 unexpected novel results: OCPs can induce NCS formation in the follicular phase and robust midluteal NCS presence is dependent on a minimum threshold of serum progesterone.
Method
Participants
This prospective observational cohort study was approved by the institutional review board of the Albert Einstein College of Medicine. Informed consent was obtained from all participants. Women (age 18-43 years) with regular menses (23-36 days) were recruited in 2 cohorts: (1) naturally cycling women and (2) OCP users with a minimum of 2 completed OCP cycles. Exclusion criteria were current use of an intrauterine device, glucocorticoids 3 months or less prior to enrollment, ovarian masses, symptomatic endometriosis, submucosal myomas, endometrial polyps, and breastfeeding. Participants were seen for 2 appointments. At the first appointment (cycle day [CD] 9-11), ovarian and uterine abnormalities were ruled out by physical examination and hysterosonogram. Endometrial biopsy (EMB) was performed using a Pipelle (Cooper Surgical, Trumbull, Connecticut). Blood was drawn for hormonal evaluation. The naturally cycling group returned on LH +6/7. The OCP group returned on CD19 to 21. At the second appointment, EMB and blood draw were again performed. A third group of participants with characteristics similar to the other study participants (ie, age 21-32 years, regular 24-35 day cycles and no gynecologic pathology) consisted of 6 naturally cycling women recruited at East Coast Fertility, Plainview, New York, for a different study as described.20 Endometrial biopsies and blood draws were performed on LH +7 using the methods described previously. There was no significant difference between the groups regarding age (range 21-41), body mass index (18.6-44.5), and parity (6/18). The OCPs used in the study contained 3 types of synthetic progestins, 2 each of norethindrone, drospirenone, and norgestimate. All OCPs also contained estradiol in the following combinations: 1 mg norethindrone, 10 or 20 µg ethinyl estradiol (Lo Loestrin Fe or Loestrin Fe; Warner Chilcott, Rockaway, New Jersey); 3 mg drospirenone, 20 or 30 µg ethinyl estradiol (Yaz or Yasmin; Bayer HealthCare Pharmaceuticals, Inc, Wayne, New Jersey) and 0.18, 0.215, and 0.25 mg norgestimate, 35 µg ethinyl estradiol (Ortho Tri-Cyclen, Ortho-McNeil-Janssen Pharmaceuticals, Inc, Raritan, New Jersey). Patients using a variety of OCPs were recruited because the main purpose of the study was to manipulate endogenous progesterone levels that were monitored and were lowered independent of the formulation of the OCP. The OCP compliance was confirmed verbally at each appointment.
Processing and Immunostaining of Tissue Specimens
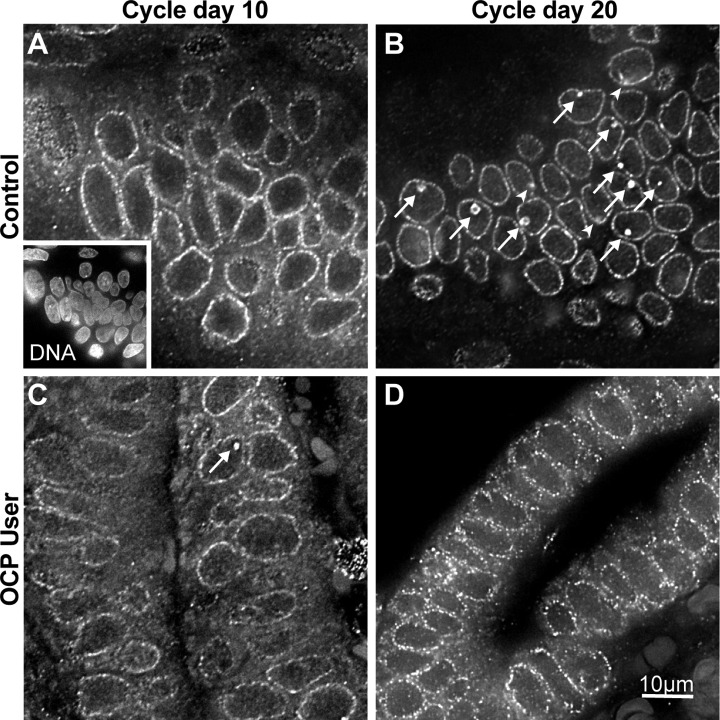
Endometrial biopsies were fixed in 4% paraformaldehyde diluted in 1× phosphate-buffered saline, paraffin embedded, sectioned (7 µm), and mounted on glass slides. Immunostaining was performed as described previously.17 Briefly, tissue sections were deparaffinized, rehydrated, and treated with 10 mmol/L sodium citrate (pH 6.0) for antigen retrieval. Nucleolar channel systems were detected by indirect immunofluorescence with monoclonal antibody 414 (1:5000, Covance, Princeton, New Jersey) and DyLight488-labeled secondary antibodies (1:500, Jackson ImmunoResearch, West Grove, Pennsylvania; Figure 1). The specificity of NCS labeling was confirmed by the following negative controls: lack of NCS staining in the absence of primary antibodies, when analyzing epithelial cells of different provenance or cycle phase and when labeling adjacent stromal cell nuclei, as described previously.1 Nuclei were identified by DNA staining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma, St Louis, Missouri; Figure 1A, inset).
Figure 1.
Detection of NCSs in human endometrial biopsies by indirect immunofluorescence. Examples of single optical planes (0.3 µm thick) from endometrial biopsies of the control (A and B) and the OCP user group (C and D) on CD10 (A and C) and CD20 (B and D) of paraffin sections stained with antibodies against a subset of nuclear pore complex proteins (note the punctate rim staining of the nuclear envelope outlining nuclei) that are enriched in NCSs (arrows and arrowheads in B and C). The inset in (A) shows a miniature of the same panel, double labeled for DNA, which was used to count EECs. Note the arrowheads in (B) point out NCSs that were confirmed in optical planes above or below but that are barely detectable in this plane, whereas others that are farther removed are not visible at all (but were counted). Moreover, NCSs stain much more brightly than the nuclear pore complexes (making them readily identifiable), although this is less obvious in this rendering, which was adjusted for clarity (to identify the EEC nuclei). Finally, each panel corresponds only to one-fourth of 1 of 10 fields that were analyzed for these and all other biopsies. CD indicates cycle day; EEC, endometrial epithelial cell; NCS, nucleolar channel system; OCP, oral contraceptive pill.
Imaging and Quantification of NCSs
All slides were analyzed by an observer who was blinded regarding the CD of biopsy and treatment of patient. The slides were imaged on a DeltaVision Core system (Applied Precision, Issaquah, Washington) as described.17 Briefly, 10 fields with endometrial glands were randomly selected from each specimen based on nuclear DNA staining with DAPI (Figure 1, inset; this ensured blindness to NCS presence). All EECs and NCSs within these 10 fields of each paraffin section were counted and summed. Endometrial epithelial cells were counted by their nuclei in DNA stain (Figure 1A, inset). Nucleolar channel systems were labeled and counted by manually scrolling through each 0.3 µm-thick optical plane of each of the 10 randomly selected fields to ascertain that they were truly spherical and not tubular in nature, that is, only in focus in 4 to 5 consecutive planes, allowing for some bleed through of signal from planes above and below (Figure 1B and C, arrows and arrow heads). Note each panel in Figure 1 represents only one-fourth of the field analyzed of a single optical plane of between 20 and 40 total for each of the 10 fields depending on the local thickness of the paraffin section. Overall, 2201 NCSs in 20 839 EEC nuclei were counted in 24 endometrial biopsies assessing between 571 and 1165 (834 ± 142, mean ± standard deviation [SD]) EEC nuclei in each. The output measure was NCS prevalence (percentage of NCSs per EECs) for each biopsy. We previously showed the method to be observer independent with agreement on NCS prevalence between 2 observers in 25 samples being excellent (r 2 = .99; linear regression).17 After our two recent publications,17,20 this is the third study that successfully applies this quantitative NCS detection methodology, documenting its reproducibility. For more details on the statistical evaluation of the approach see reference.17
Processing of Blood Specimens
Serum samples were analyzed for progesterone (ng/mL) and estradiol (pg/mL) using a commercially available solid-phase, competitive chemiluminescent enzyme immunoassay (Immulite 2000, Siemens, Erlangen, Germany).
Statistical Analyses
Mann Whitney, Student t, and Fisher exact tests were used for data analysis as appropriate (Prism 6, GraphPad Software, La Jolla, California).
Results
Nucleolar channel systems were identified in paraffin sections of human endometrial biopsies by their enrichment of a subset of related nuclear pore complex proteins (Figure 1B and C, arrows and arrowheads) and EECs counted by their nuclei (Figure 1A, inset). An example for each patient group and CD is depicted (Figure 1).
Natural CD10 biopsies contained no NCSs (median prevalence 0.0%, range 0.0-0.2), whereas 3 of the 6 CD10 biopsies from women taking OCPs exhibited some NCSs (2.7%, 2.1-4.8; Figure 2, CD10). Median serum P levels and range on CD10 for the corresponding participants (0.0, 0.0-0.6, and 0.0 ng/mL, 0.0-0.3, respectively) did not differ significantly. The CD10 biopsies that had NCSs corresponded to 2 of the 3 different progestins taken by the participants, drospirenone and norethindrone.
Figure 2.
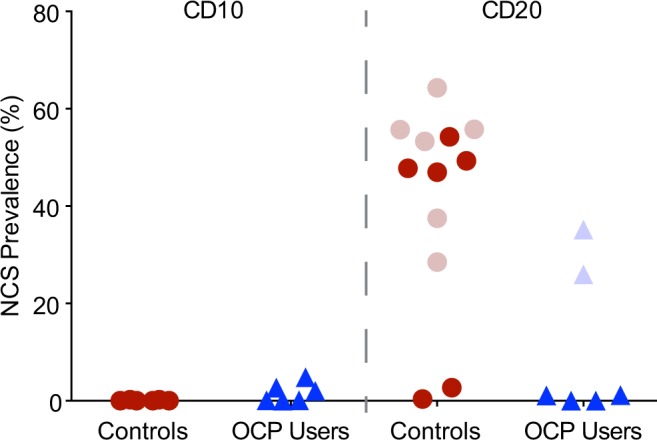
NCS prevalence in control participants and OCP users on CD10 and CD20. Control participants were recruited at Albert Einstein College of Medicine (dark dots) and East Coast Fertility (light dots). On CD10 they lacked NCSs but showed robust NCS prevalence on CD20 (LH +6/7), except for 2, which had correspondingly low serum P levels <4 ng/mL (see Figure 3). Of the 6 OCP users, (triangles; taking norethindrone, drospirenone, or norgestimate, 2 each, in combination with ethinyl estradiol) 3 exhibited some NCSs already on CD10 when they are never present in natural cycles. On CD20, the OCP users showed no or minimal NCS prevalence (dark triangles), except for 2, which had correspondingly high serum P levels, indicative of escape ovulatory events (light triangles). CD indicates cycle day; NCS, nucleolar channel system; OCP, oral contraceptive pill.
Of the natural CD20 biopsies, 10 had robust NCS prevalence (51.3%, 28.5-64.3) but 2 barely contained NCSs (0.4 and 2.7%; Figure 2, CD20). The patients with robust NCS prevalence exhibited increased serum P levels (8.9 ng/mL, 4.2-16.4) on CD20, whereas the 2 with minimal NCS prevalence had low serum P levels (2.5 and 3.5 ng/mL; Figure 3A, dots). Of the OCP cohort, 4 biopsies contained no or few NCSs on CD20 (0.6%, 0.0-1.2), but 2 exhibited robust NCS prevalence (26.0 and 35.2%; Figure 2, CD20). Serum P levels of the 4 participants with minimal NCS prevalence were low on CD20 (0.0 ng/mL, 0.0-1.9; Figure 3A, dark triangles), whereas the serum P levels of the other 2 were increased (4.4 and 7.9 ng/mL; Figure 3A, light triangles), indicative of escape ovulatory events. The NCS prevalence on CD20 did not linearly correlate with P levels but indicated a serum P threshold for robust NCS prevalence (4 ng/mL; Figure 3A, dashed line), irrespective of whether the biopsies were from natural cycle (dots) or OCP cohorts (triangles). Dichotomization of NCS prevalence on CD20 according to this P threshold was highly significant (0.89% ± 1.0% vs 46.21% ± 11.9; mean ± SD; P < .0001; Figure 3B).
Figure 3.
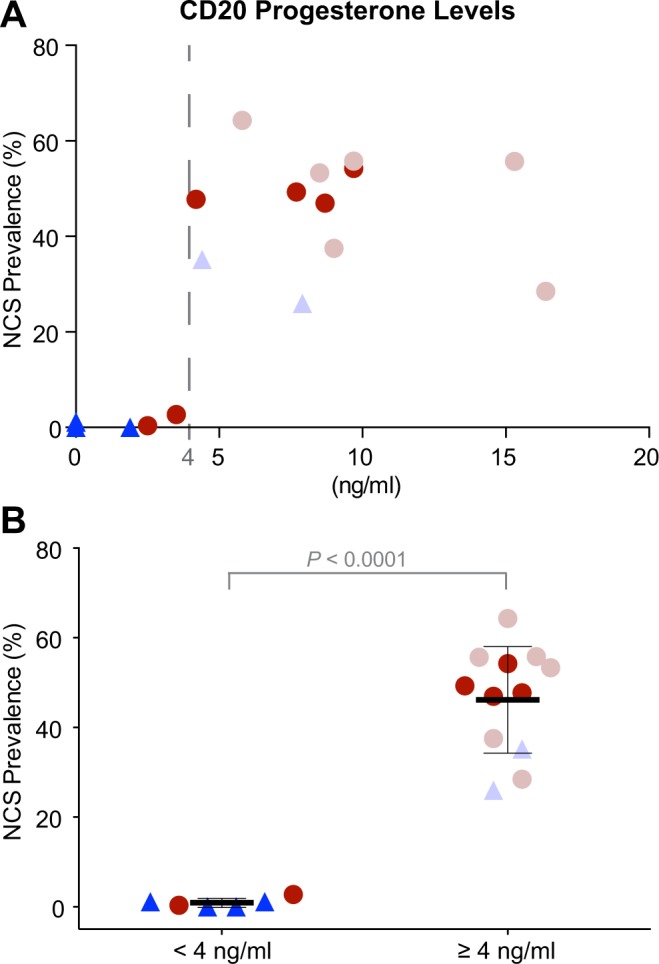
NCS prevalence versus serum P level on CD20. A, NCS prevalence of all CD20 biopsies is plotted against the serum P levels of the corresponding participants on that day. Note the low NCS prevalence below 4 ng/mL serum P levels but the robust NCS presence above that value (dashed line), irrespective of whether the biopsies are from control participants (dots) or OCP users (triangles). B, The data in (A) dichotomized according to the 4 ng/mL serum P level threshold, indicating the significant difference in NCS prevalence between the 2 groups. CD indicates cycle day; NCS, nucleolar channel system; OCP, oral contraceptive pill.
Discussion
Our data show that OCPs both interfere with robust NCS formation in the midluteal phase and induce NCSs in the follicular phase, when they are not normally present. Moreover, for the first time, we demonstrate that midluteal NCS appearance is dependent on a minimum serum progesterone level that marks a threshold for robust NCS formation.
The progesterone threshold for NCS formation (4 ng/mL) coincides nearly with that indicating ovulation (3 ng/mL).21 This finding together with the fact that midluteal NCS presence is robust, independent of fertility status, and uniform throughout the upper uterine cavity16,17 reveals NCSs as reliable histological indicators for ovulation in natural cycles.
Nucleolar channel system response to OCPs has been previously investigated based on the lower sampling detection method of electron microscopy. The OCPs used in those studies contained the following progesterone receptor antagonists, low-dose progestins, and high-dose estrogens: mifepristone (RU486), 13-ethyl-17-hydroxy-18,19-dinor-17alpha-pregna-4,9,11-trien-20-yn-3-one (R-2323), lynestrenol, megestrol, norethindrone, or ethynodiol with mestranol, and ethinyl estradiol followed by dimethisterone.11,22–26 Consistent with our study, all these regimens interfered with midluteal NCS formation supporting the hormone sensitivity of the NCS.
A surprising finding of our study was the minor but distinct induction of NCSs during the proliferative phase, when NCSs are not normally observed, in response to 2 of the 3 progestins employed. Follicular NCSs had previously only been reported in response to placement of an inert plastic intrauterine device (Lippes loop)26 and in 1 other study27 that since has been questioned.11 Apparently, the synthetic progestins, drospirenone, and norethindrone, which according to the manufacturers already after a single dose reach serum levels exceeding 4 ng/mL, contribute to the minimal follicular NCS presence. Nevertheless, we note that the serum level of these progestins is at least twice that on CD20 when they completely suppress NCS formation. Preovulatory induction of NCSs by synthetic progestins is similar to that reported in organ culture using endometrial tissue from the proliferative phase.5 In that system, NCS induction required an acyl group off carbon 17 of the steroid ring of the progestin.28 In contrast, neither of the 2 progesterone analogs that induced follicular NCSs in vivo in this study possesses an acyl group in the 17-β position. Similarly, NCS-inducing progestins used in hormone replacement therapy lacked the acyl group,18,29 although medroxyprogesterone induced NCSs in adenocarcinomas where they are not normally observed.1,30 Overall, it appears that synthetic progestins can induce follicular NCSs in vivo but that in vitro this ability is limited. Nevertheless, robust NCS induction occurs only in the luteal phase and requires endogenous progesterone.
Our study further supports the necessity of progesterone for NCS induction. Interestingly, there is no linear correlation between NCS prevalence and progesterone levels, but simply a progesterone threshold, above which NCSs form. However, whether that is sufficient for NCS induction remains to be seen—our recent study on NCS prevalence following controlled ovarian hyperstimulation suggests not.20
Acknowledgments
We are grateful to Dr David Kreiner for support of the study at East Coast Fertility. All samples were embedded and sectioned at the Histotechnology and Comparative Pathology Facility and imaged at the Analytical Imaging Facility of the Albert Einstein College of Medicine.
Footnotes
Authors’ Note: The research was conducted at Albert Einstein College of Medicine, Bronx, New York and East Coast Fertility, Plainview, New York.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The study was supported by the March of Dimes Birth Defects foundation (1-FY09-363 to U.T.M.); Ferring Pharmaceuticals, Parsippany, New Jersey, and East Coast Fertility, Plainview, New York (to GZ); and the CMBG Training Program (T32 GM007491 to MJS).
References
- 1. Guffanti E, Kittur N, Brodt ZN, et al. Nuclear pore complex proteins mark the implantation window in human endometrium. J Cell Sci. 2008;121(pt 12):2037–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dubrauszky V, Pohlmann G. Strukturveränderungen am Nukleolus von Korpusendometriumzellen während der Sekretionsphase. Naturwissenschaften. 1960;47(22):523–524 [Google Scholar]
- 3. Clyman MJ. A new structure observed in the nucleolus of the human endometrial epithelial cell. Am J Obstet Gynecol. 1963;86:430–432 [DOI] [PubMed] [Google Scholar]
- 4. Terzakis JA. The nucleolar channel system of human endometrium. J Cell Biol. 1965;27(2):293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kohorn EI, Rice SI, Gordon MM. In vitro production of nucleolar channel system by progesterone in human endometrium. Nature. 1970;228(5272):671–672 [DOI] [PubMed] [Google Scholar]
- 6. Luginbuhl WH. Electron microscopic study of the effects of tissue culture on human endometrium. Am J Obstet Gynecol. 1968;102(2):192–201 [DOI] [PubMed] [Google Scholar]
- 7. Gore BZ, Gordon M. Fine structure of epithelial cell of secretory endometrium in unexplained primary infertility. Fertil Steril. 1974;25(2):103–107 [DOI] [PubMed] [Google Scholar]
- 8. More IA, McSeveney D. The three dimensional structure of the nucleolar channel system in the endometrial glandular cell: serial sectioning and high voltage electron microscopic studies. J Anat. 1980;130(pt 4):673–682 [PMC free article] [PubMed] [Google Scholar]
- 9. Wynn RM, Woolley RS. Ultrastructural cyclic changes in the human endometrium. II. Normal postovulatory phase. Fertil Steril. 1967;18(6):721–738 [DOI] [PubMed] [Google Scholar]
- 10. Dockery P, Pritchard K, Warren MA, Li TC, Cooke ID. Changes in nuclear morphology in the human endometrial glandular epithelium in women with unexplained infertility. Hum Reprod. 1996;11(10):2251–2256 [DOI] [PubMed] [Google Scholar]
- 11. Spornitz UM. The functional morphology of the human endometrium and decidua. Adv Anat Embryol Cell Biol. 1992;124:1–99 [DOI] [PubMed] [Google Scholar]
- 12. Cornillie FJ, Lauweryns JM, Brosens IA. Normal human endometrium. an ultrastructural survey. Gynecol Obstet Invest. 1985;20(3):113–129 [DOI] [PubMed] [Google Scholar]
- 13. Dehou MF, Lejeune B, Arijs C, Leroy F. Endometrial morphology in stimulated in vitro fertilization cycles and after steroid replacement therapy in cases of primary ovarian failure. Fertil Steril. 1987;48(6):995–1000 [DOI] [PubMed] [Google Scholar]
- 14. Novotný R, Malínský J, Oborná I, Dostál J. Nuclear channel system (NCS) in normal endometrium and after hormonal stimulation. Acta Univ Palacki Olomuc Fac Med. 1999;142:41–16 [PubMed] [Google Scholar]
- 15. Kittur N, Zapantis G, Aubuchon M, Santoro N, Bazett-Jones DP, Meier UT. The nucleolar channel system of human endometrium is related to endoplasmic reticulum and R-rings. Mol Biol Cell. 2007;18(6):2296–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rybak EA, Szmyga MJ, Zapantis G, et al. The nucleolar channel system reliably marks the midluteal endometrium regardless of fertility status: a fresh look at an old organelle. Fertil Steril. 2011;95(4):1385–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szmyga MJ, Rybak EA, Nejat EJ, et al. Quantification of nucleolar channel systems: uniform presence throughout the upper endometrial cavity. Fertil Steril. 2013;99(2):558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pryse-Davies J, Ryder TA, MacKenzie ML. In vivo production of the nucleolar channel system in post menopausal endometrium. Cell Tissue Res. 1979;203(3):493–498 [DOI] [PubMed] [Google Scholar]
- 19. Roberts DK, Horbelt DV, Powell LC. The ultrastructural response of human endometrium to medroxyprogesterone acetate. Am J Obstet Gynecol. 1975;123(8):811–818 [DOI] [PubMed] [Google Scholar]
- 20. Zapantis G, Szmyga MJ, Rybak E, Meier UT. Premature formation of nucleolar channel systems indicates advanced endometrial maturation following controlled ovarian hyperstimulation. Hum Reprod. 2013;28(12):3292–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Israel R, Mishell DR, Stone SC, Thorneycroft IH, Moyer DL. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol. 1972;112(8):1043–1046 [DOI] [PubMed] [Google Scholar]
- 22. Dockery P, Ismail RM, Li TC, Warren MA, Cooke ID. The effect of a single dose of mifepristone (RU486) on the fine structure of the human endometrium during the early luteal phase. Hum Reprod. 1997;12(8):1778–1784 [DOI] [PubMed] [Google Scholar]
- 23. Azadian-Boulanger G, Secchi J, Laraque F, Raynaud JP, Sakiz E. Action of midcycle contraceptive (R 2323) on the human endometrium. Am J Obstet Gynecol. 1976;125(8):1049–1056 [DOI] [PubMed] [Google Scholar]
- 24. Feria-Velasco A, Aznar-Ramos R, González-Angulo A. Ultrastructural changes found in the endometrium of women using megestrol acetate for contraception. Contraception. 1972;5(3):187–201 [DOI] [PubMed] [Google Scholar]
- 25. van Santen MR, Haspels AA, Heijnen HF, Rademakers LH. Interfering with implantation by postcoital estrogen administration. II. Endometrium epithelial cell ultrastructure. Contraception. 1988;38(6):711–724 [DOI] [PubMed] [Google Scholar]
- 26. Wynn RM, Sawaragi I. Effects of intra-uterine and oral contraceptives on the ultrastructure of the human endometrium. J Reprod Fertil. 1969;suppl 8:45–57 [PubMed] [Google Scholar]
- 27. Feldhaus FJ, Themann H, Wagner H, Verhagen A. [Ultrastructural investigations on the nuclear channel system in the human endometrium [in German]. Arch Gynakol. 1977;223(3):195–204 [DOI] [PubMed] [Google Scholar]
- 28. Kohorn EI, Rice SI, Hemperly SS, Gordon MM. The relation of the structure of progestational steroids to nucleolar differentiation in human endometrium. J Clin Endocrinol Metab. 1972;34(2):257–264 [DOI] [PubMed] [Google Scholar]
- 29. Ryder TA, Mobberley MA, Whitehead MI. The endometrial nucleolar channel system as an indicator of progestin potency in HRT. Maturitas. 1995;22(1):31–36 [DOI] [PubMed] [Google Scholar]
- 30. Horbelt DV, Delmore JE, Parmley TH, Roberts DK, Walker N. The nuclear channel system in endometrial adenocarcinoma exposed to medroxyprogesterone acetate. Hum Pathol. 1996;27(1):9–14 [DOI] [PubMed] [Google Scholar]