Abstract
Over the past several years, there has been increasing recognition that pathogenesis of adhesion development includes significant contributions of hypoxia induced at the site of surgery, the resulting oxidative stress, and the subsequent free radical production. Mitochondrial dysfunction generated by surgically induced tissue hypoxia and inflammation can lead to the production of reactive oxygen and nitrogen species as well as antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase which when optimal have the potential to abrogate mitochondrial dysfunction and oxidative stress, preventing the cascade of events leading to the development of adhesions in injured peritoneum. There is a significant cross talk between the several processes leading to whether or not adhesions would eventually develop. Several of these processes present avenues for the development of measures that can help in abrogating adhesion formation or reformation after intraabdominal surgery.
Keywords: adhesion markers, hypoxia, postoperative adhesions, oxidative stress
Introduction
Oxidative stress is defined as an imbalance between the production of oxidants and the biological system’s inability to eliminate these oxidants by antioxidants. Oxidative stress generated reactive oxygen species (ROS) include superoxide (O2 •−), hydroxyl radical (HO•), hydrogen peroxide (H2O2), peroxynitrite (ONOO−), and hypohalous acids (HOX, where X = Cl–, Br–, I–, Br–, or SCN–); these are free radical molecules that are highly destructive to cellular functions.1,2 Under normal physiological conditions, eukaryotic cells are under aerobic conditions and have an innate defense system against ROS which contributes to the maintenance of the balance between prooxidants (free radical species) and the body’s scavenging ability (antioxidants). When an excess of ROS is produced or defective elimination of the excess ROS occurs, oxidative stress arises, during which ROS accumulate and damage important biomolecules such as nucleic acids, proteins, and lipids, as well as cells within the body.3 Under hypoxic conditions, the mitochondrial respiratory chain also produces nitric oxide (NO) that can generate reactive nitrogen species (RNS; ONOO−, nitrite [NO2 −], nitrate [NO3 −]), which further generate other reactive species such as reactive aldehydes—malondialdehyde and 4-hydroxynonenal—which can attack membrane lipids to initiate a free radical chain reaction known as lipid peroxidation (Figure 1). Reactive oxygen species and RNS are also known to contribute to vascular dysfunction and remodeling through oxidative damage by reducing the bioavailability of NO, impairing endothelium-dependent vasodilatation and endothelial cell growth, causing apoptosis, stimulating endothelial cell migration, and activating adhesion molecules.4 These functions are strictly linked with the mediators of the inflammatory pathways.5
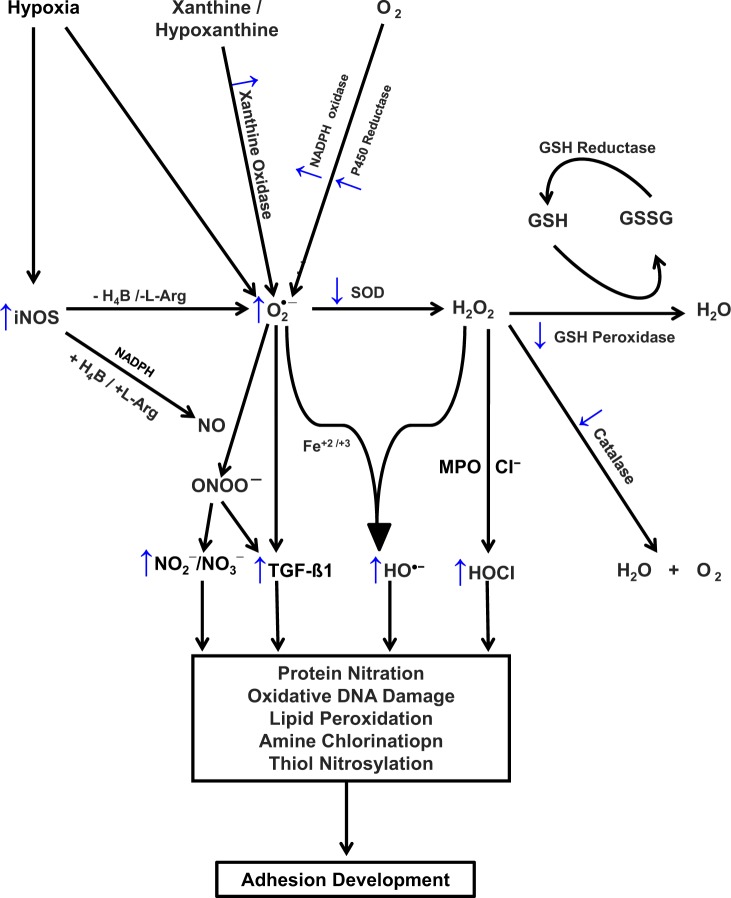
Figure 1.
Proposed scheme for redox balance in postoperative adhesions development. ↑ denotes an increase; ↓, a decrease; Cl−, chloride ion; Fe+2 and Fe+3, elemental iron; GSH, glutathione; GSSG, glutathione disulfide; H2O, water; H4B, tetrahydrobiopterin; H2O2, hydrogen peroxide; HOCI, hypochlorous acid; l-Arg, l-arginine; MPO, myeloperoxidase; O2, molecular oxygen; O2 •–, superoxide anion; HO•, hydroxyl radical; ONOO−, peroxynitrite; NADPH, nicotine adenine dinucleotide phosphate; NO, nitric oxide; NO2 −, nitrite; NO3 −, nitrate; iNOS, inducible nitric oxide synthase; ROS, reactive oxygen species; SOD, superoxide dismutase; TGF-β, transforming growth factor β.
In reproductive health, it is now known that imbalance of oxidative stress (excess ROS or depleted antioxidants) affects multiple physiological processes such as age-related decline in fertility,6 impairment in oocyte maturation, fertilization and embryo development,7–9 and pregnancy and normal parturition.10 In pathological states, derangement of the oxidative balance can lead to the improper activation of inflammatory changes, triggering complications such as premature labor, fetal growth restriction, and preeclampsia,11 development of endometriosis,12 and cancer progression.13 Although inflammatory processes, immunological and degenerative disorders, and cancer play a role in the production of free radicals, this review will focus on hypoxia-induced oxidative stress following intra-abdominal and pelvic surgery and subsequent adhesion development.
Postoperative adhesions represent tissue adherence at locations where there should be no such attachments. Specifically, abdominopelvic adhesions occur in 24% to 83% following cesarean and 55% to 100% after gynecological operations14–17 and are associated with potential devastating complications such as infertility,18 ectopic pregnancy,19 bowel obstruction,20 abdominal and pelvic pain,21 and difficult reoperation.22 The pathogenesis of oxidative stress-triggered postoperative adhesion development remains poorly understood.
Herein, we synopsize the available information regarding the role of oxidative stress in the development of abdominopelvic adhesions. This review addresses the pathological roles exerted by ROS and their scavenging systems in adhesion development and has 3 main objectives. First, we summarize the molecular processes involved in the generation of free radicals leading to oxidative stress. Second, we discuss the link between hypoxia, oxidative stress, and the pathophysiology of adhesion development. Third, we describe the possible role of antioxidants and enzymes that reduce oxidative stress and other important documented measures in the amelioration of adhesion development and describe their potential role in formulating strategies to decrease the burden of this often debilitating disease.
A Medline search was performed to identify articles published in English that deal with oxidative stress and adhesion development. Relevant articles dated up to February 2013 were selected and checked for previously unidentified articles. The following key words were used: (oxidative stress OR reactive oxidative species OR RNS) AND (Adhesions OR adhesion development) AND (abdominal or pelvic surgery). We reviewed all the available literature on oxidative stress, ROS and RNS, and their effect on adhesion development and also articles that described the mechanisms underlying these phenomena and how it can be prevented or reduced. We believe that such a review on this topic would be valuable and lead to a better understanding of how free radicals generated by oxidative stress serve as the “conductor” leading to the cascading activation of many proinflammatory pathways that promote adhesion development. A full understanding of the pathological mechanisms involved in adhesion development will be of benefit to the clinician who is challenged by patients at risk of postoperative adhesion development and the desire to reduce or eliminate their occurrence following intraabdominal surgery. With this understanding, the clinician can comprehend the hitherto mysterious but significant effects of oxidative stress, and other diverse influences, on adhesion development.
Molecular Processes Involved in the Generation of Free Radicals Leading to Oxidative Stress
Tissue injury, with its associated disruption of blood supply, results in deprivation of tissue perfusion and local hypoxia whereupon neutrophils and macrophages are recruited from the circulation and are activated to undergo a respiratory burst releasing O2 •− and NO.23 These reactive oxygen and nitrogen radicals are produced after oxygen supply interruption and/or restoration.3,24 The major intracellular sources of O2 •− are the electron transport chain in the mitochondria, the nicotinamide adenine dinucleotide dihydrophosphate (NADPH) oxidase, cytochrome P450 reductase system in the cellular plasma membrane,1,2 and the xanthine oxidase25 and NO synthase (NOS)26,27 enzyme systems (Figure 1).
Xanthine oxidase synthesizes O2 •− and is one of the major O2 •−-producing enzymes.25 McNally and his group28 used oscillatory shear stress to determine the enzymes responsible for the increased endothelial production of ROS. Xanthine-dependent O2 •− production was associated with decreased xanthine dehydrogenase (XDH) protein levels and enzymatic activity, resulting in an elevated ratio of xanthine oxidase (XO) to XDH. These authors also used endothelial cells lacking the p47phox subunit of the NADPH oxidase and found that inhibition of this enzyme with apocynin confirmed that NADPH oxidase maintains endothelial cell XO levels.28
Nitric oxide, also known as endothelium-derived relaxing factor, is a key biological messenger that is synthesized endogenously during conversion of arginine to citrulline in a process that required molecular oxygen and NADPH with tetrahydrobiopterin (H4B) acting as a cofactor. Bioregulatory NO is generated by 3 isoforms of NOS enzymes,26,27 namely, neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). Although the former 2 are calcium dependent for their regulation, iNOS is insensitive to endogenous calcium, likely due to its tight noncovalent interaction with calmodulin (CaM) and Ca2+. Thus, unlike eNOS and nNOS, iNOS is fully active in the absence of Ca2+ or added CaM29,30 and is insensitive to CaM inhibitors such as trifluoperazine. Inducible NOS is a homodimer and combines a carboxyl-terminal reductase domain homologs to the cytochrome P450 reductase and an amino-terminal oxygenase catalytic domain containing a heme prosthetic group, which is linked in the middle of the protein to a CaM-binding domain. Binding of CaM appears to act as a “molecular switch” to enable electron flow from flavin prosthetic groups in the reductase domain to heme. This facilitates the conversion of O2 and l-arginine (L-Arg) to NO and l-citrulline. The oxygenase domain also contains an H4B prosthetic group, which is required for the efficient generation of NO.31,32 In the absence of L-Arg and H4B, iNOS leads to the production of O2 •−.
The synthesis of NO can be inhibited by endogenously produced methylated analogs of arginine, which are competitive inhibitors of NOS, namely, asymmetric dimethyl arginine and monomethyl arginine. Nitric oxide may itself regulate iNOS expression and activity by acting in a negative feedback regulatory role on iNOS in a process referred to as S-nitrosylation. This process is regulated by cellular redox conditions and may provide a mechanism for the association between “oxidative stress” and macrophage dysfunction. Mitochondria, in addition to serving as a site of oxidative stress induction, is also highly susceptible to ROS. Inducible NOS has a protective role in host immunity, enabling its participation in antimicrobial33 and antitumor32 activities as part of the oxidative burst of macrophages.
Inbuilt into biological systems are enzymes that ameliorate the deleterious actions of ROS. One such enzyme is superoxide dismutase (SOD) that catalyzes the dismutation of O2 •− to H2O2, which is then utilized in combination with chloride ions (Cl−) by myeloperoxidase (MPO), a highly cationic heme protein that catalyzes the formation of potent oxidants such as cytotoxic hypochlorous acid (HOCl) and diffusible radical species34–36 (Figure 1). Hypochlorous acid is a potent oxidant which functions as a powerful antimicrobial agent and can also damage the host tissue by the same mechanism it uses to destroy invading pathogens.37 Additionally, there are other groups of enzymes such as glutathione (GSH) peroxidase, catalase (CAT), and indoleamine dioxygenase that help to protect cells from the toxic effects of H2O2 throughout the female reproductive tract.1,2,38,39 Catalase is a major H2O2 scavenger and acts by catalyzing its decomposition into molecular oxygen and water (Figure 1). In normal wound healing, there is decreased production and efficient elimination of free radicals and hence no oxidative DNA damage, protein nitration, or lipid peroxidation occur, thereby allowing normal healing.
A major pathway for NO removal is through near-diffusion controlled interaction with O2 •− yielding ONOO− (Figure 1).40 Peroxynitrite, like HOCl, is a powerful oxidant that can react with tyrosine residues to form the stable adduct nitrotyrosine.41,42 Hypochlorous acid also induces the production of transforming growth factor (TGF)-β1 and type 1 collagen, which are known markers of adhesion fibroblasts in vitro43 and its decay can be monitored by an increase in the NO2 ––NO3 – ratio.
Similar to other systems, ROS may be overproduced in injured peritoneal surfaces in response to several conditions such as surgical trauma, ongoing acute or chronic infections or inflammation, degenerative and autoimmune diseases, cancer, or radiation. On the other hand, a decrease in free radical scavengers may contribute to accumulation of ROS.44,45 Likewise, perturbation in the cellular mechanisms that eliminate the ROS may also result in accumulation of ROS. Such compromise could occur either by defect in the expression of genes encoding antioxidant enzymes46 or due to the deficiency of low-molecular-weight substances such as vitamin E (α-tocopherol), vitamin C, uric acid, GSH, taurine, hypotaurine, and albumin, all of which help scavenge free radicals.
In summary, therefore, oxidative stress represents an imbalance between increased O2 •− production, increased ROS and RNS production, and inefficient antioxidants such as scavenging (SOD) mechanism, reduced redox (GSH), and reduced CAT enzyme. Collectively, these result in failure to prevent increases in oxidative stress, resulting in the development of adhesions (Figure 1).
Tissue Injury, Hypoxia, Oxidative Stress, and Transcription Factors and Their Role in the Development of Postoperative Adhesions
Adhesions are known to develop as a response to hypoxia, whereby the body tries to reestablish oxygen and nutrient supply to tissues that have been injured by surgery or previous pathology.47 Tissue injury results in bleeding and the resulting local hypoxia perpetuates endothelial permeability48 with serosanguinous tissue exudation and leakage of lymphatic fluid, in addition to collection of blood, which occurs from transected vessels. Hypoxia resulting from injury acutely promotes O2 •− generation while the trauma itself disrupts stromal mast cells with release of vasoactive substances such as histamine and kinins, which enhance underlying fibroblasts migration and neutrophil recruitment to the wounded sites,49 as well as decreased fibrinolytic activity.50
The accumulation of red and white blood cells, macrophages, platelets, and tissue exudates forms a fibrinous mass, which, if persists, provides a fibrin matrix from which adhesion development occurs. A major determinant of persistence of the fibrinous mass is the degree of plasminogen activator activity (PAA), a process that is suppressed by local trauma. Plasminogen activator activity, which can be considered to be represented by the ratio of tissue plasminogen activator (tPA) to its inhibitor plasminogen activator inhibitor 1 (PAI-1), resides in both the peritoneal mesothelial cells and the underlying fibroblasts. If these cells are functioning normally, PAA persists, with dissolution of the fibrinous mass by fibrinolysis within 72 hours.51 Fibroblasts that begin to migrate to the site of tissue injury must then stop at the tissue surface. Concurrent with the fibroblast migration into the fibrinous mass is the deposition of mesothelial cells from the underlying mesenchyme, resulting in a monolayer mesothelial cell covering the injured site. Importantly, this process of remesotheliazation is thought to be completed in 3 to 5 days after tissue injury (unless there is persisting tissue injury). Therefore, during normal healing accumulation of red and white blood cells and platelets decreases while the activated fibroblasts are removed by apoptosis,52,53 allowing tissue to heal without inappropriate attachments to other tissues. However, if the ensuing local hypoxia persists, it initiates a series of gene signaling pathways that may result in alteration of the balance between extracellular matrix (ECM) deposition and degradation.54 Therefore, in the presence of tissue hypoxia, adjacent fibroblasts proliferate, become myofibroblasts, deposit collagen matrix, and migrate toward the site of tissue injury and into the persisting fibrinous mass overlying the injured tissue.
Use of a cell culture model has allowed the examination of the impact of hypoxia on cells involved with adhesion development. We have shown that exposure of normal peritoneal fibroblasts to hypoxia irreversibly induces TGF-β1 and type I collagen expression to levels seen in adhesion fibroblasts.55,56 Similarly, the stimulatory effect of hypoxia on procollagen messenger RNA (mRNA) levels in fibroblast monolayers was diminished by antibodies to TGF-β1.57 Transforming growth factor-β1 is a major profibrotic factor and has been described as a direct inductor of the myofibroblastic differentiation by controlling α-smooth muscle cell actin expression both in vitro58 and in vivo59 (Figure 2).
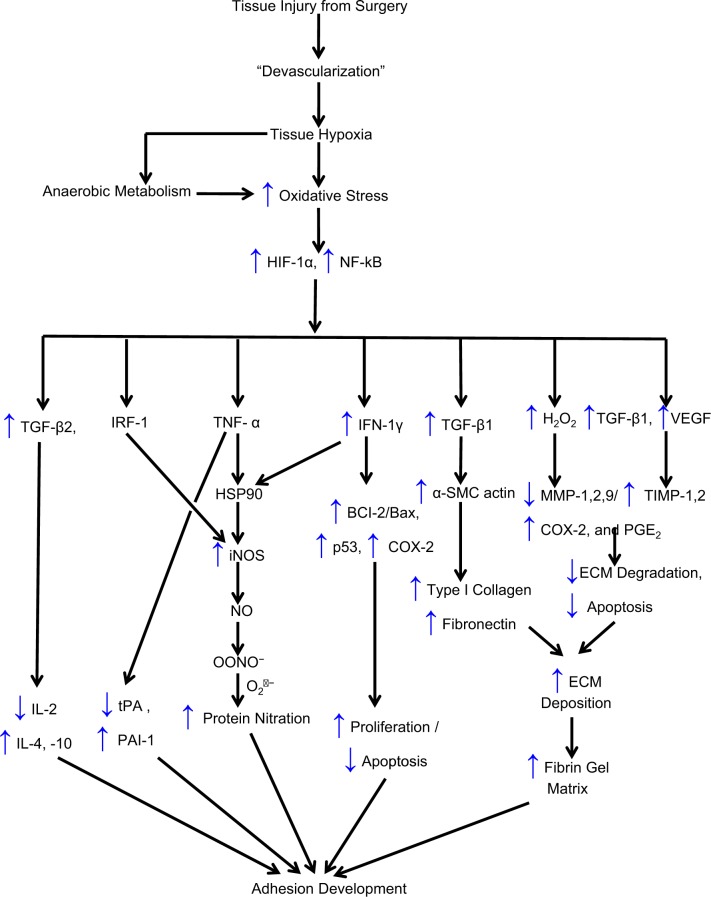
Figure 2.
Proposed chain of events that lead to postoperative adhesion development: tissue injury, hypoxia, oxidative stress, and transcription factors and their role in the development of postoperative adhesions. ↑ denotes an increase; ↓, a decrease; BCl-2, B-cell CLL/lymphoma 2; BAX, BCl2-associated X; COX-2, cyclooxygenase 2; ECM, extracellular matrix; HIF, hypoxia-induced factor; IFN-γ, interferon γ; IL, interleukin; iNOS, inducible nitrous oxide synthase; MMP, matrix metalloproteinases; NADP, nicotine adenine dinucleotide phosphate; NO, nitric oxide; NOS, nitric oxide synthase; NF-κB, nuclear factor κB; P53, tumor protein 53; PAI-1, plasminogen activator inhibitor; TGF-β1, transforming growth factor β; TIMP, tissue inhibitor of matrix metalloproteinases; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor.
Adhesion fibroblasts produce less NO than normal fibroblasts60 and hypoxia, through the production of O2 •−, causes normal fibroblasts to acquire the adhesion phenotype.43 The adhesion fibroblast phenotype manifests elevated expression of adhesiogenic factors such as increased production of adhesiogenic transcription factors and their protein (hypoxia-inducible factor [HIF]-1α,61 the nuclear factor-κB,62 increased B-cell leukemia/lymphoma 2 [BCl-2]/BCl2-associated X63), increased cytokine production (TGF-β1 and TGF-β2,55,64,65 interleukin [IL]-4 and IL-10,66 lipopolysaccharide, and tumor necrosis factor α [TNF-α])67, increased angiogenesis (increased vascular endothelial growth factor [VEGF]68), increased glycoprotein (α-smooth muscle actin59), increased components of the ECM (type I and type III collagen and fibronectin),69 increased expression of cyclooxygenase 2 (COX-2),70 reduced proteolytic enzymes production (decreased ratios of tPA/PAI-1,71 and matrix metalloproteinase [MMP] 1/tissue inhibitor of metalloproteinase [TIMP] 1),47,66 and higher protein nitration (Figure 2).
Studies have also implicated oxidative stress in the activation of MMPs.72 We have shown that decrease in MMP-1 and in the ratio of MMP-1/TIMP-1 occurs with resultant decrease in the ability to clear the injured site of ECM, leading to increase propensity for the development of adhesions. It has also been reported that H2O2 augments the production of MMP-1, COX-2, and prostaglandin E2 and the mechanism by which H2O2 affects these mediators was through enhancement of inhibitory NF-κBα degradation, with subsequent increases in NF-κB activation and NF-κB p50 translocation to the nucleus72 (Figure 2). In addition, evidence of an interferon regulatory factor 1 and NF-κB-dependent activation of the iNOS promoter supports an inflammation-mediated stimulation of the iNOS transcript. High-output iNOS usually occurs in an oxidative environment, allowing high levels of NO production to react with O2 •−, leading to ONOO− formation and resultant cell toxicity (Figures 2 and 3). Moreover, we have shown that the deficiency of the iNOS substrate, L-Arg, the cofactor H4B, or the molecular oxygen may lead to lower NO levels, which enhances the development of the adhesion phenotype.73–75 Adhesion fibroblasts also exhibit lower apoptosis and higher protein nitration than those of normal peritoneal fibroblasts, potentiating adhesion development.47,76 This mechanism involves caspase 3 S-nitrosylation, which we have previously reported to be significantly higher in adhesion fibroblasts than in normal peritoneal fibroblasts.76 We have also demonstrated similar changes in cytokine and ECM production in mesothelial cells and macrophages in response to hypoxia, which may further exaggerate the adhesion phenotype and facilitate adhesion development.69
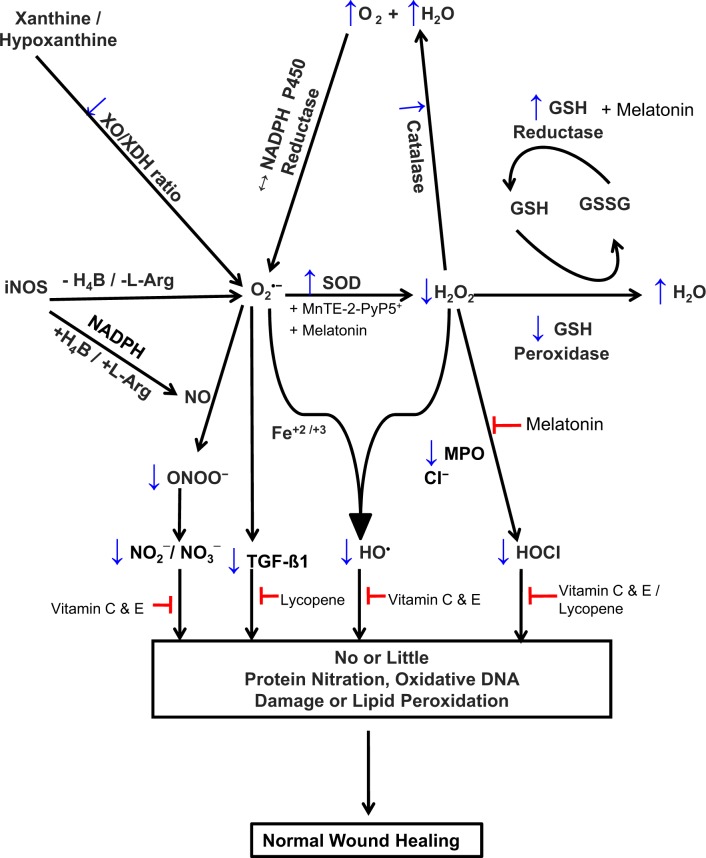
Figure 3.
Proposed scheme for potential targets for the prevention of postoperative adhesion development. ↑ denotes an increase; ↓, a decrease; Cl−, chloride ion; Fe+2 and Fe+3, elemental iron; GSH, glutathione; GSSG, glutathione disulfide; H2O, water; H4B, tetrahydrobiopterin; H2O2, hydrogen peroxide; HOCI, hypochlorous acid; l-Arg, l-arginine; MPO, myeloperoxidase; O2, molecular oxygen; O2 •–, superoxide anion; HO•, hydroxyl radical; ONOO−, peroxynitrite; NADPH, nicotine adenine dinucleotide phosphate; NO, nitric oxide; NO2 −, nitrite; NO3 −, nitrate; iNOS, inducible nitric oxide synthase; ROS, reactive oxygen species; SOD, superoxide dismutase; TGF-β, transforming growth factor β; XO, xanthine oxidase; XDH, xanthine dehydrogenase.
Oxidative stress also results in a build-up of intracellular ROS, resulting in mutations, inactivation or loss of mitochondrial DNA and synthesis, and accumulation of abnormal or oxidized proteins. Similarly, oxidative stress could change the membrane lipid composition, decrease the concentrations of ascorbic acid, cause a drop in the GSH–oxidized GSH (GSSG) ratio, and increase cytosolic Ca2+.77–80 There is increasing evidence to suggest that apart from NO, and O2 •−, lipid peroxidation produced under oxidative stress may also be associated with the development of postoperative adhesions47,81–83 (Figure 1). Under these conditions, the enzyme iNOS may play a potential role in accelerating adhesion development by serving as an O2 •−-generating system instead of producing NO, by causing steady state catalysis of NADPH oxidation.74,75 Increase in O2 •− production as a result of hypoxia may also significantly accelerate adhesion development through its rapid reaction with NO yielding ONOO– (Figure 1).43,84 Peroxynitrite is a much more toxic reagent and attacks many cellular components, reacting with thiols and iron–sulfur centers, initiating lipid peroxidation, and promoting protein nitration via reactions with peroxidases85,86 (Figure 1). Peroxynitrite also nitrates tyrosine by a reaction catalyzed by SOD.42 Peroxynitrite alone induces the production of TGF-β1 and type 1 collagen, both of which are among the phenotype markers of peritoneal adhesion fibroblasts in vitro.43,47
As mentioned previously, H2O2 generated by dismutation of O2 •− is utilized in combination with Cl− by MPO to form potent HOX such as cytotoxic HOCl and diffusible radical species34–36 through the formation of a ferryl π cation radical (E-Fe-(IV)—O+π) intermediate87–89 (Figure 1). Hypohalous acids are potent cytotoxic oxidants that directly oxidize reactive groups, including sulphydryls, iron–sulfur centers, and hemes, or react with amines-forming chloramines.1,34,90–93 Hypohalous acid is known to contribute to tissue damage and may mediate oxidative modification/fragmentation of normal fibroblasts through its ability to undergo numerous reactions with biomolecules, including aromatic chlorination, double bond addition, and chloramine formation. It also compromises antioxidant machinery, for example, by decreasing the GSH–GSSG ratio, which could affect optimal chromatin decondensation and consequently altered gene expression.40,94 The presence of Cl− enhances the catalytic activity of MPO and switches the enzyme activity from CAT to peroxidase-like activity. Recently, we have demonstrated that mammalian peroxidases (MPO, lactoperoxidase, and eosinophil peroxidase) may operate as alternative pathways for catalytic removal of NO91,92,94,95 with predisposition to adhesion development. In addition, the heme prosthetic groups of peroxidases accommodate a large variety of molecules as ligands of the Fe cation, causing enzyme inhibition.91,92,94,96,97 Thus, mammalian peroxidases may accelerate adhesion development through more than 1 pathway such as by generating HOCl, by consuming NO, or by mediating protein nitration, lipid peroxidation, amine chlorination, and thiol nitrosylation98–100 (Figures 1 and 2). Midwinter and collaborators101 found that HOCl is also able to activate mitogen-activated protein kinase (MAPK), but not Jun kinase (JNK), in human fibroblasts suggesting that the development of an adhesion fibroblasts may occur via MAPK signaling. These authors also found that effective doses of HOCl were considerably lower than with H2O2 and the lack of JNK activation contrasts with the activation frequently seen with H2O2.101
Hydrogen peroxide is freely diffusible through biological membranes, and its overproduction is extremely destructive to tissues and have the ability to react with cellular constituents, including amino acid residues, purine and pyrimidine bases of DNA, and membrane lipids, to initiate lipid peroxidation.1,102,103 Hydrogen peroxide may also react with O2 •− and is physiologically important both because it is the precursor of the more toxic HO• radical and because its lifetime in the intracellular space is relatively long. Hydroxyl radicals may also be generated through Fenton-type chemistry and Fe catalyzes its production from H2O2.104 Hydroxyl radicals can extract hydrogen atoms from DNA and RNA, causing mutations or cleavage of the phosphodiester backbone105,106 predisposing to adhesion formation following tissue damage (Figure 1).
Associated with tissue injury and adhesions is the anaerobic metabolism which is in part necessitated by the lack of oxygen supply for oxidative metabolism through the Kreb cycle. Consequently, adhesions manifest collection of lactic acid with a tendency for lowering of tissue pH, which if severe could interfere with protein and enzyme functions. If this was true, stimulation of aerobic metabolism would reverse this process and prevent adhesion development. Dichloroacetic acid (DCA) stimulates the pyruvate dehydrogenase complex that causes pyruvate to be metabolized in the Kreb cycle rather than being converted into lactate, thereby switching anaerobic to aerobic metabolism. Experiments using DCA should therefore reverse the oxidative stress changes that are associated with adhesion development. We have shown that DCA increases tPA–PAI-1 ratios, which favor the development of a fibrinolytic milieu that would be expected potentially to create an environment likely to be less favorable to postoperative adhesion development.107
As the developing adhesion continues to be relatively hypoxic, there is continuing production of prooxidants, such as O2 •− and NO, which maintain continuous signaling by HIF-1α61 and the NF-κB family of proteins,62 which continue to stimulate the cascading pathways (Figure 2). This includes continued increased expression of TGF-β1, TNF-α, and IL-6 as well as increased expression of VEGF. Of note, this enhancement of VEGF expression is also associated with neoangiogenesis, which develops within the now established adhesion.
Possible Role of Enzymes, Polypeptides, and Antioxidants in the Prevention of Adhesion Development and Their Potential Role in Formulating Strategies to Decrease the Burden of This Disease
To prevent adhesion development, one has to appreciate that it takes 3 to 5 days after surgical injury for peritoneal repair (eg, remesotheliazation) to be completed. Therefore, approaches to reduce postoperative adhesions (including mode of entry into the abdomen, surgical instrumentation, and antiadhesion adjuvants) need to affect peritoneal repair processes during this early short-time interval. In addition, the observation that adhesions become denser and more vascular over a time interval of 1 to 2 years is consistent with the concept that the adhesion continues to be relatively hypoxic over this time period, with increasing vascularity as a teleological effort of the body to reestablish the supply of oxygen and nutrients to these tissues.
There are several possible ways to reduce adhesion development. First, traditionally most surgeons recognize the value of the principles of microsurgical technique that emphasizes the importance of tissue handling, air drying of exposed tissues, good hemostasis, and peritoneal raw areas as precursors of later adhesion formation. Second, is the use of scavengers of ROS and RNS such as SODs and measures that could induce the phenotypic reversion of adhesion fibroblasts to normal fibroblasts. Third, is the use of other antioxidant proteins, such as CAT, GSH, and peroxidases. Fourth, is utilization of nonenzymatic compounds such as tocopherol, β-carotene, lycopene, vitamin E and C, and ascorbate in mammals including humans. Fifth, are measures that could increase degradation of the ECM and promote apoptosis of adhesion fibroblasts. Sixth, are measures that modulate the immune response to peritoneal injury. Seventh, is the use of barriers to separate adjacent denuded areas that persist until healing is completed.
Improved surgical techniques outlined by the use of microsurgical principles can reduce postoperative surgical adhesions.108,109 These techniques are incooperated as a matter of course in laparoscopic pelvic and abdominal surgery. Therefore, laparoscopic surgery is often theorized by its nature to cause less adhesion than laparotomy because it has the potential to fulfill the requirements prescribed by microsurgical technique.110 Nevertheless, the role of laparoscopy in adhesion development and prevention is less well clear with some suggesting that laparoscopy surgery reduces adhesion development111 while others disagree.112,113 Furthermore, laparoscopy is often incorrectly thought to be associated with less tissue drying. In fact, Ott114 has shown electron microscopic damage from tissue drying during laparoscopy, especially with the use of high velocity flow and dry cold gas. Also, insufflations alone have been shown to cause induction of adhesion development in mice by Yesildaglar and collaborators,115 in both a time and flow dependent manner.
We have shown that scavenging O2 •– with SOD, even in the presence of hypoxia, prevented the development of the adhesion phenotype in vitro.43 There is evidence in the literature to suggest that SODs exhibit potent antiinflammatory properties116 and could potentially be used to prevent the development of adhesions. Consistent with this hypothesis, SOD has been shown to have a therapeutic effect on radiation-induced fibrosis,117 an effect that could be related to downregulation of TGF-β1 expression and activity.118 In addition, all isoforms of SODs (SOD1, SOD2, and SOD3) have been shown to induce 70% regression of established fibrotic tissue and its replacement by regenerated normal tissue.117 Transforming growth factor β provides a strong inhibitory signal to iNOS whereas IL-4 and IL-10 provide weak inhibitory signals. In this way, the immune system may regulate the armamentarium of phagocytes that play a role in inflammation and immune responses. In vitro experiments in our laboratory on primary cultures of fibroblasts isolated from normal peritoneal and adhesion tissues43 to evaluate the antifibrotic and/or antioxidant potential of SOD and its action on TGF-β1 and type I collagen expression showed that normal peritoneal fibroblasts treated with SOD under hypoxic conditions had no change in these adhesion markers, while adhesion fibroblasts did not have a further increase in these markers as previously seen after hypoxia treatment alone.43,55 MnTE-2-PyP5+ is a catalytic metalloporphyrin antioxidant with SOD mimetic properties that affects transcription factor activation by eliminating ROS signaling molecules.119,120 It has been shown that MnTE-2-PyP5+ is a potent scavenger of ROS and RNS, particularly O2 •– and ONOO−,119,120 and it reduces hypoxia-induced O2 •–, TGF-β, and VEGF production by macrophages in vitro119,120 and in vivo121 (Figure 3).
Apart from SOD, intracellular ROS levels are kept under tight control by enzymatic activities of other antioxidant proteins, such as CAT, GSH, and peroxidases, as well as by nonenzymatic compounds such as tocopherol, β-carotene, vitamin E, ω-3 fatty acid and ascorbate,122–124 and by the action of low-efficiency ROS scavengers like free amino acids, peptides, and proteins125 (Figure 3). Vitamin C-dependent inhibition of the HIF pathway may provide alternative or additional approaches for controlling inflammation and hence adhesion development.126 Vitamin C also plays a role in the function of eNOS by recycling the eNOS cofactor, H4B.126 As an antioxidant, vitamin C provides protection against oxidative stress-induced cellular damage by scavenging ROS, by vitamin E-dependent neutralization of lipid hydroperoxyl radicals, and by protecting proteins from alkylation by electrophilic lipid peroxidation products.126 Lycopene belongs to the family of carotenoids found in tomatoes and although known to be an efficient free radical scavenger, the exact mechanism by which it accomplishes its scavenging function has only recently been addressed.127 Work in our laboratory has shown that lycopene reduces mRNA levels of adhesion-related markers such as type I collagen, TGF-β1, and VEGF.128 In addition, we have shown that lycopene exerts its antioxidant effect in vitro by acting as a potent scavenger of HOCl.129
Given that postoperative oxidative stress is linked to neutrophil recruitment49 and decreased fibrinolytic activity,50 and subsequently the development of intraabdominal adhesions, antioxidants, through the reduction in levels of oxidative stress and increasing PAA and MMP activities postoperatively, may contribute to reduction in adhesion development.130 Consistent with this hypothesis, in the rat model experiments, antioxidants such as methylene blue,50,131 indigo carmine,132 pentoxifylline,133 and neurokinin 1 receptor CJ-12-255 (Pfizer) antagonist49 have been shown to inhibit postoperative adhesion development.
Modulation of the immune response to peritoneal injury may modify postoperative adhesion formation. Polypeptides, such as interferon (IFN) α and IFN-γ, relaxin, and hepatocyte growth factor, all show an ability to limit fibrogenesis in either clinical or experimental settings. Several in vitro and in vivo findings ascertain the antifibrogenic effect of IFN-γ. For example, IL-10 inhibits IFN-γ expression in most cell systems, and adhesion tissues have been shown to contain a significantly lower amount of IFN-γ and a higher amount of IL-10 as compared with normal peritoneal tissue.134 Our group has shown that expression of IFN-γ was significantly reduced with the simultaneous increase in IL-10 expression in adhesion fibroblasts under hypoxic conditions.66 We have also shown that IFN-γ can block the effects of hypoxia on apoptosis.135 In addition, IFN-γ treatment of both adhesion and normal fibroblasts resulted in a dose-response decrease in type I collagen and fibronectin mRNA levels and hypoxia had no effect on type I collagen and fibronectin mRNA levels in the presence of increasing dose of IFN-γ in both cell types.136 Collectively, these suggest that IFN-γ is able to block the stimulating effects of hypoxia on type I collagen and fibronectin, supporting its antifibrogenic effect. Although the mechanisms by which exogenous IFN-γ treatment inhibits hypoxia-induced type I collagen synthesis in normal peritoneal and adhesion fibroblasts remain to be determined, Yamanaka and collaborators137 showed that IFN-γ modulation of the Smads 2-4 pathway of TGF-β1 signal transduction prevent the upregulation of ECM components following ocular surgery. This suggests that IFN-γ may overcome the upregulation of the production of ECM by TGF-β in vivo in fibroblasts and would be a good candidate for consideration for intervention in the prevention of peritoneal adhesions.
Melatonin or 5-methoxy-N-acetyltryptamine is a neurohormone secreted by the pineal gland in the brain and is synthesized from the amino acid tryptophan. It plays an important role in the regulation of various body functions including circadian sleep rhythms, blood pressure, seasonal reproduction, and immunity. It has been demonstrated that melatonin in the presence of Cl− serves as a potent inhibitor of MPO under physiological-like conditions by modulating the formation of MPO intermediates and their decay rates138 (Figure 3). It has also been shown that MPO has 2 halide-binding sites that have a distinct impact on its heme iron microenvironment and its inhibition requires the occupation of both MPO binding sites, 1 site with Cl− and the other site with melatonin.138,139 Thus, the presence of Cl− enhanced the affinity of MPO toward melatonin, which switches the enzyme activity from peroxidation to CAT-like activity.138 The inhibition of MPO and its downstream inflammatory byproducts could be attractive targets in the development of therapeutic interventions for the prevention of postoperative adhesions.
These aforementioned studies indicate that scavenging free radicals with SOD mimetics, use of dietary supplements such as ω-3 fatty acids, vitamin C, lycopene, and melatonin, or increasing fibrinolytic activity of MMP and PAA as well as modulation of the immune response with IFN-α and IFN-γ mimetics may theoretically be useful in the development of new therapeutic strategies for preventing the adhesion formation and reformation after surgery. Finally, Saed et al140 transfected normal and adhesion fibroblasts with an adenovirus COX-2 mRNA in sense or antisense orientation and were able to demonstrate that adhesion fibroblasts transfected with COX-2 antisense exhibited markedly reduced mRNA levels of type I collagen, type III collagen, fibronectin, and TGF-β1 suggesting that inhibition of COX-2 may reduce the development of postoperative adhesions by preventing the formation of the adhesion phenotype.
Conclusion
A discrete and modifiable parameter such as oxidative stress represents a potential compelling upstream factor of injured peritoneum, which may have downstream implications for adhesion development. Increase in ROS and subsequent oxidative stress are potential candidates responsible for the initiation of adhesion development. Enhancement in ROS in injured peritoneum is associated with a modification in proteins and other moieties crucial for maintaining viability and integrity of various organelles and cytoskeleton and thus may affect their activity, organization, and distribution. It is also associated with a significant decrease in NO bioavailability, which subsequently disturbs the intracellular signal transduction pathways. Finally, O2 •–, H2O2, HO•, and HOCl significantly enhanced adhesion development. Although some measures identified to reduce adhesions may be able to be administered systemically leading to biological modification of the cascade of events that leads to adhesions, it is likely that local delivery, such as with an antiadhesion adjuvant that has its own inherent ability to reduce postoperative adhesions as well as acting as a physical barrier to separate tissues during the early phase of healing,141 will allow for prolonged delivery during the healing process, thereby improving efficacy. The adhesion prevention material that acts as a physical barrier must remain in place during remesothelization to prevent migration of the proliferating fibroblasts at the tissue edge. This process theoretically forces remesothelization to occur only over the injured surfaces without adhesion development bridging adjacent tissue surfaces.
From available studies, detailed biological information is enlightening our understanding of the pathogenesis of adhesion development and the role of oxidative stress. There remain important deficits in our knowledge about this disorder; and again, these have been highlighted. New therapeutic strategies are emerging but, as yet, require longer evaluation before being introduced into routine practice. Understanding the mechanisms underlying the process of adhesion development is crucial in designing targeted therapy that could help in the prevention of adhesion development following gynecological surgery. These targets could be the subjects of future randomized studies on the prevention of adhesion development. In the meantime, surgeon should focus on ensuring meticulous hemostasis during surgery, avoid air drying of exposed tissues, and use microsurgical techniques whenever possible.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work is supported in part by NIH/NICHD grant number-K12HD001254.
References
- 1. Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9(3):338–347 [DOI] [PubMed] [Google Scholar]
- 2. Halliwell BG, John Gutteridge, eds. Free Radicals in Biology and Medicine. Oxford: Clarendon; 1989:1–20 [Google Scholar]
- 3. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84 [DOI] [PubMed] [Google Scholar]
- 4. Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6(1):1–19 [DOI] [PubMed] [Google Scholar]
- 5. Crimi E, Ignarro LJ, Napoli C. Microcirculation and oxidative stress. Free Radic Res. 2007;41(12):1364–1375 [DOI] [PubMed] [Google Scholar]
- 6. Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility—a clinician's perspective. Reprod Biomed Online. 2005;11(5):641–650 [DOI] [PubMed] [Google Scholar]
- 7. Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124(6):745–754 [DOI] [PubMed] [Google Scholar]
- 8. Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med. 2008;44(7):1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goud AP, Goud PT, Diamond MP, Abu-Soud HM. Nitric oxide delays oocyte aging. Biochemistry. 2005;44(34):11361–11368 [DOI] [PubMed] [Google Scholar]
- 10. Rizzo A, Roscino M, Binetti F, Sciorsci R. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 2012;47(2):344–352 [DOI] [PubMed] [Google Scholar]
- 11. Clerici G, Slavescu C, Fiengo S, et al. Oxidative stress in pathological pregnancies. J Obstet Gynaecol. 2012;32(2):124–127 [DOI] [PubMed] [Google Scholar]
- 12. Yildirim G, Attar R, Ozkan F, Kumbak B, Ficicioglu C, Yesildaglar N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: a preliminary study. Fertil Steril. 2010;93(6):1787–1792 [DOI] [PubMed] [Google Scholar]
- 13. Taddei ML, Giannoni E, Raugei G, et al. Mitochondrial oxidative stress due to complex I dysfunction promotes fibroblast activation and melanoma cell invasiveness. J Signal Transduct. 2012;2012:684592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diamond MP. Surgical aspects of infertility. In: Sciarra JW, ed. Gynecology and Obstetrics. Vol 2. Philadelphia: Harper & Row; 1988:1–23 [Google Scholar]
- 15. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. seprafilm adhesion study group. Fertil Steril. 1996;66(6):904–910 [PubMed] [Google Scholar]
- 16. Diamond MP. Incidence of postsurgical adhesions. In: GS d diZerega, ed. Peritoneal Surgery. New York: Spriger-Verlag; 2000:217–220 [Google Scholar]
- 17. Awonuga AO, Fletcher NM, Saed GM, Diamond MP. Postoperative adhesion development following cesarean and open intra-abdominal gynecological operations: a review. Reprod Sci. 2011;18(12):1166–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vrijland WW JJ, van Geldorp HJ, Swank DJ, Bonjer HJ. Abdominal adhesions: intestinal obstruction, pain, and infertility. Surg Endosc. 2003;17(7):1017–1022 [DOI] [PubMed] [Google Scholar]
- 19. Marana R, Catalano GF, Muzii L. Salpingoscopy. Curr Opin Obstet Gynecol. 2003;15(4):333–336 [DOI] [PubMed] [Google Scholar]
- 20. Barmparas G, Branco BC, Schnuriger B, Lam L, Inaba K, Demetriades D. The incidence and risk factors of post-laparotomy adhesive small bowel obstruction. J Gastrointest Surg. 2010;14(10):1619–1628 [DOI] [PubMed] [Google Scholar]
- 21. Duffy DM, diZerega GS. Adhesion controversies: pelvic pain as a cause of adhesions, crystalloids in preventing them. J Reprod Med. 1996;41(1):19–26 [PubMed] [Google Scholar]
- 22. Holmdahl L, Risberg B. Adhesions: prevention and complications in general surgery. Eur J Surg. 1997;163(3):169–174 [PubMed] [Google Scholar]
- 23. Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117(5):701–708 [DOI] [PubMed] [Google Scholar]
- 24. Terada LS, Guidot DM, Leff JA, et al. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci U S A. 1992;89(8):3362–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fridovich I. The biology of oxygen radicals. Science. 1978;201(4359):875–880 [DOI] [PubMed] [Google Scholar]
- 26. Yamamoto Y, Konig P, Henrich M, Dedio J, Kummer W. Hypoxia induces production of nitric oxide and reactive oxygen species in glomus cells of rat carotid body. Cell Tissue Res. 2006;325(1):3–11 [DOI] [PubMed] [Google Scholar]
- 27. Zhu H, Bunn HF. Oxygen sensing and signaling: impact on the regulation of physiologically important genes. Respir Physiol. 1999;115(2):239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McNally JS, Davis ME, Giddens DP, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285(6):H2290–H2297 [DOI] [PubMed] [Google Scholar]
- 29. Stuehr DJ, Cho HJ, Kwon NS, Weise MF, Nathan CF. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991;88(17):7773–7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Kawai C. Purification of nitric oxide synthase from rat macrophages. J Biol Chem. 1991;266(19):12544–12547 [PubMed] [Google Scholar]
- 31. Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Annu Rev Pharmacol Toxicol. 1997;37:339–359 [DOI] [PubMed] [Google Scholar]
- 32. Chinje EC, Stratford IJ. Role of nitric oxide in growth of solid tumours: a balancing act. Essays Biochem. 1997;32:61–72 [PubMed] [Google Scholar]
- 33. MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81(4):641–650 [DOI] [PubMed] [Google Scholar]
- 34. Kettle AJ, van Dalen CJ, Winterbourn CC. Peroxynitrite and myeloperoxidase leave the same footprint in protein nitration. Redox Rep. 1997;3(5-6):257–258 [DOI] [PubMed] [Google Scholar]
- 35. Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. evidence for hypochlorous acid generation. J Clin Invest. 1982;70(3):598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tahboub YR, Galijasevic S, Diamond MP, Abu-Soud HM. Thiocyanate modulates the catalytic activity of mammalian peroxidases. J Biol Chem. 2005;280(28):26129–26136 [DOI] [PubMed] [Google Scholar]
- 37. Pullar JM, Vissers MC, Winterbourn CC. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life. 2000;50(4-5):259–266 [DOI] [PubMed] [Google Scholar]
- 38. Pascoe GA, Fariss MW, Olafsdottir K, Reed DJ. A. role of vitamin E in protection against cell injury. Maintenance of intracellular glutathione precursors and biosynthesis. Eur J Biochem. 1987;166(1):241–247 [DOI] [PubMed] [Google Scholar]
- 39. Harrison FE, Meredith ME, Dawes SM, Saskowski JL, May JM. Low ascorbic acid and increased oxidative stress in gulo(-/-) mice during development. Brain Res. 2010;1349:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240 [DOI] [PubMed] [Google Scholar]
- 41. Akaike T, Suga M, Maeda H. Free radicals in viral pathogenesis: molecular mechanisms involving superoxide and NO. Proc Soc Exp Biol Med. 1998;217(1):64–73 [DOI] [PubMed] [Google Scholar]
- 42. Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54(4):619–634 [DOI] [PubMed] [Google Scholar]
- 43. Fletcher NM, Jiang ZL, Diamond MP, Abu Soud HM, Saed GM. Hypoxia-generated superoxide induces the development of the adhesion phenotype. Free Radic Biol Med. 2008;45(4):530–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Szczepanska M, Kozlik J, Skrzypczak J, Mikolajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril. 2003;79(6):1288–1293 [DOI] [PubMed] [Google Scholar]
- 45. Van Langendonckt A, Casanas Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002;77(5):861–870 [DOI] [PubMed] [Google Scholar]
- 46. El Mouatassim S, Guerin P, Menezo Y. Mammalian oviduct and protection against free oxygen radicals: expression of genes encoding antioxidant enzymes in human and mouse. Eur J Obstet Gynecol Reprod Biol. 2000;89(1):1–6 [DOI] [PubMed] [Google Scholar]
- 47. Saed GM, Diamond MP. Molecular characterization of postoperative adhesions: the adhesion phenotype. J Am Assoc Gynecol Laparosc. 2004;11(3):307–314 [DOI] [PubMed] [Google Scholar]
- 48. Li YQ BJ, Nordal RA, Su ZF, Wong CS. Hypoxia in radiation-induced blood spinal cord barrier breakdown. Cancer Res Treat. 2000;61(8):3348–3354 [PubMed] [Google Scholar]
- 49. Reed KL, Heydrick SJ, Aarons CB, et al. A neurokinin-1 receptor antagonist that reduces intra-abdominal adhesion formation decreases oxidative stress in the peritoneum. Am J Physiol Gastrointest Liver Physiol. 2007;293(3):G544–G551 [DOI] [PubMed] [Google Scholar]
- 50. Heydrick SJ, Reed KL, Cohen PA, et al. Intraperitoneal administration of methylene blue attenuates oxidative stress, increases peritoneal fibrinolysis, and inhibits intraabdominal adhesion formation. J Surg Res. 2007;143(2):311–319 [DOI] [PubMed] [Google Scholar]
- 51. Dunn RC, Buttram VC, Jr. Tissue-type plasminogen activator as an adjuvant for post surgical adhesions. Prog Clin Biol Res. 1990;358:113–118 [PubMed] [Google Scholar]
- 52. Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146(1):56–66 [PMC free article] [PubMed] [Google Scholar]
- 53. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277(2 pt 1):C183–C201 [DOI] [PubMed] [Google Scholar]
- 54. Sogawa K. Overview: hypoxia [in Japanese]. Tanpakushitsu Kakusan Koso. 1999;44(15 suppl):2470–2471 [PubMed] [Google Scholar]
- 55. Saed GM, Diamond MP. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-beta1 in human peritoneal fibroblasts. Fertil Steril. 2002;78(1):144–147 [DOI] [PubMed] [Google Scholar]
- 56. Alpay Z, Ozgonenel M, Savasan S, Buck S, Saed GM, Diamond MP. Altered in vitro immune response to hypoxia-treated normal peritoneal fibroblasts. Fertil Steril. 2007;87(2):426–429 [DOI] [PubMed] [Google Scholar]
- 57. Falanga V. Wound healing. an overview. J Dermatol Surg Oncol. 1993;19(8):689–690 [DOI] [PubMed] [Google Scholar]
- 58. Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saed GM, Diamond MP. Differential expression of alpha smooth muscle cell actin in human fibroblasts isolated from intraperitoneal adhesions and normal peritoneal tissues. Fertil Steril. 2004;82 suppl 3:1188–1192 [DOI] [PubMed] [Google Scholar]
- 60. Saed GM, Abu-Soud HM, Diamond MP. Role of nitric oxide in apoptosis of human peritoneal and adhesion fibroblasts after hypoxia. Fertil Steril. 2004;82 suppl 3:1198–1205 [DOI] [PubMed] [Google Scholar]
- 61. Shavell VI, Saed GM, Diamond MP. Review: cellular metabolism: contribution to postoperative adhesion development. Reprod Sci. 2009;16(7):627–634 [DOI] [PubMed] [Google Scholar]
- 62. Cookson VJ, Chapman NR. NF-kappaB function in the human myometrium during pregnancy and parturition. Histol Histopathol. 2010;25(7):945–956 [DOI] [PubMed] [Google Scholar]
- 63. Saed GM, Diamond MP. Apoptosis and proliferation of human peritoneal fibroblasts in response to hypoxia. Fertil Steril. 2002;78(1):137–143 [DOI] [PubMed] [Google Scholar]
- 64. Chegini N. The role of growth factors in peritoneal healing: transforming growth factor beta (TGF-beta). Eur J Surg Suppl. 1997;(577):17–23 [PubMed] [Google Scholar]
- 65. Idell S, Zwieb C, Boggaram J, Holiday D, Johnson AR, Raghu G. Mechanisms of fibrin formation and lysis by human lung fibroblasts: influence of TGF-beta and TNF-alpha. Am J Physiol. 1992;263(4 pt 1):L487–L494 [DOI] [PubMed] [Google Scholar]
- 66. Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75(4):763–768 [DOI] [PubMed] [Google Scholar]
- 67. Ivarsson ML, Holmdahl L, Falk P, Molne J, Risberg B. Characterization and fibrinolytic properties of mesothelial cells isolated from peritoneal lavage. Scand J Clin Lab Invest. 1998;58(3):195–203 [DOI] [PubMed] [Google Scholar]
- 68. Diamond MP, El-Hammady E, Munkarah A, Bieber EJ, Saed G. Modulation of the expression of vascular endothelial growth factor in human fibroblasts. Fertil Steril. 2005;83(2):405–409 [DOI] [PubMed] [Google Scholar]
- 69. Saed GM, Zhang W, Chegini N, Holmdahl L, Diamond MP. Alteration of type I and III collagen expression in human peritoneal mesothelial cells in response to hypoxia and transforming growth factor-beta1. Wound Repair Regen. 1999;7(6):504–510 [DOI] [PubMed] [Google Scholar]
- 70. Saed GM, Munkarah AR, Abu-Soud HM, Diamond MP. Hypoxia upregulates cyclooxygenase-2 and prostaglandin E(2) levels in human peritoneal fibroblasts. Fertil Steril. 2005;83 suppl 1:1216–1219 [DOI] [PubMed] [Google Scholar]
- 71. Saed GM, Diamond MP. Modulation of the expression of tissue plasminogen activator and its inhibitor by hypoxia in human peritoneal and adhesion fibroblasts. Fertil Steril. 2003;79(1):164–168 [DOI] [PubMed] [Google Scholar]
- 72. Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol. 2005;175(8):5423–5429 [DOI] [PubMed] [Google Scholar]
- 73. Saed GM, Zhao M, Diamond MP, Abu-Soud HM. Regulation of inducible nitric oxide synthase in post-operative adhesions. Hum Reprod. 2006;21(6):1605–1611 [DOI] [PubMed] [Google Scholar]
- 74. Abu-Soud HM, Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A. 1993;90(22):10769–10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rosen GM, Tsai P, Weaver J, et al. The role of tetrahydrobiopterin in the regulation of neuronal nitric-oxide synthase-generated superoxide. J Biol Chem. 2002;277(43):40275–40280 [DOI] [PubMed] [Google Scholar]
- 76. Jiang ZL, Fletcher NM, Diamond MP, Abu-Soud HM, Saed GM. S-nitrosylation of caspase-3 is the mechanism by which adhesion fibroblasts manifest lower apoptosis. Wound Repair Regen. 2009;17(2):224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med. 1999;26(3-4):463–471 [DOI] [PubMed] [Google Scholar]
- 78. Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13(4):998–1002 [DOI] [PubMed] [Google Scholar]
- 79. Tarin JJ, Gomez-Piquer V, Pertusa JF, Hermenegildo C, Cano A. Association of female aging with decreased parthenogenetic activation, raised MPF, and MAPKs activities and reduced levels of glutathione S-transferases activity and thiols in mouse oocytes. Mol Reprod Dev. 2004;69(4):402–410 [DOI] [PubMed] [Google Scholar]
- 80. Gardiner CS, Salmen JJ, Brandt CJ, Stover SK. Glutathione is present in reproductive tract secretions and improves development of mouse embryos after chemically induced glutathione depletion. Biol Reprod. 1998;59(2):431–436 [DOI] [PubMed] [Google Scholar]
- 81. Shukla A, Rasik AM, Shankar R. Nitric oxide inhibits wounds collagen synthesis. Mol Cell Biochem. 1999;200(1-2):27–33 [DOI] [PubMed] [Google Scholar]
- 82. Ferrini MG, Vernet D, Magee TR, et al. Antifibrotic role of inducible nitric oxide synthase. Nitric Oxide. 2002;6(3):283–294 [DOI] [PubMed] [Google Scholar]
- 83. Jiang ZL, Zhu X, Diamond MP, Abu-Soud HM, Saed GM. Nitric oxide synthase isoforms expression in fibroblasts isolated from human normal peritoneum and adhesion tissues. Fertil Steril. 2008;90(3):769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cudd A, Fridovich I. Electrostatic interactions in the reaction mechanism of bovine erythrocyte superoxide dismutase. J Biol Chem. 1982;257(19):11443–11447 [PubMed] [Google Scholar]
- 85. van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272(12):7617–7625 [DOI] [PubMed] [Google Scholar]
- 86. Hazen SL, Zhang R, Shen Z, et al. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation In vivo. Circ Res. 1999;85(10):950–958 [DOI] [PubMed] [Google Scholar]
- 87. Jong EC, Klebanoff SJ. Eosinophil-mediated mammalian tumor cell cytotoxicity: role of the peroxidase system. J Immunol. 1980;124(4):1949–1953 [PubMed] [Google Scholar]
- 88. Mayeno AN, Curran AJ, Roberts RL, Foote CS. Eosinophils preferentially use bromide to generate halogenating agents. J Biol Chem. 1989;264(10):5660–5668 [PubMed] [Google Scholar]
- 89. Klebanoff SJ, Waltersdorph AM, Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403 [DOI] [PubMed] [Google Scholar]
- 90. Stocker R, Keaney JF, Jr. Role of oxidative modification in atherosclerosis. Physiol Rev. 2004;84(4):1381–1478 [DOI] [PubMed] [Google Scholar]
- 91. Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275(48):37524–37532 [DOI] [PubMed] [Google Scholar]
- 92. Abu-Soud HM, Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 2000;275(8):5425–5430 [DOI] [PubMed] [Google Scholar]
- 93. Abu-Soud HM, Hazen SL. Interrogation of heme pocket environment of mammalian peroxidases with diatomic ligands. Biochemistry. 2001;40(36):10747–10755 [DOI] [PubMed] [Google Scholar]
- 94. Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc Natl Acad Sci U S A. 2003;100(25):14766–14771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Abu-Soud HM, Khassawneh MY, Sohn JT, Murray P, Haxhiu MA, Hazen SL. Peroxidases inhibit nitric oxide (NO) dependent bronchodilation: development of a model describing NO-peroxidase interactions. Biochemistry. 2001;40(39):11866–11875 [DOI] [PubMed] [Google Scholar]
- 96. Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3(3):3–15 [DOI] [PubMed] [Google Scholar]
- 97. Abu-Soud HM, Raushel FM, Hazen SL. A novel multistep mechanism for oxygen binding to ferrous hemoproteins: rapid kinetic analysis of ferrous-dioxy myeloperoxidase (compound III) formation. Biochemistry. 2004;43(36):11589–11595 [DOI] [PubMed] [Google Scholar]
- 98. Shao B, Oda MN, Vaisar T, Oram JF, Heinecke JW. Pathways for oxidation of high-density lipoprotein in human cardiovascular disease. Curr Opin Mol Ther. 2006;8(3):198–205 [PubMed] [Google Scholar]
- 99. Xu W, Zheng S, Dweik RA, Erzurum SC. Role of epithelial nitric oxide in airway viral infection. Free Radic Biol Med. 2006;41(1):19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Westman E, Lundberg K, Erlandsson Harris H. Arthritogenicity of collagen type II is increased by chlorination. Clin Exp Immunol. 2006;145(2):339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Midwinter RG, Vissers MC, Winterbourn CC. Hypochlorous acid stimulation of the mitogen-activated protein kinase pathway enhances cell survival. Arch Biochem Biophys. 2001;394(1):13–20 [DOI] [PubMed] [Google Scholar]
- 102. McCord JM, Fridovich I. Superoxide dismutase. an enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049–6055 [PubMed] [Google Scholar]
- 103. Schallreuter KU, Moore J, Wood JM, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc. 1999;4(1):91–96 [DOI] [PubMed] [Google Scholar]
- 104. Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59(3):527–605 [DOI] [PubMed] [Google Scholar]
- 105. Halliwell B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat Res. 1999;443(1-2):37–52 [DOI] [PubMed] [Google Scholar]
- 106. Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci U S A. 1998;95(17):9738–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Diamond MP, El-Hammady E, Wang R, Kruger M, Saed G. Regulation of expression of tissue plasminogen activator and plasminogen activator inhibitor-1 by dichloroacetic acid in human fibroblasts from normal peritoneum and adhesions. Am J Obstet Gynecol. 2004;190(4):926–934 [DOI] [PubMed] [Google Scholar]
- 108. Gomel V. The impact of microsurgery in gynecology. Clin Obstet Gynecol. 1980;23(4):1301–1310 [DOI] [PubMed] [Google Scholar]
- 109. Winston RM. Microsurgery of the fallopian tube: from fantasy to reality. Fertil Steril. 1980;34(6):521–530 [DOI] [PubMed] [Google Scholar]
- 110. Awonuga AO, Saed GM, Diamond MP. Laparoscopy in gynecologic surgery: adhesion development, prevention, and use of adjunctive therapies. Clin Obstet Gynecol. 2009;52(3):412–422 [DOI] [PubMed] [Google Scholar]
- 111. Lundorff P, Hahlin M, Kallfelt B, Thorburn J, Lindblom B. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55(5):911–915 [DOI] [PubMed] [Google Scholar]
- 112. Milingos S, Kallipolitis G, Loutradis D, et al. Adhesions: laparoscopic surgery versus laparotomy. Ann N Y Acad Sci. 2000;900:272–285 [DOI] [PubMed] [Google Scholar]
- 113. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. operative laparoscopy study group. Fertil Steril. 1991;55(4):700–704 [PubMed] [Google Scholar]
- 114. Ott DE. Desertification of the peritoneum by thin-film evaporation during laparoscopy. JSLS. 2003;7(3):189–195 [PMC free article] [PubMed] [Google Scholar]
- 115. Yesildaglar N, Ordonez JL, Laermans I, Koninckx PR. The mouse as a model to study adhesion formation following endoscopic surgery: a preliminary report. Hum Reprod. 1999;14(1):55–59 [DOI] [PubMed] [Google Scholar]
- 116. Michelson AM, Puget K. [Medical aspects of superoxide dismutases]. C R Seances Soc Biol Fil. 1979;173(2):380–393 [PubMed] [Google Scholar]
- 117. Lefaix JL, Delanian S, Leplat JJ, et al. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys. 1996;35(2):305–312 [DOI] [PubMed] [Google Scholar]
- 118. Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47(2):277–290 [DOI] [PubMed] [Google Scholar]
- 119. Batinic-Haberle I, Spasojevic I, Stevens RD, et al. New PEG-ylated Mn(III) porphyrins approaching catalytic activity of SOD enzyme. Dalton Trans. 2006;(4):617–624 [DOI] [PubMed] [Google Scholar]
- 120. Ferrer-Sueta G, Hannibal L, Batinic-Haberle I, Radi R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic Biol Med. 2006;41(3):503–512 [DOI] [PubMed] [Google Scholar]
- 121. Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33(6):857–863 [DOI] [PubMed] [Google Scholar]
- 122. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95 [DOI] [PubMed] [Google Scholar]
- 123. Rier SE, Martin DC, Bowman RE, Dmowski WP, Becker JL. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1993;21(4):433–441 [DOI] [PubMed] [Google Scholar]
- 124. Victory R, Saed GM, Diamond MP. Antiadhesion effects of docosahexaenoic acid on normal human peritoneal and adhesion fibroblasts. Fertil Steril. 2007;88(6):1657–1662 [DOI] [PubMed] [Google Scholar]
- 125. Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821 [DOI] [PubMed] [Google Scholar]
- 126. Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51(5):1000–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992;669:7–20 [DOI] [PubMed] [Google Scholar]
- 128. Fletcher NM, Awonuga AO, Saed MG, Abu-Soud HM, Diamond MP, Saed GM. Lycopene, a powerful antioxidant, significantly reduces the development of the adhesion phenotype. Syst Biol Reprod Med. 2014;60(1):14–20 [DOI] [PubMed] [Google Scholar]
- 129. Pennathur S, Maitra D, Byun J, et al. Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid. Free Radic Biol Med. 2010;49(2):205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Reed KL, Stucchi AF, Leeman SE, Becker JM. Inhibitory effects of a neurokinin-1 receptor antagonist on postoperative peritoneal adhesion formation. Ann N Y Acad Sci. 2008;1144:116–126 [DOI] [PubMed] [Google Scholar]
- 131. Kluger Y, Weinbroum A, Ben-Avraham R, Galili Y, Klausner J, Rabau M. Reduction in formation of peritoneal adhesions by methylene blue in rats: a dose response study. Eur J Surg. 2000;166(7):568–571 [DOI] [PubMed] [Google Scholar]
- 132. Gul A, Kotan C, Dilek I, Gul T, Tas A, Berktas M. Effects of methylene blue, indigo carmine solution and autologous erythrocyte suspension on formation of adhesions after injection into rats. J Reprod Fertil. 2000;120(2):225–229 [DOI] [PubMed] [Google Scholar]
- 133. Mendes JB, Campos PP, Rocha MA, Andrade SP. Cilostazol and pentoxifylline decrease angiogenesis, inflammation, and fibrosis in sponge-induced intraperitoneal adhesion in mice. Life Sci. 2009;84(15-16):537–543 [DOI] [PubMed] [Google Scholar]
- 134. Chegini N, Rong H, Bennett B, Stone IK. Peritoneal fluid cytokine and eicosanoid levels and their relation to the incidence of peritoneal adhesion. J Soc Gynecol Investig. 1999;6(3):153–157 [DOI] [PubMed] [Google Scholar]
- 135. Saed GM, Jiang Z, Fletcher NM, Diamond MP. Modulation of the BCL-2/BAX ratio by interferon-gamma and hypoxia in human peritoneal and adhesion fibroblasts. Fertil Steril. 2008;90(5):1925–1930 [DOI] [PubMed] [Google Scholar]
- 136. Saed GM, Diamond MP. Effects of interferon-gamma reverse hypoxia-stimulated extracellular matrix expression in human peritoneal and adhesion fibroblasts. Fertil Steril. 2006;85 suppl 1:1300–1305 [DOI] [PubMed] [Google Scholar]
- 137. Yamanaka O, Saika S, Okada Y, Ooshima A, Ohnishi Y. Effects of interferon-gamma on human subconjunctival fibroblasts in the presence of TGFbeta1: reversal of TGFbeta-stimulated collagen production. Graefes Arch Clin Exp Ophthalmol. 2003;241(2):116–124 [DOI] [PubMed] [Google Scholar]
- 138. Galijasevic S, Abdulhamid I, Abu-Soud HM. Melatonin is a potent inhibitor for myeloperoxidase. Biochemistry. 2008;47(8):2668–2677 [DOI] [PubMed] [Google Scholar]
- 139. Proteasa G, Tahboub YR, Galijasevic S, Raushel FM, Abu-Soud HM. Kinetic evidence supports the existence of two halide binding sites that have a distinct impact on the heme iron microenvironment in myeloperoxidase. Biochemistry. 2007;46(2):398–405 [DOI] [PubMed] [Google Scholar]
- 140. Saed GM, Al-Hendy A, Salama SA, Diamond MP. Adenovirus-mediated expression of cyclooxygenase-2 antisense reverse abnormal genetic profile of human adhesion fibroblasts. Fertil Steril. 2008;89(5 suppl):1455–1460 [DOI] [PubMed] [Google Scholar]
- 141. Ahmad G, Duffy JM, Farquhar C, et al. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2008;(2):CD000475. [DOI] [PubMed] [Google Scholar]