Abstract
Bone marrow cells for the treatment of ischemic brain injury may depend on the secretion of a large number of neurotrophic factors. Bone marrow regenerative cells are capable of increasing the secretion of neurotrophic factors. In this study, after tail vein injection of 5-fluorouracil for 7 days, bone marrow cells and bone marrow regenerative cells were isolated from the tibias and femurs of rats, and then administered intravenously via the tail vein after focal cerebral ischemia. Immunohistological staining and reverse transcription-PCR detection showed that transplanted bone marrow cells and bone marrow regenerative cells could migrate and survive in the ischemic regions, such as the cortical and striatal infarction zone. These cells promote vascular endothelial cell growth factor mRNA expression in the ischemic marginal zone surrounding the ischemic penumbra of the cortical and striatal infarction zone, and have great advantages in promoting the recovery of neurological function, reducing infarct size and promoting angiogenesis. Bone marrow regenerative cells exhibited stronger neuroprotective effects than bone marrow cells. Our experimental findings indicate that bone marrow regenerative cells are preferable over bone marrow cells for cell therapy for neural regeneration after cerebral ischemia. Their neuroprotective effect is largely due to their ability to induce the secretion of factors that promote vascular regeneration, such as vascular endothelial growth factor.
Keywords: neural regeneration, brain injury, cerebral ischemia, seed cells, bone marrow, transplantation, bone marrow cells, bone marrow regenerative cells, vascular regeneration factor, brain, neuroregeneration
Research Highlights
-
(1)
Bone marrow regenerative cells are superior to bone marrow cells in neuroprotection; they can enhance vascular endothelial cell growth factor mRNA expression in the ischemic penumbra, promote the recovery of neurological function, reduce infarct size and promote angiogenesis.
-
(2)
Bone marrow regenerative cells are a preferable choice for cell therapy for neural regeneration after cerebral ischemia. Their neuroprotective effect is in a large part due to their ability to induce the secretion of factors that promote vascular regeneration, such as vascular endothelial growth factor.
INTRODUCTION
Bone marrow-derived cells are multipotent cells that are capable of being chemoattracted to lesioned tissues, and can release many cytokines and trophic factors[1,2,3,4,5]. Several studies have shown beneficial effects of treatment with these cells in animal models of ischemia and also in a few clinical trials of ischemic stroke, suggesting that this therapeutic strategy is promising[2,6,7]. The cells used in these studies were bone marrow-derived mesenchymal stem cells, a population of cells obtained after culturing bone marrow aspirate for weeks. Alternatively, other studies have used bone marrow cells, a fraction of the bone marrow that contains two types of adult stem cells (bone marrow-derived mesenchymal stem cells and hematopoietic stem cells)[8,9], hematopoietic progenitor cells[9] and endothelial progenitor cells[10], which have also been shown to be beneficial in animal models of stroke[11,12,13,14,15,16,17,18]. The feasibility and safety of autologous bone marrow cell transplantation have also been evaluated in a clinical trial in patients with ischemic stroke[19,20,21]. Compared with bone marrow-derived mesenchymal stem cells, bone marrow cells have similar efficacy when applied in the acute/subacute phase of stroke[22]. While the preparation of autologous bone marrow-derived mesenchymal stem cells requires cell cultivation that might delay treatment beyond the therapeutic time window within which therapy is effective[23,24], bone marrow cells can be rapidly derived from the bone marrow of animals and patients. They can be separated and isolated within 3 hours and then returned to the animals or patients, and therefore permit autologous testing. Furthermore, bone marrow cells do not require growth in culture, so they hold promise for treating acute stroke. The mechanisms by which transplanted bone marrow cells provide therapeutic benefit for brain damage remain unknown, although it is well known that neurotrophic factors play critical roles.
Bone marrow-derived mesenchymal stem cells produce different trophic factors (e.g. brain derived neurotrophic factor, vascular endothelial growth factor, nerve growth factor and hepatocellular growth factor) and cytokines, decrease apoptosis in the ischemic boundary zone and activate endogenous restorative responses such as angiogenesis, synaptogenesis and neurogenesis[25,26,27,28]. Bone marrow cells contain several types of cells, including stem cells and precursors, which can produce large amounts of cytokines and trophic factors that promote angiogenesis, neuroprotection and neuroregeneration after central nervous system injury in animal models of neurological disease[13,22,29,30,31,32].
Studies indicate that bone marrow regenerative cells treated with 5-fluorouracil increase the secretion of neurotrophic factors, proangiogenic factors and cytokines[33]. Thus, bone marrow regenerative cells are potentially more effective than bone marrow cells in treating stroke. In the present study, we investigated whether bone marrow regenerative cells are more efficient in treating focal brain ischemia. The underlying mechanisms were also investigated.
RESULTS
Quantitative analysis of experimental animals
A total of 86 healthy Sprague-Dawley rats were used for the middle cerebral artery occlusion model of cerebral ischemia, and 75 rats with successfully established middle cerebral artery occlusion were randomly assigned to three groups: control group (n = 21), bone marrow cells group (n = 27) and bone marrow regenerative cells group (n = 27). Rats were intravenously transplanted with PBS, bone marrow cells or bone marrow regenerative cells via the tail vein. After transplantation, 17 rats in each group were selected for the experiments, and 54 rats were included in the final analysis.
Assessment of transplanted bone marrow cells and bone marrow regenerative cells
4’,6-diamidine-2’-phenylindole (DAPI) has a high affinity for DNA and can emit intense fluorescence upon binding. In addition, DAPI is nontoxic to living cells and induces no changes in organelle ultrastructure, and fluorescence is maintained for a long period, so it is often used for in vivo tracing of cells. In this study, transplanted cells whose nuclei were stained sapphire with DAPI were visible under the fluorescence microscope. Three days and 2 weeks after transplantation, the bone marrow cells (Figure 1A) and bone marrow regenerative cells (Figure 1B) were observed to migrate to the ischemic lesion area and maintained viability. However, there was no significant difference in the survival numbers between these two types of cells at 3 and 14 days after transplantation (Figure 2).
Figure 1.

Bone marrow cells (A) and bone marrow regenerative cells (B) migrated to the ischemic lesion area and remained alive 3 days after transplantation (× 200).
Cells were stained with 4’,6-diamidino-2’-phenylindole (DAPI; blue).
Figure 2.
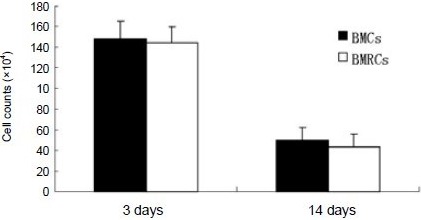
BMC and BMRC survival in the ischemic cerebral region at 3 and 14 days after cell transplantation.
There was no significant difference in the survival numbers of two types of cells. Five sections were taken from each rat and observed under the microscope (× 200). DAPI-positive cells were then counted for every section. Calculation of the total number of DAPI-positive cells was for five sections from each group. Data were expressed as mean ± SD of three rats for each group at each time point (one-way analysis of variance).
DAPI: 4’,6-Diamidino-2’-phenylindole; BMCs: bone marrow cells; BMRCs: bone marrow regenerative cells.
Neuroprotective effect of bone marrow cells and bone marrow regenerative cells
After middle cerebral artery occlusion, all rats exhibited decreasing grip strength of the right limb, fell down, and showed an inability to walk straight with an obvious impairment of balance. Before transplantation, no differences were observed in the Modified Neurological Severity Score; however, significant functional improvement was shown by the bone marrow cells/bone marrow regenerative cells-treated groups 7 and 14 days after middle cerebral artery occlusion, compared with the control group (P < 0.05). The bone marrow regenerative cells group showed a significant improvement in neurological function compared with the bone marrow cells group (P < 0.05; Figure 3).
Figure 3.
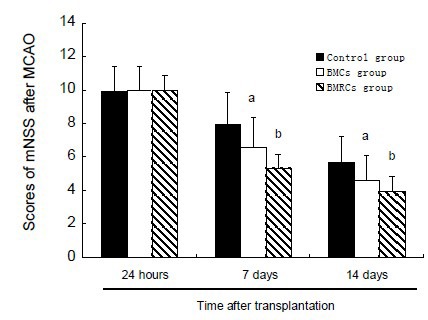
Effects of BMCs and BMRCs administration on neurological function (Modified Neurological Severity Score) in rats with cerebral ischemia at 24 hours, 7 and 14 days after transplantation.
aP < 0.05, vs. control group at the same time; bP < 0.05, vs. BMCs group at the same time point. Data were expressed as mean ± SD of six rats for each group at each time point (one-way analysis of variance and least significant difference t-test). Higher scores indicates more severe neurological function deficit.
BMCs: Bone marrow cells; BMRCs: bone marrow regenerative cells.
Effects of bone marrow cells and bone marrow regenerative cell transplantation on infarct size
As shown by 2,3,5-triphenyltetrazolium chloride (TTC) staining (Figure 4), infarct volumes in the bone marrow cells and bone marrow regenerative cells groups were significantly decreased compared with the control group 14 days after middle cerebral artery occlusion (P < 0.01). The infarct volume in the bone marrow regenerative cells group was significantly lower than in the bone marrow cells group 14 days after middle cerebral artery occlusion (P < 0.05; Figure 5).
Figure 4.
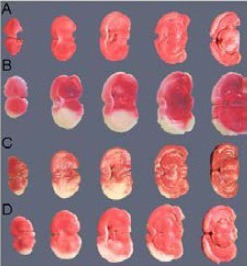
Representative photographs of coronal brain sections stained with TTC.
The infarct lesions are shown as white areas.
(A) TTC staining of coronal sections in normal rat brain, no infarct lesions are visible.
(B) TTC staining of coronal sections in MCAO rats (control group) without cell administration, infarct lesions are obvious.
(C) TTC staining of coronal sections in MCAO rats with BMCs administration, infarct lesions are significantly decreased compared with the control group.
(D) TTC staining of coronal sections in MCAO rats with BMRCs administration, infarct lesions are significantly decreased compared with the BMCs group.
TTC: 2,3,5-Triphenyltetra zolium chloride; BMCs: bone marrow cells; BMRCs: bone marrow regenerative cells; MCAO: middle cerebral artery occlusion.
Figure 5.
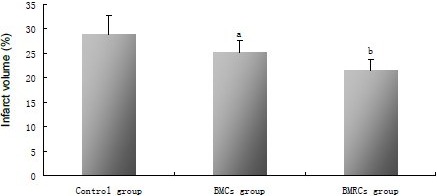
Effects of BMCs and BMRCs administration on infarct volume in rats with cerebral ischemia.
The infarction volumes of the lesioned structures were expressed as a percentage of the volume of the structures in the control hemispheres (mean, %). aP < 0.05, vs. control group; bP < 0.05, vs. BMCs group. Data were expressed as mean ± SD of six rats for each group (one-way analysis of variance and least significant difference t-test). BMCs: Bone marrow cells; BMRCs: bone marrow regenerative cells.
Administration of bone marrow cells and bone marrow regenerative cells increased endogenous levels of vascular endothelial growth factor
There was a very low level of vascular endothelial growth factor mRNA expression in the normal group at each time point. In the control group, the expression of vascular endothelial growth factor reached a maximum level at 24 hours and then decreased to a level similar to that in the normal group. There was no significant difference in vascular endothelial growth factor expression among the control, bone marrow cells and bone marrow regenerative cells groups 24 hours after transplantation. The expression of vascular endothelial growth factor in the bone marrow cells and bone marrow regenerative cells groups reached a peak at day 3 (P < 0.01), and then decreased, but was still maintained at a higher level than in the control group (P < 0.05). Interestingly, the expression of vascular endothelial growth factor in the bone marrow regenerative cells group was significantly higher than in the bone marrow cells group at days 3 and 7 (P < 0.05; Figure 6).
Figure 6.
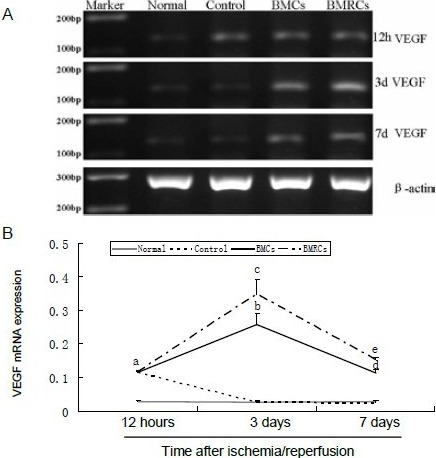
Effect of BMCs and BMRCs transplantation on vascular endothelial growth factor (VEGF) mRNA expression levels in the ischemic penumbra.
(A) Representative reverse transcription-PCR images of VEGF mRNA expression at 12 hours, 3 and 7 days.
(B) Quantification of VEGF mRNA expression. Data are expressed as absorbance value (VEGF/β-actin). aP < 0.05, vs. normal group; bP < 0.05, vs. control and normal groups; cP < 0.05, vs. BMCs group. Data were expressed as mean ± SD of six rats for each group (one-way analysis of variance and least significant difference t-test). BMCs: Bone marrow cells; BMRCs: bone marrow regenerative cells.
Effects of bone marrow cells and bone marrow regenerative cell treatment on angiogenesis
Immunohistochemical staining showed that the number of vessels positive for von Willebrand factor in the ischemic penumbra increased 106% in the bone marrow cells group and 218% in the bone marrow regenerative cells group which was significantly higher than in the control group (P < 0.05). Compared with the bone marrow cells group, the number of vessels stained positive for von Willebrand factor in the bone marrow regenerative cells group was significantly increased (P < 0.05; Figure 7).
Figure 7.
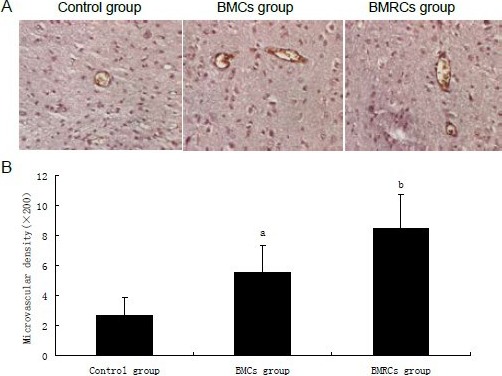
Effect of BMCs and BMRCs transplantation on angiogenesis in the ischemic penumbra.
(A) Von Willebrand factor immunohistochemistry showing vessel walls of rats with cerebral ischemia stained brown (× 200).
(B) BMCs and BMRCs significantly increased vessel density in the ischemic penumbra, with BMRCs having a better effect than BMCs. aP < 0.05, vs. control group; bP < 0.05, vs. BMCs group. Data were expressed as mean ± SD of six rats for each group (one-way analysis of variance and least significant difference t-test). BMCs: Bone marrow cells; BMRCs: bone marrow regenerative cells.
DISCUSSION
Survival and migration of intravenously administrated bone marrow cells and bone marrow regenerative cells
The migration and survival of bone marrow cells and bone marrow regenerative cells after intravenous administration were observed.
In cell transplantation therapy, the migration and survival of transplanted cells are important indices for evaluating the efficacy of this treatment. Previous reports have shown that chemokines, increased permeability of the blood-brain barrier and changes in the microenvironment after cerebral ischemia can facilitate the migration of bone marrow cells and bone marrow regenerative cells into the ischemic areas and promote their survival[34,35]. In this study, there was no difference in the number of surviving cells between the bone marrow cells and bone marrow regenerative cells groups at days 3 and 14 after cell transplantation. Interestingly, however, compared with the number of surviving cells at 3 days, the number of surviving cells at 14 days was reduced. This suggests that inflammation probably creates a more unfavorable environment for circulating bone marrow cells and bone marrow regenerative cells to migrate into, repopulate, survive and differentiate.
Bone marrow cells and bone marrow regenerative cells reduced infarct size and improved recovery of neurological function
The Modified Neurological Severity Score measurement demonstrated that the recovery of neurological function in the bone marrow cells and bone marrow regenerative cells groups was significantly better than in the control group. Two weeks after middle cerebral artery occlusion, partial recovery of neurological function in the control group without any treatment was observed. However, from 1 to 2 weeks after transplantation of the bone marrow cells and bone marrow regenerative cells, the Modified Neurological Severity Scores were significantly decreased in the bone marrow cells and bone marrow regenerative cells groups compared with the control group. Importantly, the Modified Neurological Severity Score in the bone marrow regenerative cells group was significantly lower than in the bone marrow cells group. In addition, TTC staining revealed that administration of bone marrow cells and bone marrow regenerative cells reduced brain infarct size. The infarct size in the bone marrow regenerative cells group was significantly smaller than in the bone marrow cells group.
The present study showed that intravenous (but not intra-arterial) administration of bone marrow cells results in decreased infarct volume and functional improvement in rats subjected to middle cerebral artery occlusion. Importantly, this study shows that bone marrow regenerative cells are also neuroprotective against ischemia-induced damage and have greater efficacy than bone marrow cells.
Administration of bone marrow cells and bone marrow regenerative cells increased vascular endothelial growth factor mRNA expression
Endothelial cells can specifically respond to vascular endothelial growth factor, which plays an important role in blood vessel formation and promotes the proliferation and migration of endothelial cells. Even a slight up-regulation of vascular endothelial growth factor has a significant impact on local neovascularization in ischemic tissues[36]. Vascular endothelial growth factor has strong angiogenic effects in brain and is required for the initiation of immature vessel formation during vasculogenesis/angiogenesis. In the present study, we found that the expression of vascular endothelial growth factor mRNA peaked at 24 hours, and then decreased to normal levels at day 3, which is consistent with a previous study[37]. Vascular endothelial growth factor mRNA in both of the transplantation groups was significantly higher than in the control group, peaking at day 3, and remaining at elevated levels at day 7. The highest level of vascular endothelial growth factor was observed in the bone marrow regenerative cells group. Cultured bone marrow-derived mesenchymal stem cells secrete angiogenic cytokines, including vascular endothelial growth factor. Miki et al[38] reported that bone marrow stromal cells genetically modified to express vascular endothelial growth factor may have a greater therapeutic effect than non-modified cells. Therefore, the level of vascular endothelial growth factor expression may be critical in terms of potential therapeutic effects. So we can deduce that bone marrow regenerative cells secrete more angiogenic cytokines, such as vascular endothelial growth factor, than bone marrow cells and have a greater therapeutic effect as well.
Administration of bone marrow cells and bone marrow regenerative cells promoted vascular regeneration
Vascular regeneration is closely related with the restoration of neurological functioning after stroke. Stroke can stimulate vasculogenesis, which is also closely related to the recovery of neurological functions[39]. The newly formed vessels can deliver more oxygen and nutrients to the local ischemic region, and this promotes the recovery and repair of neurons, which are still viable after the ischemic event. The microenvironment associated with vascular regeneration also benefits nerve formation. However, vessel regeneration induced by stroke has a negligible impact on microvessel formation, and consequently does not sufficiently help neurological functional recovery[28]. Bone marrow cells can work efficiently to promote vessel regeneration because various cytokines (e.g. vascular endothelial growth factor, basic fibroblast growth factor and fibroblast growth factor) can be secreted by bone marrow cells. In the present study, von Willebrand factor was used as a marker to assess the density of microvessels by immunohistochemical staining. Compared with the control group, the number of von Willebrand factor-positive microvessels in both of the transplanted groups in the ischemic penumbra was significantly higher. Zhang et al[40] proposed that circulating endothelial progenitor cells may participate in the process of vessel regeneration, thereby significantly improving cerebral blood flow. However, the number of endothelial progenitor cells in peripheral blood was only 0.2% of that in bone marrow, which contains many more endothelial progenitor cells than peripheral blood. Bone marrow cells, without separation of the endothelial progenitor cell pool, may be a more efficient resource for treatment. Hematopoietic stem cells are also very important cells present among bone marrow-derived mesenchymal stem cells. Ang-1, a ligand of the Tie-2 receptor, can be released by hematopoietic stem cells and promote vascularization. Other types of non-hematopoietic stromal cells, such as mature stromal stem cells, fibroblasts and adipocytes, can also proliferate and serve as a feeder layer for endothelial progenitor cells. The concept that peripheral blood mononuclear cells have a vascular regeneration effect was also suggested by Iba et al[41]. The greater vascular regeneration following bone marrow-derived mesenchymal stem cell transplantation may have been stimulated by the decrease in blood cells after 5-fluorouracil treatment. In response, stem cells located in the bone marrow may have been mobilized to enhance bone marrow regeneration. In addition, neurotrophic factors, such as vascular endothelial growth factor, were likely secreted in abundance. Furthermore, a large number of cells, such as endothelial progenitor cells and hematopoietic stem cells, regenerated. The combination of these various factors and regenerative cells likely contributed to vascular regeneration and functional recovery.
In summary, the present study demonstrated that intravenously administered bone marrow cells and bone marrow regenerative cells can migrate into and survive in the ischemic area, promote vascular endothelial growth factor mRNA expression in the ischemic penumbra, increase vascular regeneration, decrease infarct volume, and improve neurological function. Moreover, bone marrow regenerative cells have better effects on neuroprotection and vascular regeneration, and a greater amount of neurotrophic factors are secreted by these cells, accounting for the greater therapeutic efficacy. Thus, bone marrow regenerative cells may be an excellent source of seed cells, serving as a simple, effective and feasible therapeutic strategy for ischemic brain injury.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The present study was performed at the Experimental Center of Xinhua Hospital, Affiliated to Shanghai Jiao Tong University School of Medicine, China, from December 2009 to March 2011.
Materials
A total of 86 adult male Sprague-Dawley rats, aged 12 weeks, weighing 270–290 g, were provided by Shanghai Laboratory Animals Co., Ltd., Shanghai, China, (certification No. SCXK-2006-0005). Ten male rats aged 4–6 weeks, weighing 100–150 g, were used to prepare bone marrow cells and bone marrow regenerative cells. All the rats were housed under 12-hour light/dark cycles. All experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[42].
Methods
Isolation and staining of bone marrow cells and bone marrow regenerative cells
After tail vein injection with or without 5-fluorouracil (125 mg/kg; Sigma, St. Louis, MO, USA) for 7 days, bone marrow cells and bone marrow regenerative cells were isolated from the tibias and femurs of normal and 5-fluorouracil-injected syngeneic donor rats. Briefly, bone marrow was aspirated from the bones, dissociated with serum-free Dulbecco's modified Eagle's medium/Ham's Nutrient Mixture F12 and collected in sterile tubes. Bone marrow cells were then mechanically dissociated, centrifuged for 5 minutes at 250 × g, and resuspended in 4 mL of Dulbecco's modified Eagle's medium/Ham's Nutrient Mixture F12. This volume was gently poured over 4 mL of Histopaque 1083 and centrifuged for 30 minutes at 400 × g. The cells were collected from the mononuclear cell layer and washed with PBS (pH 7.4) over three consecutive centrifugations at 250 × g for 5 minutes each. Following a final centrifugation, bone marrow cells and bone marrow regenerative cells were stained with DAPI (50 mg/L; Sigma) at 4°C for 60 minutes, and these cells were used for transplantation.
Middle cerebral artery occlusion model and cell transplantation
Adult male rats were anesthetized with an intraperitoneal injection of chloral hydrate (0.4 g/kg; Sigma) before the operation. Based on the method described by Longa et al[43], the right middle cerebral artery was occluded for 2 hours and then blood reperfusion was started. Stroke onset was identified when the rats fell to the left and exhibited circling behavior. Subsequently, the rats were kept under constant housing conditions and had free access to food and water. Twenty-four hours after middle cerebral artery occlusion, rats were transplanted with 1 × 107 bone marrow cells or bone marrow regenerative cells by tail vein injection, and in the control group the same volume of PBS was injected.
Determination of transplant cell survival, neurological function and infarct volume
Two weeks after transplantation, 20-μm serial coronal sections of the infarct regions were processed for the experiment. Five sections were taken from each rat and observed under the microscope (Olympus IX50, Tokyo, Japan). DAPI-positive cells were then counted. At 24 hours, 7 and 14 days after transplantation, neurological functional deficits were measured with the double blind Modified Neurological Severity Score[44]. This measurement is a composite of motor, sensory and reflex tests. In the severity scores of injury, a score of 1 is awarded for the inability to perform the test or for the lack of a tested reflex; thus, the higher the score, the more severe the injury. Neurological function was graded on a scale of 0–18 (normal score: 0; maximal deficit score: 18). To calculate the infarct volume, 14 days after middle cerebral artery occlusion rats were killed under deep anesthesia, the brains were removed quickly and then sectioned. Afterwards, slices were immersed in 2% TTC solution, and kept at 37°C in a water bath for 30 minutes. The slices were then transferred to 10% buffered formalin. About 24 hours later, slices were photographed, and the infarct areas were first measured using Image Tool software (Image Tool 2.0). The infarct volume of each slice was calculated as follows: V = t × (A1+ A2 + A3 + A4 + A5) – (A1 + A2) × t/2 (A represents the infarct area of the slice, t represents the slice thickness). The lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere.
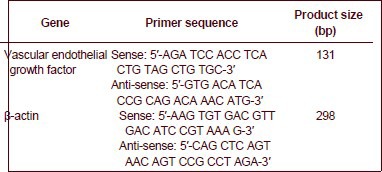
Expression of vascular endothelial growth factor as detected by reverse transcription-PCR
Rat ischemic penumbras from the three groups were isolated as previously described, and total RNA was exacted using Trizol reagent. Complementary DNA was prepared from the RNA using reverse transcriptase. Quantification PCR reactions were run using rat vascular endothelial growth factor-specific primers. The primers were designed and synthesized by Shanghai Sangon Biological Engineering Technology and Service Co., Ltd., Shanghai, China.
The amplification conditions were: 3 minutes at 94°C, 35 cycles of 30 seconds at 94°C, 30 seconds at 55°C and 50 seconds at 72°C; followed by 10 minutes at 72°C. PCR products were visualized on 1.5% agarose gels stained with ethidium bromide and placed under UV transillumination (Sigma). PCR product band intensities were measured using semi-quantitative analysis using a computerized densitometric imager (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Measurement of vascular density and immunohistochemical staining for von Willebrand factor
The expression of factor VIII from vascular endothelial cells was detected using the ABC immunohistochemical staining method. A standard paraffin-embedded block (within the center of the lesion of middle cerebral artery occlusion), corresponding to coronal coordinates bregma –1.0 to +1.0 mm was obtained, and a series of 5-μm-thick sections were processed for immunostaining. Sections were placed in boiling citrate buffer (pH 6) in a microwave oven (700 W). Following blocking in normal serum, sections were incubated with mouse anti-rat von Willebrand factor (1:400; Dako, Carpinteria, CA, USA) at 37°C for 60 minutes, followed by 30-minute incubation with a biotinylated goat anti-mouse second antibody (1:500; Antibody Diagnostica Inc., Stamford, CA, USA). The sections were color-developed with a StreptABComplex/DAB kit (Lab Vision Corporation, Fremont, CA, USA) according to the company's instructions. Five serial sections were taken for determining microvessel densities (the number of von Willebrand factor-positive vessels in the field of view), and counting was performed using a 200 × objective within the cortex and the penumbra regions. In total, five fields of view were observed.
Statistical analysis
All the data were expressed as mean ± SD. All statistics were performed using SPSS 14.0 software package (SPSS, Chicago, IL, USA). Differences between individual groups were compared using one-way analysis of variance followed by least significant difference t-test. A value of P < 0.05 was considered statistically significant.
Footnotes
Conflicts of interest: None declared.
Ethical approval: The project received full ethical approval by the Animal Ethics Committee of Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, China.
(Reviewed by Patel B, Wysong S, Zhang RX, Yuan B)
(Edited by Wang J, Yang Y, Li CH, Song LP)
REFERENCES
- 1.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 2.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1(2):92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Crain BJ, Tran SD, Mezey E. Transplanted human bone marrow cells generate new brain cells. J Neurol Sci. 2005;233(1-2):121–123. doi: 10.1016/j.jns.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Shyu WC, Lee YJ, Liu DD, et al. Homing genes, cell therapy and stroke. Front Biosci. 2006;11:899–907. doi: 10.2741/1846. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med. 2008;18(1):33–37. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 7.Mezey E. Bone marrow-derived stem cells in neurological diseases: stones or masons? Regen Med. 2007;2(1):37–49. doi: 10.2217/17460751.2.1.37. [DOI] [PubMed] [Google Scholar]
- 8.Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet. 2000;1(1):57–64. doi: 10.1038/35049577. [DOI] [PubMed] [Google Scholar]
- 9.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 10.Wang QR, Wang BH, Huang YH, et al. Purification and growth of endothelial progenitor cells from murine bone marrow mononuclear cells. J Cell Biochem. 2008;103(1):21–29. doi: 10.1002/jcb.21377. [DOI] [PubMed] [Google Scholar]
- 11.Iihoshi S, Honmou O, Houkin K, et al. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007(1-2):1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 12.Kamiya N, Ueda M, Igarashi H, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;83(11-12):433–437. doi: 10.1016/j.lfs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Giraldi-Guimaraes A, Rezende-Lima M, Bruno FP, et al. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 14.Yang B, Strong R, Sharma S, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89(6):833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasconcelos-Dos-Santos A, Rosado-de-Castro PH, Lopes de Souza SA, et al. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem Cell Res. 2012;9(1):1–8. doi: 10.1016/j.scr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Taguchi A, Zhu P, Cao F, et al. Reduced ischemic brain injury by partial rejuvenation of bone marrow cells in aged rats. J Cereb Blood Flow Metab. 2011;31(3):855–867. doi: 10.1038/jcbfm.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita Y, Ihara M, Ushiki T, et al. Early protective effect of bone marrow mononuclear cells against ischemic white matter damage through augmentation of cerebral blood flow. Stroke. 2010;41(12):2938–2943. doi: 10.1161/STROKEAHA.110.596379. [DOI] [PubMed] [Google Scholar]
- 18.Brenneman M, Sharma S, Harting M, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30(1):140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendonca ML, Freitas GR, Silva SA, et al. Safety of intra-arterial autologous bone marrow mononuclear cell transplantation for acute ischemic stroke. Arq Bras Cardiol. 2006;86(1):52–55. doi: 10.1590/s0066-782x2006000100008. [DOI] [PubMed] [Google Scholar]
- 20.Battistella V, de Freitas GR, da Fonseca LM, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011;6(1):45–52. doi: 10.2217/rme.10.97. [DOI] [PubMed] [Google Scholar]
- 21.Savitz SI, Misra V, Kasam M, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70(1):59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 22.de Vasconcelos Dos Santos A, da Costa Reis J, Diaz Paredes B, et al. Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res. 2010;1306:149–158. doi: 10.1016/j.brainres.2009.09.094. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32(11):2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Li Y, Zhang ZG, et al. Bone marrow stromal cells increase oligodendrogenesis after stroke. J Cereb Blood Flow Metab. 2009;29(6):1166–1174. doi: 10.1038/jcbfm.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59(4):514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189(1-2):49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92(6):692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel-Coelho PM, Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev. 2010;19(3):299–310. doi: 10.1089/scd.2009.0403. [DOI] [PubMed] [Google Scholar]
- 30.Pimentel-Coelho PM, Magalhaes ES, Lopes LM, et al. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2010;19(3):351–358. doi: 10.1089/scd.2009.0049. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro-Resende VT, Pimentel-Coelho PM, Mesentier-Louro LA, et al. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience. 2009;159(2):540–549. doi: 10.1016/j.neuroscience.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 32.Nakano-Doi A, Nakagomi T, Fujikawa M, et al. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells. 2010;28(7):1292–1302. doi: 10.1002/stem.454. [DOI] [PubMed] [Google Scholar]
- 33.Han W, Yu Y, Liu XY. Local signals in stem cell-based bone marrow regeneration. Cell Res. 2006;16(2):189–195. doi: 10.1038/sj.cr.7310026. [DOI] [PubMed] [Google Scholar]
- 34.Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8(8):1193–1201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locatelli F, Bersano A, Ballabio E, et al. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66(5):757–772. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 37.Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156(3):965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miki Y, Nonoguchi N, Ikeda N, et al. Vascular endothelial growth factor gene-transferred bone marrow stromal cells engineered with a herpes simplex virus type 1 vector can improve neurological deficits and reduce infarction volume in rat brain ischemia. Neurosurgery. 2007;61(3):586–594. doi: 10.1227/01.NEU.0000290907.30814.42. discussion 594-585. [DOI] [PubMed] [Google Scholar]
- 39.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang ZG, Zhang L, Jiang Q, et al. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90(3):284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 41.Iba O, Matsubara H, Nozawa Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106(15):2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 42.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions For the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- 43.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]


