Abstract
Transplantation of neural stem cells has been reported as a possible approach for replacing impaired dopaminergic neurons. In this study, we tested the efficacy of early-stage human dental papilla-derived stem cells and human brain-derived neural stem cells in rat models of 6-hydroxydopamine-induced Parkinson's disease. Rats received a unilateral injection of 6-hydroxydopamine into right medial forebrain bundle, followed 3 weeks later by injections of PBS, early-stage human dental papilla-derived stem cells, or human brain-derived neural stem cells into the ipsilateral striatum. All of the rats in the human dental papilla-derived stem cell group died from tumor formation at around 2 weeks following cell transplantation. Postmortem examinations revealed homogeneous malignant tumors in the striatum of the human dental papilla-derived stem cell group. Stepping tests revealed that human brain-derived neural stem cell transplantation did not improve motor dysfunction. In apomorphine-induced rotation tests, neither the human brain-derived neural stem cell group nor the control groups (PBS injection) demonstrated significant changes. Glucose metabolism in the lesioned side of striatum was reduced by human brain-derived neural stem cell transplantation. [18F]-FP-CIT PET scans in the striatum did not demonstrate a significant increase in the human brain-derived neural stem cell group. Tyrosine hydroxylase (dopaminergic neuronal marker) staining and G protein-activated inward rectifier potassium channel 2 (A9 dopaminergic neuronal marker) were positive in the lesioned side of striatum in the human brain-derived neural stem cell group. The use of early-stage human dental papilla-derived stem cells confirmed its tendency to form tumors. Human brain-derived neural stem cells could be partially differentiated into dopaminergic neurons, but they did not secrete dopamine.
Keywords: neural regeneration, stem cells, cell transplantation, glucose metabolism, human brain-derived neural stem cells, human dental papilla-derived stem cells, Parkinson's disease, positron emission tomography, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
For the first time, this study tested the effects of transplantation of early-stage human dental papilla-derived stem cells and human brain-derived neural stem cells on Parkinson's disease in rats.
-
(2)
Experimental results showed that after cell transplantation, early-stage human dental papilla-derived stem cells exhibited a tendency to form tumors, while human brain-derived neural stem cells can partially differentiate into dopaminergic neurons, but they do not secrete dopamine.
-
(3)
These findings suggest that both early-stage human dental papilla-derived stem cells and human brain-derived neural stem cells are not suitable for the treatment of Parkinson's disease.
INTRODUCTION
Parkinson's disease is characterized by the degeneration of dopaminergic neurons in the substantia nigra, which largely results in motor symptoms, such as tremor, rigidity, bradykinesia, and postural instability[1]. Levodopa has been most commonly used in the treatment of Parkinson's disease since the 1960s, and has been shown to dramatically improve motor deficits in many clinical and experimental studies[1,2]. However, the long-term use of levodopa can result in levodopa-induced dyskinesia and motor fluctuations[3]. To combat these complications, the use of deep brain stimulation surgery has been proposed[4,5]. However, no treatment is currently available that can stop Parkinson's disease progression[2]. Cell replacement therapy has been performed previously for Parkinson's disease in humans and animals, but this treatment approach also carries with it some ethical and technical problems[1].
The rat model of Parkinson's disease, which involves the injection of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle causing a complete depletion of dopamine, mimics the pathological manifestations of human Parkinson's disease patients[2]. In recent cell transplantation studies that have used the Parkinson's disease rat model, apomorphine-induced rotation tests did not indicate full recovery in the animals, but only a partial reduction in rotation[6,7]. This might be due to the effects of apomorphine sensitization[8]. To overcome these limitations, we performed a microPET study that used multitracers of glucose metabolism and the dopamine transporter[9]. This can more precisely indicate the state of Parkinson's disease than drug-induced tests. In addition, this is a noninvasive in vivo method. 2-Deoxy-2-[18F]-fluoro-D-glucose ([18F]-FDG) is a marker of glucose metabolism that can be used to indicate neuronal viability[10], and [18F]-N-(3-fluoropropyl)-2-carbomethoxy-3-(4-iodophenyl) nortropane ([18F]-FP-CIT) binds to the dopamine transporter with high affinity[11].
Recently, it has been reported that stem cells from dental tissues showed high potency for neural differentiation, and several in vitro and in vivo studies showed the generation of functional neurons in neurogenic cultures of dental stem cells[12,13,14]. They are easily obtained from discarded human teeth[12]. Moreover, the use of these cells does not have ethical problems. Early-stage human dental papilla-derived stem cells have the potential for neuronal differentiation; however, their safety when they are transplanted into the striatum has rarely been studied, especially in Parkinson's disease[12]. Herein, for the first time, we tested the effects of early-stage human dental papilla-derived stem cells in the 6-hydroxydopamine-induced Parkinson's disease rat model. It was reported that human neural stem cells improve motor deficits and cognitive performance in the rat model of Parkinson's disease[15]. However, it is still unknown whether transplanted human brain-derived neural stem cells can be differentiated into dopaminergic neurons.
In this study, we evaluated human dental papilla-derived stem cell and human brain-derived neural stem cell transplantation for the treatment of 6-hydroxydopamine-induced Parkinson's disease in rats using behavioral tests, multi-tracer microPET, and immunohistological evaluations.
RESULTS
Quantitative analysis of animals
Thirty rats were initially included, and twenty-three rats were used in this study. Seven rats were excluded from data because they did not demonstrate > 6 rotations per minute after apomorphine administration. Rats received a unilateral injection of 6-hydroxydopamine into the right medial forebrain bundle, followed 3 weeks later by injections of PBS, early-stage human dental papilla-derived stem cells, or human brain-derived neural stem cells into the ipsilateral striatum.
Tumorigenesis in the human dental papilla-derived stem cell group
All rats in the human dental papilla-derived stem cell group died within 12–15 days after transplantation. These rats were quadriplegic for a couple of days before death. Staining for microtubule-associated protein 2 (MAP2), which is a neuronal-specific cytoskeletal protein, was slightly positive, but other markers such as neurofilament (NF), glial fibrillary acidic protein (GFAP), and synaptophysin were negative in the striatum (Figure 1). Human dental papilla-derived stem cells transformed into homogenous tumors that were densely packed and globular on hematoxylin-eosin staining (Figure 1E).
Figure 1.
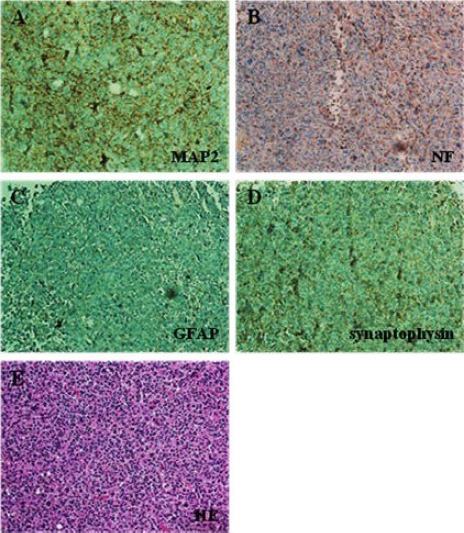
Immunostaining patterns of the human dental papilla-derived stem cell group in the striatum.
(A) These cells are slightly positive for microtubule-associated protein 2 (MAP2; A), but negative for neurofilament (NF; B), glial fibrillary acidic protein (GFAP; C), and synaptophysin(D). (E) Hematoxylin-eosin (HE) staining showed homogenous tumors that are densely packed with globular shapes (all images: × 100 magnification).
Effect of stem cell transplantation on motor function of rats
Stepping tests showed that 1 week after 6-hydroxydopamine insults, all rats showed remarkable deficits when adjusting the contralateral forelimb. Most rats demonstrated slight increases in contralateral forelimb motor function 2 weeks after cell transplantation, but these increases gradually decreased (Figure 2). There were no statistical differences among the repeated test results after transplantation in the human brain-derived neural stem cell group.
Figure 2.
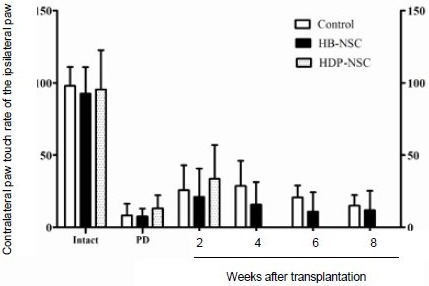
Effect of stem cell transplantation on motor function of rats (stepping tests).
These tests were performed during the intact state, 1 week after 6-hydroxydopamine (6-OHDA) injection, and at 2, 4, 6, and 8 weeks after cell transplantations.
Data are expressed as mean ± SD. The parameters were analyzed using the paired samples t-test. Two weeks after transplantation, all groups demonstrated slight increases in contralateral forelimb use, which were non-significant; these then continuously declined. HB-NSC: Human brain-derived neural stem cell; HDP-NSC: human dental papilla-derived stem cell; PD: Parkinson's disease state.
Effect of stem cell transplantation on Parkinson's disease symptom
Drug-induced rotation tests showed that rats in the control and human brain-derived neural stem cell groups showed contralateral rotations following apomorphine administration 1 week after 6-hydroxydopamine injection. These asymmetrical rotations were still persistent at 4 and 8 weeks after PBS or human brain-derived neural stem cell transplantation (Figure 3). There were no statistical differences between the groups.
Figure 3.
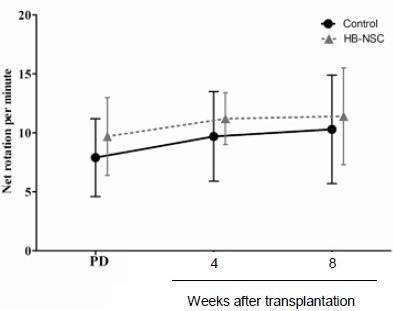
Effect of stem cell transplantation on Parkinson's disease symptom (apomorphine-induced rotation tests).
These tests were performed 1 week after 6-hydroxydopamine (6-OHDA) injection and 4 and 8 weeks after human brain-derived neural stem cell (HB-NSC) transplantation.
Data are shown as the net rotations per minute, and expressed as mean ± SD. The parameters were analyzed using the paired samples t-test. Asymmetrical rotations in the Parkinson's disease state were not reversed by human dental papilla-derived stem cell transplantation. PD: Parkinson's disease state.
Effect of stem cell transplantation on striatal glucose metabolism and dopamine transporter activities
[18F]-FDG and [18F]-FP-CIT microPET scans were performed 4 and 8 weeks after human brain-derived neural stem cell transplantation. Reconstructed volume of interest revealed that glucose metabolism in the lesional side of the striatum in the human brain-derived neural stem cell group was significantly reduced 4 weeks after transplantation compared with the control group (89.4 ± 2.0% vs. 96.6 ± 3.0%; P = 0.002; Figures 4, 5). This metabolic reduction was still persistent at 8 weeks after human brain-derived neural stem cell transplantation (90.2 ± 1.4% vs. 96.9 ± 3.6%; P = 0.003; Figures 4, 5). As determined by the volume of interest analysis of the [18F]-FP-CIT PET scans, dopamine transporter activity in the lesional side of the striatum in the human brain-derived neural stem cell group was increased 4 weeks after transplantation, but decreased by 8 weeks after transplantation (Figures 6, 7); in the control group, dopamine transporter activity gradually increased at 4 and 8 weeks after transplantation (Figures 6, 7). In the human dental papilla-derived stem cell group, we were unable to obtain PET data as all rats died within 2 weeks after cell transplantation.
Figure 4.
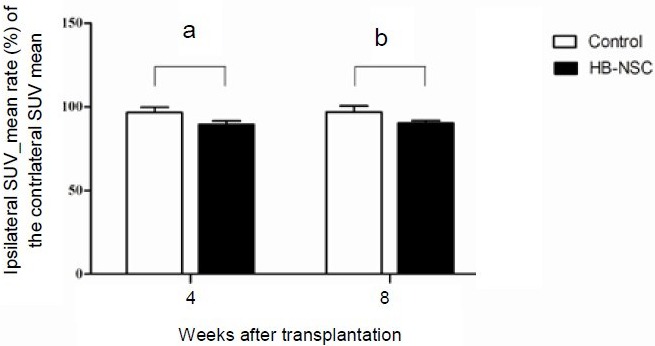
[18F]-FDG uptake determined using microPET scanning in the striatum.
Data are shown in terms of the ipsilateral standardized uptake value (SUV) mean rate (%) of contralateral SUV mean, and expressed as mean ± SD.
dThe parameters were analyzed using repeated measures analysis of variance (ANOVA). [18F]-FDG uptake in the human brain-derived neural stem cell (HB-NSC) group was significantly reduced 4 weeks after transplantation compared with the control group (aP = 0.002). This decline was still persistent 8 weeks after HB-NSC transplantation (bP = 0.003). [18F]-FDG: 2-Deoxy-2-[18F]-fluoro-D-glucose.
Figure 5.
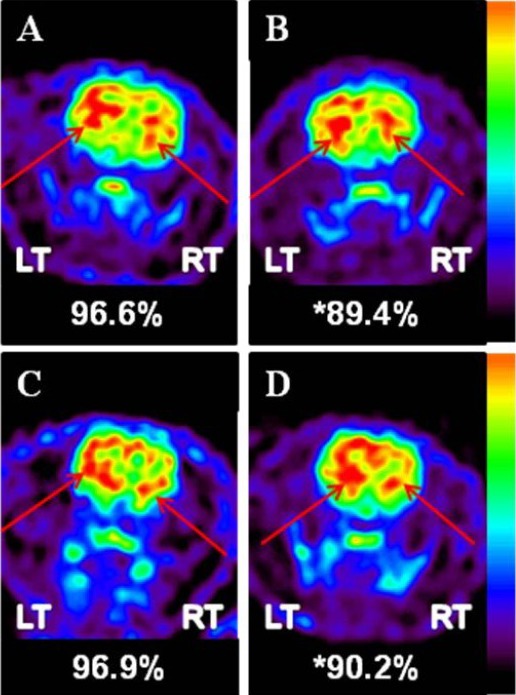
MicroPET [18F]-FDG image. Images show coronal sections through the striatum.
FDG uptake in the control (A) and in human dental papilla-derived stem cell groups (B) 4 weeks after transplantation (arrows). FDG uptake in (C) the control and (D) human brain-derived neural stem cell groups 8 weeks after transplantation (arrows). LT: Left; RT: right; [18F]-FDG:2-deoxy-2-[18F]-fluoro-D-glucose.
Figure 6.
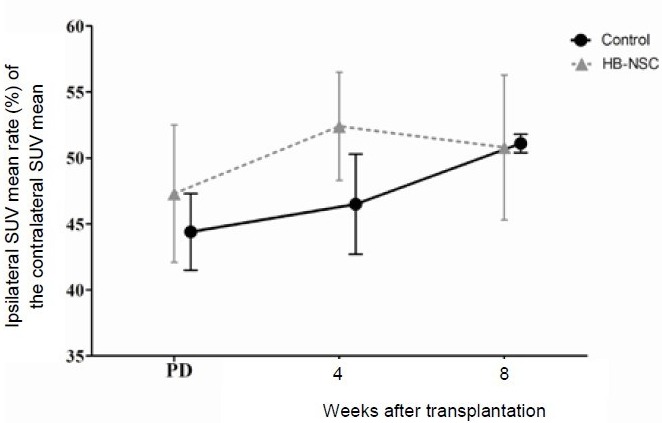
Detection of [18F]-FP-CIT using microPET scanning.
Data are shown as the ipsilateral standardized uptake value (SUV) mean rate (%) of the contralateral SUV mean, and expressed as mean ± SD. The parameters were analyzed using repeated measures analysis of variance (ANOVA). Detection of [18F]-FP-CIT in the human brain-derived neural stem cell (HB-NSC) group was increased 4 weeks after transplantation and decreased 8 weeks after transplantation. The detection of [18F]-FP-CIT in the control group gradually increased during the 8 weeks following PBS injection. PD: Parkinson's disease state. [18F]-FP-CIT: [18F]-N-(3-fluoropropyl)-2-carbomethoxy-3-(4-iodophenyl) nortropane.
Figure 7.
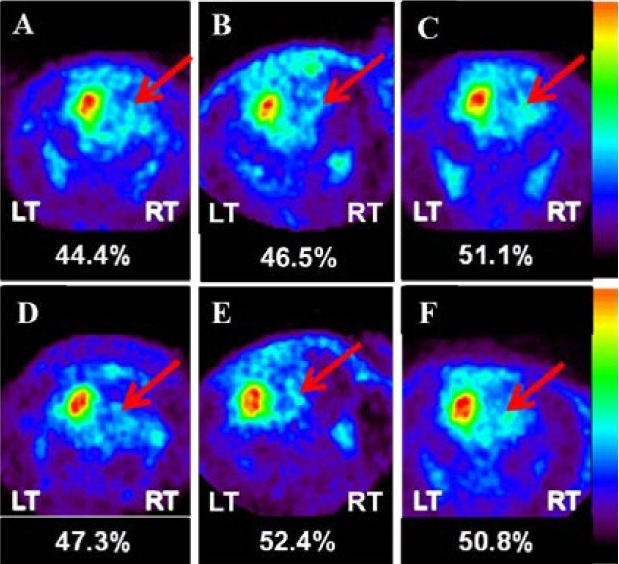
Figure 8 Tyrosine hydroxylase (TH) staining.
Immunohistochemistry for TH at 12 weeks after cell transplantation showing representative coronal sections from the striatum in the control group (A) and in the human brain-derived neural stem cell group (B).
Image of (A) showed an absence of TH-positive cells in the lesioned striatum of the control group. In contrast, the image of (B) showed the presence of TH-positive cells in the lesioned striatum of the human brain-derived neural stem cell group. (C, D) Magnified images of the lesioned striatum in the human brain-derived neural stem cell group (C: × 100, D: × 200 magnification).
Effect of stem cell transplantation on tyrosine hydroxylase and G protein-activated inward rectifier potassium channel 2 (GIRK2) expression in the lesional side of the striatum
Immunohistochemistry showed that the rats in the control group demonstrated negative tyrosine hydroxylase results in the lesional side of the striatum, while the human brain-derived neural stem cell (HB-NSC) group demonstrated positive tyrosine hydroxylase results at 12 weeks after cell transplantation. The dendrites of the tyrosine hydroxylase-positive cells in the striatum were typically extended in the same direction (Figure 8).
Figure 8.
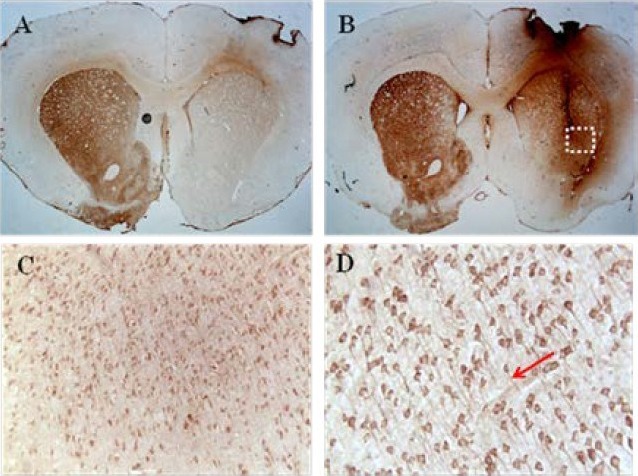
Figure 7 MicroPET [18F]-FP-CIT images.
Images showing coronal sections through the striatum. Detection of [18F]-FP-CIT in the Parkinson's disease state (A), 4 weeks after transplantation (B), and 8 weeks after transplantation (C) in the control group (arrows). Detection of [18F]-FP-CIT in the Parkinson's disease state (D), 4 weeks after transplantation (E), and 8 weeks after transplantation (F) in the human brain-derived neural stem cell group (arrows). LT: Left; RT: right; [18F]-FP-CIT: [18F]-N-(3-fluoropropyl)-2-carbomethoxy-3-(4-iodophenyl) nortropane.
Regarding the results of the immunofluorescence staining, colocalization of tyrosine hydroxylase- and G protein-activated inward rectifier potassium channel 2-positive cells was partially observed at the injection site of the striatum in the human brain-derived neural stem cell group at 12 weeks after cell transplantation. In contrast, the control group demonstrated neither tyrosine hydroxylase-nor G protein-activated inward rectifier potassium channel 2-positive cells (Figure 9).
Figure 9.
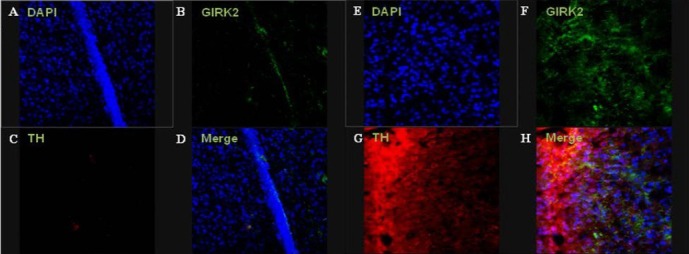
Immunofluorescence staining of the lesioned striatum at 12 weeks after cell transplantation.
Images showing colocalization of tyrosine hydroxylase (TH)- and G protein-activated inward rectifier potassium channel 2 (GIRK2)-positive cells were partially observed in the injection site of the human brain-derived neural stem cell (HB-NSC) group. Immunohistochemical results of GIRK2 (green; B, F), TH (red; C, G), and merged images (D, H). The control (A–D) and human brain-derived neural stem cell groups (E–H) are shown (all images: × 400 magnification).
DISCUSSION
In this study, we intrastriatally transplanted human dental papilla-derived stem cells and human brain-derived neural stem cells into a Parkinson's disease rat model. Several in vitro and in vivo studies showed the generation of functional neurons in neurogenic cultures of dental stem cells[12,13,14] and endogenous nerve regeneration via paracrine effects of the implanted dental stem cells[16,17]. In this study, we used teeth obtained from 6–11-year-old children because the functional and self-renewal abilities of stem cells tend to decrease with age[18,19]. Most ex vivo expanded cells from developing dental papilla tissue expressed Stro-1, and a subpopulation of cells expressed neural stem cell markers, such as nestin and polysialic acid-neural cell adhesion molecule. The polysialic acid-neural cell adhesion molecule positive dental papilla-derived stem cells could be isolated using magnetic cell sorting (MACS) and an anti-polysialic acid-neural cell adhesion molecule antibody[12,13,14]. Human brain-derived neural stem cells (Celprogen) express the neuroepithelial stem cell marker nestin, neurofilament, an astrocyte marker glial fibrillary acidic protein, and dendritic marker microtubule-associated protein 2. These cells were transplanted into the striatum of 6-hydroxydopamine-lesioned rats.
Unlike our expectations, human dental papilla-derived stem cells transplanted into the striatum transformed into homogeneous tumors that demonstrated continuous proliferation without differentiation, eventually leading to death. The postmortem study of the human dental papilla-derived stem cells group was unable to detect any neuronal markers, except microtubule-associated protein 2, which was only slightly positive. We concluded that before differentiation, early-stage human dental papilla-derived stem cells have the tendency to form tumors.
We are currently looking for an alternative method that could be used to guide human dental papilla-derived stem cells to differentiate into dopaminergic neurons. Human dental papilla-derived stem cells might still be a good candidate for use in cell therapies for the treatment of Parkinson's disease due to their availability, neuronal differentiation capacity[12], and lack of associated ethical problems.
Regarding the stepping test, we tested deficits in the use of the contralateral forelimbs to evaluate whether impaired motor activities increased. We were unable to clarify the therapeutic effects of intrastriatal human brain-derived neural stem cell injection using these tests. One week after the toxin insults, the rate of contralateral paw touches was remarkably reduced in all groups. In addition, during the follow-up period, continuous stepping deficits were noted in all groups.
The apomorphine-induced contralateral rotations that were generated by 6-hydroxydopamine injection persisted through 8 weeks after transplantation, indicating that asymmetrical dopamine release was not reversed by human brain-derived neural stem cell transplantation.
The results of the [18F]-FDG PET scans reveal that the viability of striatal neurons was reduced by human brain-derived neural stem cell transplantation, which persisted through 8 weeks after transplantation. This phenomenon might have been caused by volumetric damaged-induced cell death. This is a problem that must be solved to establish the safety of cell transplantation. In both the human brain-derived neural stem cell and control groups, we evaluated dopamine transporter activity using [18F]-FP-CIT PET 8 weeks after transplantation. [18F]-FP-CIT PET demonstrated fluctuations in the human brain-derived neural stem cell group and a gradual increase in the control group. This phenomenon might have been due to selection bias associated with the selected volume of interest. In conclusion, the results of [18F]-FP-CIT PET suggest that the human brain-derived neural stem cells transplanted into the striatum partially differentiated into dopaminergic cells that did not produce dopamine.
Regarding the immunohistochemical study, the transplanted human brain-derived neural stem cells demonstrated tyrosine hydroxylase positivity. In the immunofluorescence staining of the human brain-derived neural stem cell group, the partial colocalization of tyrosine hydroxylase and G protein-activated inward rectifier potassium channel 2 positivity was observed. These results are clearly different from the results seen in the control group. Tyrosine hydroxylase positivity indicates that the transplanted cells partially differentiated into dopaminergic cells, and there is a high probability that these cells belong to the A9 subtype of dopaminergic neurons with the expression of G protein-activated inward rectifier potassium channel 2. However, when considering dendritic alignment, which is typically shaped in the same direction, the transplanted human brain-derived neural stem cells did not form networks with each other or the host neurons.
Together, these results indicate that the human brain-derived neural stem cells transplanted into the striatum of Parkinson's disease rats can differentiate into dopaminergic neurons, but this differentiation does not result in the secretion of dopamine. Therefore, the transplanted human brain-derived neural stem cells do not improve behavioral activities or [18F]-FP-CIT uptake. Early-stage human dental papilla-derived stem cells exhibited the tendency to form tumors. Human brain-derived neural stem cells can partially differentiate into dopaminergic neurons, but they do not demonstrate dopamine secretion according to the results of stepping tests, apomorphine tests, or [18F]-FP-CIT PET scans. Therefore, more effective and safer stem cells are required to effectively treat Parkinson's disease using cell transplantation.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
This experiment was performed at Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea, from September 2011 to March 2012.
Materials
Sprague-Dawley rats, aged 7 week (Orient Bio Inc., Seongnam, Korea), each weighing between 180–210 g at the beginning of the experiment, were housed in a room under a 12-hour light/dark cycle and provided free access to food and water. All procedures complied with the guidelines of the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences (Seoul, Korea).
Methods
6-OHDA-lesion surgery
All surgical procedures were performed under general anesthesia induced by intraperitoneal injection of a mixture of 37.5 mg/kg Zoletil 50 (Virbac SA, Carros, France) and 6 mg/kg Rompun (Bayer, Leverkusen, Germany). A total of 23 rats were administered unilateral injections of 14 μg 6-OHDA hydrochloride (Sigma, St. Louis, MO, USA) in 4 μL of 0.9% saline with 0.1% ascorbic acid that was delivered into the right forebrain bundle at coordinates anteroposterior –4.4 mm, lateral +1.2 mm relative to bregma and ventral –7.8 mm from the dura by Paxinos's rat atlas, with the tooth bar set at –2.4 mm. The injections were delivered at a rate of 1 μL/min using a Hamilton syringe (33-gauge) and an automated microsyringe pump (Harvard Apparatus, Holliston, MA, USA). After injection, the needle was kept in place for 3 minutes to prevent the solution from flowing backward, and then retracted over the next 5 minutes.
Preparation of human dental papilla-derived stem cells and human brain-derived neural stem cells
Immature human premolars were extracted and collected from patients aged 6–11 years old who were undergoing orthodontic therapy. Before extraction, the patients and their guardians were informed of the procedures to be performed (the process was performed according to KHUSB IRB# 0908-01). The dental papilla tissues were procured from the extracted teeth, minced using a scalpel, and allowed to adhere to T25 flasks by culturing for 1 week in 1 mL of expansion medium that consisted of RPMI1640 (Lonza, Basel, Switzerland), 10% fetal bovine serum (FBS; Lonza), and 1% penicillin/streptomycin (Lonza). After 1 week, the stem cells were obtained from the papilla tissue fragments and maintained in an expansion medium that consisted of RPMI1640, 10% FBS, 1% penicillin/streptomycin, and 100 U/mL leukemia inhibitory factors (LIF; Invitrogen, Carlsbad, CA, USA) in a 5% CO2 humidified incubator[12].
Following ex vivo expansion, the dental papilla-derived stem cells were fixed with 4 % paraformaldehyde for 20 minutes at room temperature. After washing twice in PBS, the cells were permeabilized with 0.2 % Triton 100-X for 15 minutes and retrieved with 0.2 % sodium dodecyl sulfate polyacrylamide (SDS; Sigma) for 10 minutes. After blocking with 4 % bovine serum albumin (BSA; Millipore, Darmstadt, Germany) for 90 minutes, samples were incubated overnight with primary antibodies that had been diluted in 1% BSA solution as follows: 1:200 mouse anti-human STRO-1 antibody (Chemicon, Darmstadt, Germany), 1:250 rabbit anti-human CD44 antibody (Abcam, Cambridge, MA, USA), 1:200 mouse anti-human nestin antibody (Abcam), and 1:200 mouse anti-human polysialic acid-neural cell adhesion molecule antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). These cells were subsequently incubated for 1 hour with secondary antibodies (goat anti-mouse IgG-Alexa 488 antibody (1:1 000; Invitrogen, Carlsbad, CA, USA) or donkey anti-rabbit IgG-Alexa 594 antibody (1:2 000; Invitrogen) at room temperature. Cell nuclei were labeled with 4’,6’-diamidino-2-phenylindole dihydrochloride (DAPI; KPL, Gaithersburg, MD, USA).
To isolate the dental papilla-derived stem cells expressing polysialic acid-neural cell adhesion molecule, the cells were incubated with 1:200 mouse anti-human polysialic acid-neural cell adhesion molecule antibody (Santa Cruz Biotechnology) in 1% BSA solution for 1 hour in ice. These cells were subsequently incubated with 1:5 magnetic-activated cell sorting 2nd anti-mouse IgG microbead (MilitenyiBiotec, Bergisch Gladbach, Germany) in 0.5% BSA solution containing 2 mmol/L ethylenediamine tetraacetic acid for 45 minutes on ice, and then separated by magnetic-activated cell sorting magnetic column (MilitenyiBiotec), which was repeated three times. Separated cells were maintained in expansion medium consisting of RPMI1640 (Lonza), 10% FBS (Lonza), 1% penicillin/streptomycin (Lonza) and 100 U/mL leukemia inhibitory factor (LIF; Invitrogen) in a 5% CO2 humidified incubator (Figure 10).
Figure 10.
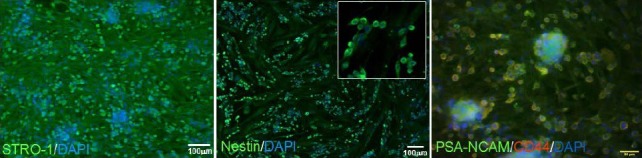
Isolation of the dental papilla-derived stem cells expressing polysialic acid-neural cell adhesion molecule (PSA-NCAM).
Most of the ex vivo expanded cells from developing dental papilla tissue expressed STRO-1 (green), and some of the cell population expressed neural stem cell markers, such as nestin and PSA-NCAM (green). The PSA-NCAM-positive dental papilla-derived stem cells could be isolated using magnetic-activated cell sorting and anti-PSA-NCAM antibody, and were transplanted into rat models of Parkinson's disease. Blue staining is 4’,6-diamidino-2-phenylindole (DAPI).
Human brain-derived neural stem cells (cat# 36109-36) and brain stem cell growth media (cat# M36109-36S) were purchased from Celprogen (San Pedro, CA, USA). Human brain-derived neural stem cells were cultured in brain stem cell growth media in accordance with the manufacturer's instructions.
Cell transplantation
Three weeks after injection of 6-hydroxydopamine, all of the rats were randomly divided into three groups. The Parkinson's disease control group (n = 7) was administered a 15-μL injection of PBS (pH 7.4). The human dental papilla-derived stem cell (n = 8) and human brain-derived neural stem cell groups (n = 8) were administered resuspended cells in sterilized PBS at a concentration of 1 × 106 cells/15 μL. The cells were injected at a rate of 1 μL/min into the ipsilateral striatum via double tracks at the following coordinates: anteroposterior +0.6/+1.6 mm, lateral +3.5/+3.2 mm, and ventral –4.5 mm with the tooth bar set at +2.3 mm. The same procedures were used for the above mentioned 6-hydroxydopamine injections. All rats received immunosuppressive treatment, including daily administration of 10 mg/kg cyclosporine A (intraperitoneal CIPOL; Chong Kun Dang Pharm, Seoul, Korea), which was initiated the day prior to cell transplantation until the animals were sacrificed.
Stepping tests
The stepping tests were performed six times: during the intact state, 1 week after 6-hydroxydopamine injections, and 2, 4, 6, and 8 weeks after cell transplantations, as previously described[20] with slight modifications. Briefly, both hind limbs were firmly fixed in one hand of the experimenter, while the other hand was used to fix one of the forelimbs. The rostral part was lowered onto a treadmill (Jeung Do Bio & Plant Co., Seoul, Korea), which was set to a rate of 0.9 m/5 s. The rat's body remained stationary while the unilateral forelimb was allowed to touch the moving treadmill track for > 5 seconds while the treadmill was operating. All of the experiments were video recorded to count the number of adjusted steps taken in the backward direction. Every rat performed the stepping test twice during every session, and the data were averaged. The data are expressed as the percentage of contralateral forelimb steps versus ipsilateral forelimb steps.
Drug-induced rotation tests
Apomorphine-induced tests were performed in transparent cylinders 1 week after 6-hydroxydopamine injection and 4 and 8 weeks after cell transplantation, as previously described[21] but with slight modifications. Briefly, as soon as 0.5 mg/kg apomorphine (dissolved in sterile water and subcutaneously administered; Sigma) was administrated, the rat was harnessed to an automated rotometer (Panlab, Barcelona, Spain) and its rotational behavior was assessed over 45 minutes. Data are expressed as the net (contralateral – ipsilateral turns) average rotations per minute. 6-OHDA-lesioned rats that demonstrated > 6 rotations per minute were selected for inclusion in the present study.
PET scanning in the striatum
All animals were scanned using the microPET Focus 120 system (Siemens Medical Solutions, Inc., Erlangen, Germany), which includes a 12 × 12 array of lutetium oxyorthosilicate and 96 detector blocks. Metabolic activity was assessed in the Parkinson's disease control and human brain-derived neural stem cell groups (n = 5, respectively) using [18F]-FDG at 4 and 8 weeks after cell transplantations were performed.
Dopamine transporter function was assessed in the Parkinson's disease control and human brain-derived neural stem cell groups (n = 3, respectively) using [18F]-FP-CIT, which binds to dopamine transporter with high affinity, at 1 and 2 weeks after 6-hydroxydopamine injection and 4 and 8 weeks after cell transplantations. After fasting for at least 12 hours, the rats were injected with 1 mCi [18F]-FDG in 0.2 mL of sterile saline via the tail vein. One hour following injection, the rats were scanned for 30 minutes under isoflurane anesthesia. In [18F]-FP-CIT scans, rats were injected with 1 mCi [18F]-FP-CIT in 0.4 mL sterile saline via the tail vein, immediately followed by dynamic scanning for 90 minutes. The [18F]-FDG PET data were reconstructed using a 3-dimensional (3D) maximum a posteriori (MAP) algorithm, and a 2D ordered-subset expectation maximization (OSEM 2D) algorithm was used to reconstruct the [18F]-FP-CIT data[11]. PET images were analyzed using ASIPro software (Siemens Preclinical Solutions, Knoxville, TN, USA) to generate a 3D volume of interest that was combined with three regions of interest at different axial slice levels. Data are expressed as the percentage of ipsilateral standardized uptake values, based on the mean uptake of volume of interest against the contralateral standardized uptake values.
Tissue processing
Twelve weeks after cell transplantation, rats were transcardially and sequentially perfused with 0.9% saline containing 10 000 IU heparin (Hanlim Pharm, Seoul, Korea) and 4% paraformaldehyde in PBS. Extracted brain tissues were postfixed overnight in the same fixative, followed by incubation and dehydration in 30% sucrose until they sank. Serial sections from frozen tissue, including the striatum (anteroposterior +2.5 to 0.0 mm), that were at least 30 μm in thickness were collected using a microtome (Leica, Wetzlar, Germany) and preserved under free-floating conditions in 0.08% sodium azide in PBS at 4°C. For the human dental papilla-derived stem cell group, the brain tissues were extracted and the tumors at the injection site of the striatum were dissected with a blade into 2 × 2 × 2 mm3 shaped pieces and used for immunohistochemical analysis.
Immunohistochemistry
The immunostaining and immunofluorescence procedures were performed as previously described[22,23]. Striatal sections were incubated overnight with primary antibodies that had been diluted in PBS (pH 7.4) containing 0.5% BSA. These sections were subsequently incubated for 2 hours at room temperature with biotinylated secondary antibodies produced in horse (1:200; Vector Laboratories, Burlingame, CA, USA) that had been diluted in the same buffer for tyrosine hydroxylase immunostaining and fluorescent-conjugated secondary antibodies (Invitrogen, Grand island, NY, USA) that had been diluted in the same buffer for immunofluorescence labeling. The complexes of primary and secondary antibodies were detected by generation of color when diaminobenzidine (R&D Systems, Minneapolis, MN, USA) was added or conjugated fluorophores. All tissues were mounted on gelatin-coated slides and dried. Diaminobenzidine-treated tissues were dehydrated in a series of alcohol and clearing agents, following which a coverslip was applied. Fluorescent-labeled tissues were coverslipped with DAKO fluorescent mounting medium (DAKO, Glostrup, Denmark). The dilution ratios of the primary antibodies were as follows: mouse anti-tyrosine hydroxylase (1:5 000; Sigma) and rabbit anti-G protein-activated inward rectifier potassium channel 2 (1:1 500; Novus Biologicals, Littleton, CO, USA). Secondary antibodies (Alexa Fluor® 555 goat anti-mouse and Alexa Fluor® 488 goat anti-rabbit IgG; Invitrogen) that had been conjugated with a florescent marker were diluted to 1:1 000. Immunohistochemical analysis of the human dental papilla-derived stem cell group was performed using an automated staining system (Ventana Medical Systems, Milan, Italy) according to manufacturer's instructions. The dilution ratios of the primary antibodies were as follows: mouse anti-microtubule-associated protein 2 (1:100; Thermo scientific, Waltham, MA, USA), mouse anti-human neurofilament protein (1:100; DakoCytomation, Glostrup, Denmark), mouse anti-glial fibrillary acidic protein (BioGenex, Fremont, CA, USA), and rabbit anti-synaptophysin (1:100; Thermo scientific).
Tyrosine hydroxylase-immunoresponsive cells were observed in the coronal sections of the striatum using a Nikon 80i microscope (Nikon, Tokyo, Japan) at ×100 magnification that employed NIS-Elements F3.0 software (Nikon, Tokyo, Japan). The fluorescent images were obtained using a confocal microscope (TCS-ST2; Leica, Wetzlar, Germany).
Statistical analysis
All data were presented as mean ± SD. All statistical analyses were performed using SPSS (version 12.0.1; SPSS Inc., Chicago, IL, USA). Behavioral parameters were analyzed using the paired samples t-test, and PET data were analyzed using repeated measures analysis of variance (ANOVA). A P value < 0.05 was regarded as statistically significant.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by a “KRCF National Agenda Project”, by an Asan Life Science Institute Grant (12-241) from the Asan Medical Center, Seoul, Korea.
Ethical approval: The Institutional Animal Care and Ethical Committee of the Asan Institute for Life Sciences (Seoul, Korea) approved this experiment.
(Reviewed by Wallace M, Haase R, Dong CX, Lee JE)
(Edited by Li CH, Song LP)
REFERENCES
- 1.Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson's disease. J Intern Med. 2009;266(4):358–371. doi: 10.1111/j.1365-2796.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 2.Chesselet MF, Richter F. Modelling of Parkinson's disease in mice. Lancet Neurol. 2011;10(12):1108–1118. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- 3.de la Fuente-Fernandez R, Schulzer M, Mak E, et al. Presynaptic mechanisms of motor fluctuations in Parkinson's disease: a probabilistic model. Brain. 2004;127(Pt 4):888–899. doi: 10.1093/brain/awh102. [DOI] [PubMed] [Google Scholar]
- 4.Benabid AL, Krack PP, Benazzouz A, et al. Deep brain stimulation of the subthalamic nucleus for Parkinson's disease: methodologic aspects and clinical criteria. Neurology. 2000;55(12)(Suppl 6):S40–44. [PubMed] [Google Scholar]
- 5.Fraix V, Pollak P, Van Blercom N, et al. Effect of subthalamic nucleus stimulation on levodopa-induced dyskinesia in Parkinson's disease. Neurology. 2000;55(12):1921–1923. doi: 10.1212/wnl.55.12.1921. [DOI] [PubMed] [Google Scholar]
- 6.Lei Z, Jiang Y, Li T, et al. Signaling of glial cell line-derived neurotrophic factor and its receptor GFRalpha1 induce Nurr1 and Pitx3 to promote survival of grafted midbrain-derived neural stem cells in a rat model of Parkinson disease. J Neuropathol Exp Neurol. 2011;70(9):736–747. doi: 10.1097/NEN.0b013e31822830e5. [DOI] [PubMed] [Google Scholar]
- 7.Ratzka A, Kalve I, Özer M, et al. The colayer method as an efficient way to genetically modify mesencephalic progenitor cells transplanted into 6-OHDA rat model of Parkinson's disease. Cell Transplant. 2012;21(4):749–762. doi: 10.3727/096368911X586774. [DOI] [PubMed] [Google Scholar]
- 8.Battisti JJ, Uretsky NJ, Wallace LJ. Sensitization of apomorphine-induced stereotyped behavior in mice is context dependent. Psychopharmacology (Berl) 1999;146(1):42–48. doi: 10.1007/s002130051086. [DOI] [PubMed] [Google Scholar]
- 9.Yoon HH, Lee CS, Hong SH, et al. Evaluation of a multiple system atrophy model in rats using multitracer microPET. Acta Neurochir (Wien) 2012;154(5):935–940. doi: 10.1007/s00701-011-1133-z. [DOI] [PubMed] [Google Scholar]
- 10.Casteels C, Lauwers E, Bormans G, et al. Metabolic-dopaminergic mapping of the 6-hydroxydopamine rat model for Parkinson's disease. Eur J Nucl Med Mol Imaging. 2008;35(1):124–134. doi: 10.1007/s00259-007-0558-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Lee JS, Im KC, et al. Performance measurement of the microPET focus 120 scanner. J Nucl Med. 2007;48(9):1527–1535. doi: 10.2967/jnumed.107.040550. [DOI] [PubMed] [Google Scholar]
- 12.Kim BC, Bae H, Kwon IK, et al. Osteoblastic/cementoblastic and neural differentiation of dental stem cells and their applications to tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2012;18(3):235–244. doi: 10.1089/ten.teb.2011.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur A, Rychkov G, Shi S, et al. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 14.Völlner F, Ernst W, Driemel O, et al. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation. 2009;77(5):433–441. doi: 10.1016/j.diff.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-Moreno T, Castillo CG, Martínez-Serrano A. Long term behavioral effects of functional dopaminergic neurons generated from human neural stem cells in the rat 6-OH-DA Parkinson's disease model. Effects of the forced expression of BCL-X(L) Behav Brain Res. 2012;232(1):225–232. doi: 10.1016/j.bbr.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Arthur A, Shi S, Zannettino AC, et al. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009;27(9):2229–2237. doi: 10.1002/stem.138. [DOI] [PubMed] [Google Scholar]
- 17.Huang AH, Snyder BR, Cheng PH, et al. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells. 2008;26(10):2654–2663. doi: 10.1634/stemcells.2008-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodson SA, Bernard GW, Kenney EB, et al. In vitro comparison of aged and young osteogenic and hemopoietic bone marrow stem cells and their derivative colonies. J Periodontol. 1996;67(3):184–196. doi: 10.1902/jop.1996.67.3.184. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji T, Hughes FJ, McCulloch CA, et al. Effects of donor age on osteogenic cells of rat bone marrow in vitro. Mech Ageing Dev. 1990;51(2):121–132. doi: 10.1016/0047-6374(90)90094-v. [DOI] [PubMed] [Google Scholar]
- 20.Paille V, Henry V, Lescaudron L, et al. Rat model of Parkinson's disease with bilateral motor abnormalities, reversible with levodopa, and dyskinesias. Mov Disord. 2007;22(4):533–539. doi: 10.1002/mds.21308. [DOI] [PubMed] [Google Scholar]
- 21.Puschban Z, Scherfler C, Granata R, et al. Autoradiographic study of striatal dopamine re-uptake sites and dopamine D1 and D2 receptors in a 6-hydroxydopamine and quinolinic acid double-lesion rat model of striatonigral degeneration (multiple system atrophy) and effects of embryonic ventral mesencephalic, striatal or co-grafts. Neuroscience. 2000;95(2):377–388. doi: 10.1016/s0306-4522(99)00457-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim ST, Choi JH, Chang JW, et al. Immobilization stress causes increases in tetrahydrobiopterin, dopamine, and neuromelanin and oxidative damage in the nigrostriatal system. J Neurochem. 2005;95(1):89–98. doi: 10.1111/j.1471-4159.2005.03342.x. [DOI] [PubMed] [Google Scholar]
- 23.Hwang O, Baker H, Gross S, et al. Localization of GTP cyclohydrolase in monoaminergic but not nitric oxide-producing cells. Synapse. 1998;28(2):140–153. doi: 10.1002/(SICI)1098-2396(199802)28:2<140::AID-SYN4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]


