Abstract
Human milk oligosaccharides (HMO) are prominent among the functional components of human breast milk. While HMO have potential applications in both infants and adults, this potential is limited by the difficulties in manufacturing these complex structures. Consequently, functional alternatives such as galacto-oligosaccharides are under investigation, and nowadays, infant formulae are supplemented with galacto-oligosaccharides to mimic the biological effects of HMO. Recently, approaches toward the production of defined human milk oligosaccharide structures using microbial, fermentative methods employing single, appropriately engineered microorganisms were introduced. Furthermore, galactose-containing hetero-oligosaccharides have attracted an increasing amount of attention because they are structurally more closely related to HMO. The synthesis of these novel oligosaccharides, which resemble the core of HMO, is of great interest for applications in the food industry.
Keywords: β-galactosidases, transgalactosylation, galacto-oligosaccharides, human milk oligosaccharides, hetero-oligosaccharides
Introduction
Certain oligosaccharides are considered to be beneficial for human and animal hosts due to their ability to stimulate selectively growth and/or activity of one or a limited number of bacteria in the colon. They are classified as ‘prebiotics’, new functional food ingredients that are of considerable interest. The prebiotic compounds are typically oligosaccharides of various compositions, and galacto-oligosaccharides (GOS), the products of transgalactosylation reactions catalyzed by β-galactosidases when using lactose as the substrate, are nondigestible carbohydrates meeting the criteria of ‘prebiotics’ (Roberfroid et al., 2010). GOS are of special interest to human nutrition because of the presence of structurally related oligosaccharides together with different complex structures in human breast milk (Sangwan et al., 2011). Several different functions are attributed to these human milk oligosaccharides (HMO). With respect to the influence on the intestinal microbiota, the neutral fraction of HMO seems to be a key factor for the development of the intestinal microbiota typical for breastfed infants and hence for the prebiotic effect. GOS together with inulin/fructo-oligosaccharides (FOS) and lactulose are among the most important and best-studied groups of prebiotic oligosaccharides. At present, these commercially important oligosaccharides with prebiotic status are available mainly in the Japanese, European and USA markets. A mixture of GOS and long-chain FOS was introduced in the market especially for the use in infant formula. This mixture can mimic HMO to some extent and shows a pronounced prebiotic effect; in that it stimulated the development of intestinal microbiota comparable with those found in breastfed infants (Boehm et al., 2008). Hence, biocatalytically produced GOS can be of significant interest for the nutrition of infants. β-galactosidases have also been used to produce hetero-oligosaccharides (HOS) with potentially extended functionality in addition to GOS. Mannose, fructose, N-acetylneuraminic acid, glucuronic acid and a number of aromatic compounds have been shown to act as galactosyl acceptor for β-galactosidases (Gänzle, 2012). The choice of suitable acceptor and enzyme allows the formation of ‘tailor-made’ HOS of high interest for applications in the food industry. This article highlights the recent progress on research in microbial production of GOS. The emerging trends in the biosynthesis of the novel oligosaccharides, which are structurally more closely related to HMO, will be reviewed as well.
Microbial production of GOS
Transgalactosylation of lactose using β-galactosidases
β-galactosidases (β-gal; EC 3.2.1.23) catalyze the hydrolysis and transgalactosylation of β-d-galactopyranosides (such as lactose). GOS are the products of transgalactosylation reactions catalyzed by β-galactosidases when using lactose or other structurally related galactosides as the substrate. β-galactosidases undergo a two-step mechanism of catalysis. First, this mechanism involves the formation of a covalently linked galactosyl-enzyme intermediate. Subsequently, the galactosyl moiety linked to the nucleophile in the active site is transferred to a nucleophilic acceptor. Water, as well as all sugar species present in the reaction mixture, can serve as a galactosyl acceptor. Hence, the resulting final mixture contains hydrolysis products of lactose, which are glucose and galactose, unconverted lactose as well as di-, tri- and higher oligosaccharides. Scheme 1 illustrates possible lactose conversion reactions catalyzed by β-galactosidases, and structures of some GOS are given in Fig. 1.
Scheme 1.
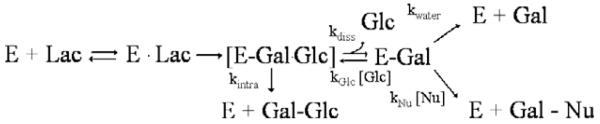
Hydrolysis and galactosyl transfer reactions, both intra- and intermolecular, during the conversion of lactose catalyzed by β-galactosidases. E, enzyme; Lac, lactose; Gal, galactose; Glc, glucose; Nu, nucleophile.
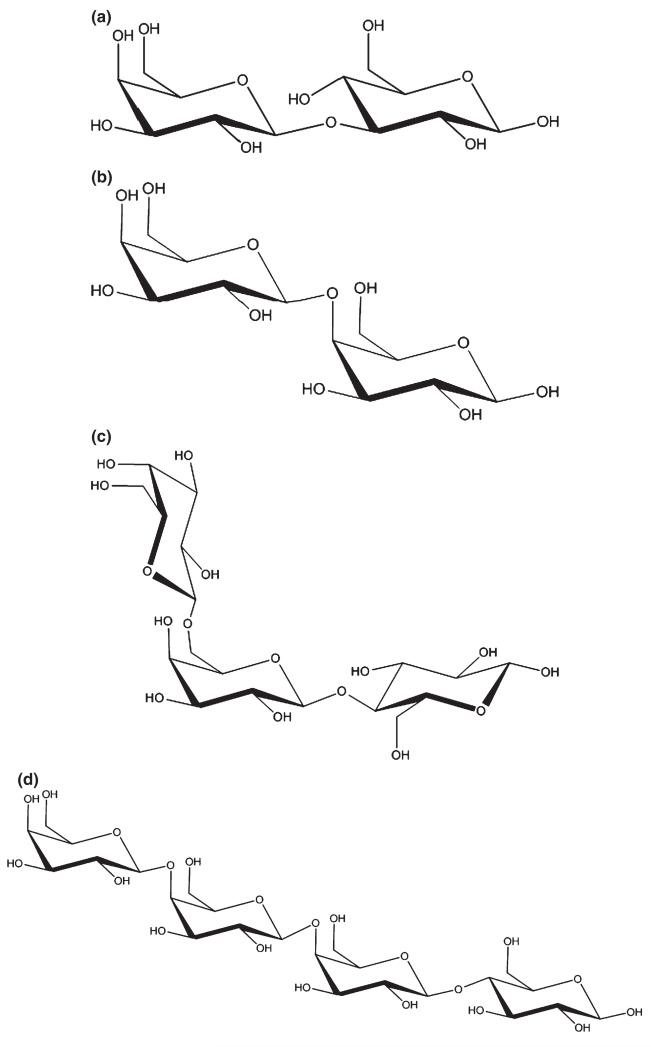
Fig. 1.
Structures of some GOS: β-d-Galp-(1→ 3)-d-Glc (a), β-d-Galp-(1→4)-d-Gal (b), β-d-Galp-(1→6)-Lac (c), β-d-Galp-(1→4)-d-Galp-(1→4)-Lac (d).
β-galactosidases can be obtained from different sources including microorganisms, plants and animals. Microbial β-galactosidases have been isolated and characterized from yeasts, fungi and bacteria. The major industrial enzymes are obtained from Aspergillus spp. and Kluyveromyces spp. where Kluyveromyces lactis is probably the most widely used source (Kim et al., 2004). Microbial sources of β-galactosidase are of great biotechnological interest because of easier handling, higher multiplication rates and production yield. Table 1 presents some of the commercially available bacterial, fungal and yeast β-galactosidases. An extensive list of bacterial and fungal sources of β-galactosidases, as well as the lactose conversion reaction conditions and GOS yields, are given in the review by Torres et al. (2010).
Table 1.
Commercial β-galactosidases
| Name | Manufacturer | Microorganism |
|---|---|---|
| BioLactase NTL-CONC | Biocon | Bacillus circulans (Rodriguez-Colinas et al., 2014) |
| Lactozym pure 6500 L | Novozymes | Kluyveromyces lactis (Rodriguez-Colinas et al., 2014) |
| Lactase F ‘Amano’ | Amano Enzyme Inc | Aspergillus oryzae (Rodriguez-Colinas et al., 2014) |
| Biolacta FN5 | Daiwa Fine Chemicals Co., Ltd | Bacillus circulans |
| LACTOLES L3 | Biocon Ltd, Japan | Bacillus circulans |
| Maxilact | DSM Food Specialties | Kluyveromyces lactis |
| Tolerase | DSM Food Specialties | Aspergillus oryzae |
β-galactosidases from different species possess very different specificities for building glycosidic linkages and therefore produce different GOS mixtures. For example, the β-galactosidase from K. lactis produced predominantly β-(1→6)-linked GOS, the β-galactosidase from Aspergillus oryzae produced mainly β-(1→3) and β-(1→6) linkages, Bacillus circulans β-galactosidase forms mainly β-(1→4)-linked GOS (Rodriguez-Colinas et al., 2014), whereas β-galactosidases from Lactobacillus spp. showed preference to form β-(1→6) as well as β-(1→3) linkages in transgalactosylation mode (Splechtna et al., 2006; Nguyen et al., 2012).
Production of GOS
Microbial sources of β-galactosidases for GOS production include crude enzymes, purified enzymes, recombinant enzymes, immobilized enzymes, whole-cell biotransformations, toluene-treated cells and immobilized cells. The enzyme sources, the process parameters as well as the yield and the productivity of these processes for GOS production are summarized in detail in recent reviews (Torres et al., 2010; Sangwan et al., 2011). The highest GOS productivity, 106 g L−1 h−1, was observed when β-galactosidase from A. oryzae immobilized on cotton cloth was used for GOS production in a packed-bed reactor (Albayrak & Yang, 2002).
Bifidobacteria and lactobacilli have been studied intensively with respect to their enzymes for various different reasons, one of which is their ‘generally recognized as safe’ status and their safe use in food applications. It is anticipated that GOS produced by these β-galactosidases will have better selectivity for growth and metabolic activity of these bacterial genera in the gut and thus will lead to improved prebiotic effects. A number of studies report the presence of multiple β-galactosidases, for example, in Bifidobacterium infantis, Bifidobacterium adolescentis or Bifidobacterium bifidum (Hung & Lee, 2002; Hinz et al., 2004; Goulas et al., 2009). It was shown that these enzymes are very different with respect to substrate specificity and regulation of gene expression. Furthermore, these reports described the cloning and characterization of these enzymes and studied their transgalactosylation activity in detail; for example, β-galactosidase BgbII from B. adolescentis showed high preference toward the formation of β-(1→4) linkages, while no β-(1→6) linkages were formed (Hinz et al., 2004). In contrast, the β-galactosidase BgbII from B. bifidum showed a clear preference for the synthesis of β-(1→6) linkages over β-(1→4) linkages (Goulas et al., 2009). A recombinant β-galactosidase from B. infantis (Hung & Lee, 2002) is an excellent biocatalyst for GOS production giving the highest GOS yield of 63% (mass of GOS of the total sugars in the reaction mixture). β-galactosidases of lactobacilli play an important role in a number of commercial processes, for example, milk processing or cheese making. Recent studies of β-galactosidases, especially with respect to their enzymatic and molecular properties, from Lactobacillus reuteri or Lactobacillus bulgaricus showed that these enzymes are very well suited for the production of GOS (Splechtna et al., 2006; Nguyen et al., 2012). Maximum GOS yields at 30 °C were c. 40% when using purified β-galactosidases from L. reuteri with initial lactose concentration of 205 g L−1 and at c. 80% lactose conversion (Splechtna et al., 2006). Purified β-galactosidase from L. bulgaricus gave the highest yield of 50% for the lactobacillal enzymes at 90% lactose conversion (Nguyen et al., 2012). To reduce enzyme costs, a crude β-galactosidase extract from Lactobacillus sp. directly obtained after cell disruption and separation of cell debris by centrifugation was used in lactose conversion for GOS production (Splechtna et al., 2007b).
Choice of process technology
The choice of process technology either for lactose hydrolysis or GOS production depends on the nature of the substrate and the characteristics of the enzyme. The primary characteristic, which determines the choice and application of a given enzyme, is the operational pH range. Acid pH enzymes, which are mainly from fungi, are suitable for processing of acid whey and whey permeate, while the neutral pH enzymes from yeasts and bacteria are suitable for processing milk and sweet whey. Depending on the enzyme source, the pH value of the reaction mixture can be acidic when using, for example, the β-galactosidase from A. oryzae with an optimum GOS yield at pH 4.5 (Iwasaki et al., 1996). The β-galactosidase from an acidophilic fungus, Teratosphaeria acidotherma AIU BGA-1, is stable over the pH range of 1.5–7.0 with optimal activity at pH 2.5–4.0 and 70 °C (Isobe et al., 2013). In contrast, the maximum yield of GOS was observed at neutral pH for most bacterial and fungal β-galactosidases. The highest GOS yields are generally observed when the reaction proceeds to 45–90% lactose conversion (Torres et al., 2010).
Studies of thermostable glycoside hydrolases have been conducted in pursuit of GOS production at high temperatures. These include β-glycosidases from Alicyclobacillus acidocaldarius, Thermus thermophilus KNOUC202 or L. bulgaricus, to name a few (Di Lauro et al., 2008; Nam et al., 2010; Nguyen et al., 2012). Cold-active β-galactosidases have also attracted attention because their applications in the industrial processes of lactose hydrolysis and oligosaccharides synthesis can lower the risk of mesophiles contamination. Cold-active β-galactosidases were isolated from different sources such as Paracoccus sp. 32d, Halorubrum lacusprofundi and Thalassospira frigidphilosprofundus (Wierzbicka-Woś et al., 2011; Karan et al., 2013; Pulicherla et al., 2013). Soluble cold-active β-galactosidase from Paracoccus sp. 32d was found to efficiently hydrolyze lactose in milk at 10 °C (Wierzbicka-Woś et al., 2011). There has been relatively little research on GOS synthesis at low temperatures by these psychrophilic enzymes.
Reactor set-up is an important factor that can influence both the yield and the composition of the GOS mixtures formed. Continuous GOS production using a continuous stirred tank reactor (CSTR) with an external cross-flow membrane was compared with the batch-wise mode of conversion using β-galactosidase from L. reuteri. Marked differences were detected for the two reactor setups. Above 65% lactose conversion, the GOS yield was lower for the CSTR due to a lower content of tri- and tetrasaccharides in the reaction mixture. In the CSTR, β-gal from L. reuteri showed up to twofold higher specificity toward the formation of β-(1→6) linked GOS with β-d-Galp-(1→6)-d-Glc and β-d-Galp-(1→6)-d-Gal being the main GOS components formed under these conditions (Splechtna et al., 2007a). A rotating disk membrane bioreactor was compared over batch mode to obtain purified GOS with high yield. It was found that GOS yield and purity were 32.4% and 77%, respectively, in batch mode followed by diafiltration-assisted nanofiltration, while in the immobilized state, they were 67.4% and 80.2% at 105 rad s−1 membrane speed. Retention of the monosaccharides that inhibit the enzyme in the reaction volume of batch mode reduced the yield of GOS. On the contrary, simultaneous production and purification of GOS in the rotating disk membrane bioreactor led to a high yield of GOS (Sen et al., 2012).
Compared with soluble β-galactosidases, immobilized β-galactosidases may provide advantages such as high enzyme reusability, higher cell densities in bioreactors, improved enzyme stability, reutilization and continuous operation, and easier separation of the products (Verma et al., 2012). Higher activity but lower thermostability was reported for β-galactosidase immobilized on chitosan nanoparticles than that bound onto macroparticles (Klein et al., 2012). β-galactosidase immobilized in polyvinyl alcohol lenses was more stable and converted more lactose than when immobilized in solgel carriers (Jovanovic-Malinovska et al., 2012). Compared with the corresponding free enzyme systems, immobilization resulted in less product inhibition by glucose and a higher stability at denaturing temperatures (Klein et al., 2012; Verma et al., 2012).
Prediction of GOS production by modeling techniques
Several kinetic mechanisms, either mechanistic, empirical or a combination of both, have been proposed to account for the transgalactosylation reactions and to subsequently define strategies to optimize GOS production. A six-parameter model was developed to describe oligosaccharide production from lactose hydrolysis by β-galactosidase from B. circulans; the model considered glucose inhibition, but ignored the formation of tetra- and higher oligosaccharides (Boon et al., 2000). A model of K. lactis β-galactosidase describing both hydrolysis and transgalactosylation reactions with glucose and lactose as acceptors fitted well to the experimental data of the time course reactions at various concentrations of lactose (Kim et al., 2004). A pseudo steady-state model for the kinetically controlled synthesis of GOS with A. oryzae β-galactosidase was presented by Vera et al. (2011). This model predicted substrate and product profiles during GOS synthesis in the temperature range between 40 and 55 °C, providing a useful tool for process scale-up and optimization. The model accounts for the total GOS production and its composition, which is a definite advantage over previously existing models. However, the model tends to underestimate disaccharides consumption and penta-GOS (GOS-5) formation and to overestimate glucose production.
Leveraging the power of protein engineering for GOS production
Protein engineering is a powerful approach to favor transgalactosylation over hydrolysis and hence to improve transgalactosylation yields. A truncated β-galactosidase from B. bifidum enhanced the transgalactosylation activity of the enzyme toward lactose, and as a result, a normal, hydrolytic β-galactosidase was converted to a highly efficient transgalactosylating enzyme (Jørgensen et al., 2001). A mutagenesis approach was applied to the galactosidase BgaB of Geobacillus stearothermophilus KVE39 to improve its enzymatic transglycosylation of lactose into oligosaccharides. Exchange of one single amino acid, arginine Arg109, in β-galactosidase BgaB to either lysine, valine or tryptophan improved significantly the formation of the main trisaccharide, that is, 3′-galactosyl-lactose. The yield of this trisaccharide increased from 2% to 12%, 21% and 23%, respectively, for these different variants compared with that of the native enzyme (Placier et al., 2009). Enhancement of the production of GOS was also achieved by mutagenesis of Sulfolobus solfataricus β-galactosidase, LacS. The GOS yield obtained from two mutants of LacS, F441Y and F359Q, was increased by 10.8% and 7.4%, respectively (Wu et al., 2013). Although protein engineering strategies were successfully applied to enhance transgalactosylation activities of different β-galactosidases, this approach has not yet been reported to alter the linkage type of the GOS products as well.
Biosynthesis of oligosaccharides structurally related to those found in human milk
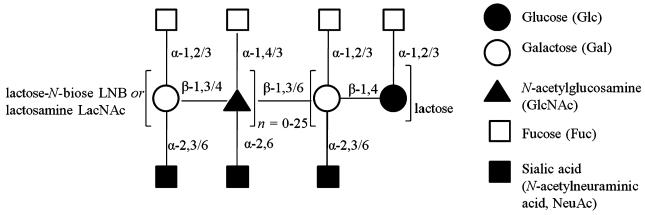
Mature human milk contains c. 7–12 g L−1 of free oligosaccharides in addition to lactose, which typically is present in concentrations of 55–70 g L−1 (Wu et al., 2011). Currently, up to 200 unique oligosaccharide structures varying from 3 to 22 sugar units have been identified (Kobata, 2010). HMO are composed of the five monosaccharide building blocks d-glucose (Glc), d-galactose (Gal), N-acetylglucosamine (GlcNAc), l-fucose (Fuc) and sialic acid (N-acetylneuraminic acid). They can be grouped into neutral and charged oligosaccharides, the latter being sialylated and comprising c. 20% of all HMO (Wu et al., 2011). The structures of HMO show typical patterns. Lactose (Gal-β-1,4-Glc) is found at the reducing end of HMO. This terminal lactose is typically elongated by lacto-N-biose units (LNB; Gal-β-1,3-GlcNAc) in type I or N-acetyl-lactosamine units (LacNAc; Gal-β-1,4-GlcNAc) in the rarer type II structures. Both LNB and LacNAc are attached via a β-1,3-linkage to the galactosyl moiety of the terminal lactose, with an additional β-1,6-linkage in branched HMO.
These LNB and LacNAc units can be repeated up to 25 times in larger HMO, forming the core region of these oligosaccharides. A further variation results from the attachment of fucosyl and sialic acid residues (Fig. 2). Thus, the simplest structures following this general scheme (apart from certain trisaccharides such as galactosyl-lactose, fucosyl-lactose and sialyl-lactose) are the tetrasaccharides lacto-N-tetraose, Gal-β-1,3-GlcNAc-β-1,3-Gal-β-1,4-Glc (type I), and lacto-N-neo-tetraose, Gal-β-1,4-GlcNAc-β-1,3-Gal-β-1,4-Glc (type II; Fig. 3). Urashima listed an HMO classification based on their 13 core structures; of which, the most abundant components are 2′-fucosyllactose (Fuc-α-1,2-Gal-β-1,4-Glc), lacto-N-tetraose (Gal-β-1,3-GlcNAc-β-1,3-Gal-β-1,4-Glc) and lacto-N-fucopentaose (Fuc-α-1,2-Gal-β-1,3-GlcNac-β-1,3-Gal-β-1,4-Glc) (Urashima et al., 2013). Despite recent modern analytical techniques, HMO identification remains a challenge for researchers.
Fig. 2.
Schematic structure of HMO.
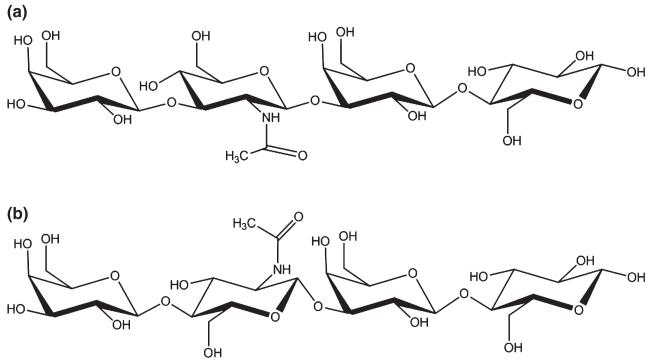
Fig. 3.
Some simple structures of HMO: tetrasaccharides lacto-N-tetraose, Gal-β-1,3-GlcNAc-β-1,3-Gal-β-1,4-Glc (a), and lacto-N-neo-tetraose, Gal-β-1,4-GlcNAc-β-1,3-Gal-β-1,4-Glc (b).
The composition and content of HMO can vary significantly between different mothers. It varies depending on both their blood group type and the time/length of lactation (Totten et al., 2012). Nevertheless, mono- and difucosyl-lactose, lacto-N-tetraose and its fucosylated derivatives as well as sialyl-lactose and the sialylated forms of lacto-N-tetraose are major HMO in human milk. The presence and absence of α-(1,3/4)- and α-(1,2)-fucosylated oligosaccharides indicate the activity of the Lewis (Le)- and secretor (Se) gene of the mother, with the latter being responsible for the expression of α-(1,2)-fucosyl transferase. Totten et al. (2012) described the four possible phenotypes expressed in HMOs and reported that West African populations have higher abundance of Lewis negative and nonsecretors than in European and American populations.
Approaches to biosynthesis of related HMO structures
Considerable interest exists in the efficient production of HMO, which is albeit hampered by the structural complexity as well as the complex mixture of HMO. Many manufacturers are trying to emulate HMO; however, essential ingredients are mostly absent from infant formula due to the lack of industrial production methods. Most often, GOS and/or FOS are added to infant formulas to mimic the effect of HMOs.
The most promising approach toward the production of defined HMO structures seems to be microbial, fermentative methods employing single, appropriately engineered microorganisms. This in vivo approach was introduced by the group of Eric Samain. They used a β-galactosidase-negative Escherichia coli strain over-expressing a β-1,3-N-acetylglucosaminyl-transferase gene from Neisseria meningitidis. When feeding lactose to this engineered strain, this disaccharide was taken up by the indigenous β-galactoside permease of E. coli. The recombinantly synthesized β-1,3-N-acetylglucosaminyl transferase then utilized the intracellular pool of UDP-GlcNAc to transfer GlcNAc residues regiospecifically to lactose, resulting in the formation of the trisaccharides GlcNac-β-1,3-Gal-β-1,4-Glc. This compound was released into the extracellular medium in yields of 6 g L−1 (Priem et al., 2002). In a similar manner, an engineered E. coli strain, over-expressing an α-1,3-fucosyltransferase from Helicobacter pylori and genetically engineered to provide sufficient GDP fucose as the intracellular substrate for the glycosyltransferase, was used to produce various fucosylated HMO from lactose added to the medium (Dumon et al., 2001). An engineered E. coli strain over-expressing the α-2,3-sialyltransferase gene from N. meningitidis, together with an engineered pathway to provide the activated sialic acid donor CMP-Neu5Ac as the substrate for the glycosyltransferase, produced 3′-sialyl-lactose in concentrations of up to 25 g L−1 in high cell density cultivations with continuous lactose feed (Fierfort & Samain, 2008). 6′-sialyl-lactose was efficiently produced when employing α-2,6-sialyltransferase from Photobacterium sp. again in metabolically engineered E. coli (Drouillard et al., 2010). In a very recent study, a recombinant Pasteurella multocida sialyltransferase exhibiting dual trans-sialidase activities catalyzed trans-sialylation using either 2-O-(p-nitrophenyl)-α-d-N-acetylneuraminic acid or casein glycomacropeptide (whey protein) as the sialyl donor and lactose as the acceptor, resulting in production of both 3′-sialyl-lactose and 6′-sialyl-lactose. The enzyme was capable of catalyzing the synthesis of both 3′- and 6′-sialylated GOS when GOS served as acceptors (Guo et al., 2014). A mutant of the sialidase from the nonpathogenic Trypanosoma rangeli expressed in Pichia pastoris after codon optimization has been reported to exhibit transsialidase activity. The enzyme catalyzed the transfer of sialic acid from cGMP (casein glycomacropeptide) to lactose at high efficiency, giving a yield at the 5 L scale of 3.6 g 3′-sialyl-lactose. The estimated molar trans-sialylation yield was 50% for the 3′-sialyl residues in cGMP without substantial hydrolysis of 3′-sialyl-lactose. Lacto-N-tetraose and lacto-N-fucopentaoses also functioned as acceptor molecules demonstrating the versatility of this trans-sialidase for catalyzing sialyl-transfer toward different HMO (Michalak et al., 2014).
LNB is a key disaccharide component of HMO such as lacto-N-tetraose and lacto-N-fucopentaose. LNB can be produced in a purely enzymatic approach, making use of the synthetic capacity of sugar phosphorylases. LNB phosphorylase, together with sucrose phosphorylase, UDP-glucose-hexose-1-phosphate uridylyltransferase and UDP-glucose 4-epimerase produced LNB from sucrose and GlcNAc in the presence of phosphate and catalytic amounts of UDP-Glc in yields of 85% (Nishimoto & Kitaoka, 2007). Recent development to enhance thermostability of galacto-N-biose/lacto-N-biose phosphorylase by directed evolution yielded a mutant that exhibited 20 °C higher thermostability than the wild type, which is suitable for industrial production of LNB at temperatures higher than 50 °C for faster reaction and prevention of microbial contamination (Koyama et al., 2013).
Galactose-containing HOS
An approach that has received some interest is the synthesis of HOS; of which, some are expected to resemble HMO-like structures, using β-galactosidases. This approach is based on β-galactosidase-catalyzed transglycosylation with lactose as donor (thus transferring galactose onto suitable acceptors) and GlcNAc as acceptor, thus obtaining N-acetyl-lactosamine (LacNAc) and its regioisomers. Using this approach and a hyperthermophilic β-galactosidase from S. solfataricus, Gal-β-1,6-GlcNAc together with an unidentified sugar were the main products starting from a mixture of 1 M lactose and 1 M GlcNAc, while LacNAc and Gal-β-1,3-GlcNAc were formed as well, yet in lower concentrations (Reuter et al., 1999). This reaction was also optimized for using β-galactosidase from B. circulans as the biocatalyst. This enzyme is known for its propensity to synthesize β-1,4-linkages in its transgalactosylation mode, and hence the main reaction product here was LacNAc together with smaller amounts of GlcNAc-containing higher oligosaccharides (one tri- and one tetrasaccharides) and Gal-β-1,6-Glc-NAc. The total yield was 40% for these GlcNAc-containing oligosaccharides when starting from 0.5 M lactose and GlcNAc each (Li et al., 2010). Crude cellular extracts of L. bulgaricus and Lactococcus lactis MG1363 expressing LacLM of Lactobacillus plantarum were used as sources of β-galactosidases for the formation of HOS by galactosylation of N-acetylglucosamine (GlcNAc) and fucose with the main products identified as Gal-β-(1→4)-GlcNAc, Gal-β-(1→6)-GlcNAc, Gal-β-(1→6)-Gal-β-(1→4)-Glc-NAc and Gal-β-(1→6)-Gal-β-(1→6)-GlcNAc (Gänzle, 2012).
Conclusion
HMO yet cannot be commercially produced due to their structural and compositional complexity; however, increased biochemical knowledge on suitable glycosyltransferases may pave the road to microbial, fermentative methods employing single, appropriately engineered microorganisms. The presence of structurally related oligosaccharides together with different complex structures in human breast milk makes GOS attract increasing interests from researchers and manufacturers. The insights into the structures and the production of GOS together with advancement in the area of biotechnology will certainly result in the enhancement of the production of GOS in the future. The use of lactic acid bacteria and Bifidobacteria as the sources of β-galactosidases offers substantial potential for the production of GOS and is an interesting approach for the production of new carbohydrate-based functional food ingredients that are of interest in applications such as infant formula. Nowadays, infant formulae are supplemented with GOS to mimic the biological effects of HMO. Some structures of novel galactose-containing HOS resemble the core of HMO, and hence, these novel functionally enhanced, prebiotic oligosaccharides could be of interest for a wide range of applications.
Acknowledgements
T.-H.N. acknowledges the support from the Austrian Science Fund (FWF Project P24868-B22). S.L.A. and M.I are thankful for a ‘Technologiestipendium Südostasien in the frame of the ASEA-Uninet granted by the OeAD – Austrian Agency for International Cooperation in Education & Research’ financed by the Austrian Federal Ministry of Science and Research. N.H.P. thanks the European Commission for the Erasmus Mundus scholarship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Albayrak N, Yang ST. Production of galacto-oligosaccharides from lactose by Aspergillus oryzae β-galactosidase immobilized on cotton cloth. Biotechnol Bioeng. 2002;77:8–19. doi: 10.1002/bit.1195. [DOI] [PubMed] [Google Scholar]
- Boehm G, Stahl B, Knol J, Garssen J. Carbohydrates in human milk and infant formulas. In: Garg HG, Cowman MK, Hales CA, editors. Carbohydrate Chemistry, Biology and Medical Applications. Elsevier Ltd; Amsterdam, the Netherlands: 2008. pp. 275–291. [Google Scholar]
- Boon MA, Janssen AEM, van’t Riet K. Effect of temperature and enzyme origin on the enzymatic synthesis of oligosaccharides. Enzyme Microb Technol. 2000;26:271–281. doi: 10.1016/s0141-0229(99)00167-2. [DOI] [PubMed] [Google Scholar]
- Di Lauro B, Strazzulli A, Perugino G, La Cara F, Bedini E, Corsaro MM, Rossi M, Moracci M. Isolation and characterization of a new family 42 β-galactosidase from the thermoacidophilic bacterium Alicyclobacillus acidocaldarius: identification of the active site residues. Biochim Biophys Acta. 2008;1784:292–301. doi: 10.1016/j.bbapap.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Drouillard S, Mine T, Kajiwara H, Yamamoto T, Samain E. Efficient synthesis of 6′-sialyllactose, 6,6′-disialyllactose, and 6′-KDO-lactose by metabolically engineered E. coli expressing a multifunctional sialyltransferase from the Photobacterium sp. JT-ISH-224. Carbohydr Res. 2010;345:1394–1399. doi: 10.1016/j.carres.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Dumon C, Priem B, Martin SL, Heyraud A, Bosso C, Samain E. In vivo fucosylation of lacto-N-neotetraose and lacto-N-neohexaose by heterologous expression of Helicobacter pylori α-1,3 fucosyltransferase in engineered Escherichia coli. Glycoconj J. 2001;18:465–474. doi: 10.1023/a:1016086118274. [DOI] [PubMed] [Google Scholar]
- Fierfort N, Samain E. Genetic engineering of Escherichia coli for the economical production of sialylated oligosaccharides. J Biotechnol. 2008;134:261–265. doi: 10.1016/j.jbiotec.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Gänzle MG. Enzymatic synthesis of galacto-oligosaccharides and other lactose derivatives (hetero-oligosaccharides) from lactose. Int Dairy J. 2012;22:116–122. [Google Scholar]
- Goulas T, Goulas A, Tzortzis G, Gibson GR. Comparative analysis of four β-galactosidases from Bifidobacterium bifidum NCIMB41171: purification and biochemical characterisation. Appl Microbiol Biotechnol. 2009;82:1079–1088. doi: 10.1007/s00253-008-1795-5. [DOI] [PubMed] [Google Scholar]
- Guo Y, Jers C, Meyer AS, Arnous A, Li H, Kirpekar F, Mikkelsen JD. A Pasteurella multocida sialyltransferase displaying dual trans-sialidase activities for production of 3′-sialyl and 6′-sialyl glycans. J Biotechnol. 2014;170:60–67. doi: 10.1016/j.jbiotec.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Hinz SWA, Van Den Broek LAM, Beldman G, Vincken JP, Voragen AGJ. β-Galactosidase from Bifidobacterium adolescentis DSM20083 prefers b(1,4)-galactosides over lactose. Appl Microbiol Biotechnol. 2004;66:276–284. doi: 10.1007/s00253-004-1745-9. [DOI] [PubMed] [Google Scholar]
- Hung MN, Lee BH. Purification and characterization of a recombinant β-galactosidase with transgalactosylation activity from Bifidobacterium infantis HL96. Appl Microbiol Biotechnol. 2002;58:439–445. doi: 10.1007/s00253-001-0911-6. [DOI] [PubMed] [Google Scholar]
- Isobe K, Yamashita M, Chiba S, Takahashi N, Koyama T. Characterization of new β-galactosidase from acidophilic fungus, Teratosphaeria acidotherma AIU BGA-1. J Biosci Bioeng. 2013;116:293–297. doi: 10.1016/j.jbiosc.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Nakajima M, Nakao S. Galacto-oligosaccharide production from lactose by an enzymic batch reaction using β-galactosidase. Process Biochem. 1996;31:69–76. [Google Scholar]
- Jørgensen F, Hansen OC, Stougaard P. High-efficiency synthesis of oligosaccharides with a truncated β-galactosidase from Bifidobacterium bifidum. Appl Microbiol Biotechnol. 2001;57:647–652. doi: 10.1007/s00253-001-0845-z. [DOI] [PubMed] [Google Scholar]
- Jovanovic-Malinovska R, Fernandes P, Winkelhausen E, Fonseca L. Galacto-oligosaccharides synthesis from lactose and whey by β-galactosidase immobilized in PVA. Appl Biochem Biotechnol. 2012;168:1197–1211. doi: 10.1007/s12010-012-9850-1. [DOI] [PubMed] [Google Scholar]
- Karan R, Capes MD, DasSarma P, DasSarma S. Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. BMC Biotechnol. 2013;13:3. doi: 10.1186/1472-6750-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Ji E-S, Oh D-K. A new kinetic model of recombinant β-galactosidase from Kluyveromyces lactis for both hydrolysis and transgalactosylation reactions. Biochem Biophys Res Commun. 2004;316:738–743. doi: 10.1016/j.bbrc.2004.02.118. [DOI] [PubMed] [Google Scholar]
- Klein MP, Nunes MR, Rodrigues RC, Benvenutti EV, Costa TMH, Hertz PF, Ninow JL. Effect of the support size on the properties of β-galactosidase immobilized on chitosan: advantages and disadvantages of macro and nanoparticles. Biomacromolecules. 2012;13:2456–2464. doi: 10.1021/bm3006984. [DOI] [PubMed] [Google Scholar]
- Kobata A. Structures and application of oligosaccharides in human milk. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:731–747. doi: 10.2183/pjab.86.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Hidaka M, Nishimoto M, Kitaoka M. Directed evolution to enhance thermostability of galacto-N-biose/lacto-N-biose I phosphorylase. Protein Eng Des Sel. 2013;26:755–761. doi: 10.1093/protein/gzt049. [DOI] [PubMed] [Google Scholar]
- Li W, Sun Y, Ye H, Zeng X. Synthesis of oligosaccharides with lactose and N-acetylglucosamine as substrates by using β-d-galactosidase from Bacillus circulans. Eur Food Res Technol. 2010;231:55–63. [Google Scholar]
- Michalak M, Larsen DM, Jers C, et al. Biocatalytic production of 3′-sialyllactose by use of a modified sialidase with superior trans-sialidase activity. Process Biochem. 2014;49:265–270. [Google Scholar]
- Nam ES, Kim MS, Lee HB, Ahn JK. β-Glycosidase of Thermus thermophilus KNOUC202: gene and biochemical properties of the enzyme expressed in Escherichia coli. Prikl Biokhim Mikrobiol. 2010;46:562–571. [PubMed] [Google Scholar]
- Nguyen TT, Nguyen HA, Arreola SL, Mlynek G, Djinovi c-Carugo K, Mathiesen G, Nguyen TH, Haltrich D. Homodimeric β-galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: expression in Lactobacillus plantarum and biochemical characterization. J Agric Food Chem. 2012;60:1713–1721. doi: 10.1021/jf203909e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto M, Kitaoka M. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum. Appl Environ Microbiol. 2007;73:6444–6449. doi: 10.1128/AEM.01425-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placier G, Watzlawick H, Rabiller C, Mattes R. Evolved β-galactosidases from Geobacillus stearothermophilus with improved transgalactosylation yield for galacto-oligosaccharide production. Appl Environ Microbiol. 2009;75:6312–6321. doi: 10.1128/AEM.00714-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priem B, Gilbert M, Wakarchuk WW, Heyraud A, Samain E. A new fermentation process allows large-scale production of human milk oligosaccharides by metabolically engineered bacteria. Glycobiology. 2002;12:235–240. doi: 10.1093/glycob/12.4.235. [DOI] [PubMed] [Google Scholar]
- Pulicherla KK, Kumar PS, Manideep K, Rekha VPB, Ghosh M, Sambasiva Rao KRS. Statistical approach for the enhanced production of cold-active β-galactosidase from Thalassospira frigidphilosprofundus: a novel marine psychrophile from deep waters of Bay of Bengal. Prep Biochem Biotechnol. 2013;43:766–780. doi: 10.1080/10826068.2013.773341. [DOI] [PubMed] [Google Scholar]
- Reuter S, Rusborg Nygaard A, Zimmermann W. β-Galactooligosaccharide synthesis with β-galactosidases from Sulfolobus solfataricus, Aspergillus oryzae, and Escherichia coli. Enzyme Microb Technol. 1999;25:509–516. [Google Scholar]
- Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Colinas B, Fernandez-Arrojo L, Ballesteros AO, Plou FJ. Galactooligosaccharides formation during enzymatic hydrolysis of lactose: towards a prebiotic-enriched milk. Food Chem. 2014;145:388–394. doi: 10.1016/j.foodchem.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Sangwan V, Tomar SK, Singh RRB, Singh AK, Ali B. Galactooligosaccharides: novel components of designer foods. J Food Sci. 2011;76:R103–R111. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- Sen D, Sarkar A, Das S, Chowdhury R, Bhattacharjee C. Batch hydrolysis and rotating disk membrane bioreactor for the production of galacto-oligosaccharides: a comparative study. Ind Eng Chem Res. 2012;51:10671–10681. [Google Scholar]
- Splechtna B, Nguyen TH, Steinböck M, Kulbe KD, Lorenz W, Haltrich D. Production of prebiotic galacto-oligosaccharides from lactose using β-galactosidases from Lactobacillus reuteri. J Agric Food Chem. 2006;54:4999–5006. doi: 10.1021/jf053127m. [DOI] [PubMed] [Google Scholar]
- Splechtna B, Nguyen TH, Haltrich D. Comparison between discontinuous and continuous lactose conversion processes for the production of prebiotic galacto-oligosaccharides using β-galactosidase from Lactobacilius reuteri. J Agric Food Chem. 2007a;55:6772–6777. doi: 10.1021/jf070643z. [DOI] [PubMed] [Google Scholar]
- Splechtna B, Nguyen TH, Zehetner R, Lettner HP, Lorenz W, Haltrich D. Process development for the production of prebiotic galacto-oligosaccharides from lactose using β-galactosidase from Lactobacillus sp. Biotechnol J. 2007b;2:480–485. doi: 10.1002/biot.200600230. [DOI] [PubMed] [Google Scholar]
- Torres DPM, Gonçalves MDPF, Teixeira JA, Rodrigues LR. Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics. Compr Rev Food Sci Food Saf. 2010;9:438–454. doi: 10.1111/j.1541-4337.2010.00119.x. [DOI] [PubMed] [Google Scholar]
- Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, Ruhaak LR, Darboe MK, German JB, Prentice AM, Lebrilla CB. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res. 2012;11:6124–6133. doi: 10.1021/pr300769g. [DOI] [PubMed] [Google Scholar]
- Urashima T, Taufik E, Fukuda K, Asakuma S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci Biotechnol Biochem. 2013;77:455–466. doi: 10.1271/bbb.120810. [DOI] [PubMed] [Google Scholar]
- Vera C, Guerrero C, Illanes A, Conejeros R. A pseudo steady-state model for galacto-oligosaccharides synthesis with β-galactosidase from Aspergillus oryzae. Biotechnol Bioeng. 2011;108:2270–2279. doi: 10.1002/bit.23201. [DOI] [PubMed] [Google Scholar]
- Verma ML, Barrow CJ, Kennedy JF, Puri M. Immobilization of β-d-galactosidase from Kluyveromyces lactis on functionalized silicon dioxide nanoparticles: characterization and lactose hydrolysis. Int J Biol Macromol. 2012;50:432–437. doi: 10.1016/j.ijbiomac.2011.12.029. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Woś A, Cieśliński H, Wanarska M, Kozlowska-Tylingo K, Hildebrandt P, Kur J. A novel cold-active β-d-galactosidase from the Paracoccus sp. 32d-gene cloning, purification and characterization. Microb Cell Fact. 2011;10:108. doi: 10.1186/1475-2859-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10:856–868. doi: 10.1021/pr101006u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yuan S, Chen S, Wu D, Chen J, Wu J. Enhancing the production of galacto-oligosaccharides by mutagenesis of Sulfolobus solfataricus β-galactosidase. Food Chem. 2013;138:1588–1595. doi: 10.1016/j.foodchem.2012.11.052. [DOI] [PubMed] [Google Scholar]