Abstract
Uric acid affects endothelial and adipose cell function and has been linked to diseases such as hypertension, metabolic syndrome, and cardiovascular disease. Interestingly uric acid has been shown to increase endothelial progenitor cell (EPC) mobilization, a potential mechanism to repair endothelial injury. Since EPC mobilization is dependent on activity of the enzyme CD26/dipeptidyl peptidase (DPP)IV, we examined the effect uric acid has on CD26/DPPIV activity. Uric acid inhibited the CD26/DPPIV associated with human umbilical vein endothelial cells but not human recombinant (hr)CD26/DPPIV. However, triuret, a product of uric acid and peroxynitrite, could inhibit cell associated and hrCD26/DPPIV. Increasing or decreasing intracellular peroxynitrite levels enhanced or decreased the ability of uric acid to inhibit cell associated CD26/DPPIV respectively. Last, protein modeling demonstrates how triuret can act as a small molecule inhibitor of CD26/DPPIV activity. This is the first time that uric acid or a uric acid reaction product has been shown to affect enzymatic activity and suggests a novel avenue of research in the role of uric acid in the development of clinically important diseases.
Keywords: uric acid, triuret, CD26/DPPIV
INTRODUCTION
Uric acid is a natural antioxidant formed from the enzymatic reaction of xanthine with xanthine oxidase or xanthine dehydrogenase and is the byproduct of purine catabolism in humans. In the past, uric acid was thought to be a biologically inert compound. However, uric acid is highly reactive and can react with superoxide {Kaur, 1990 #1}, nitric oxide {Gersch, 2008 #2}, and peroxynitrite {Robinson, 2004 #3;Gersch, 2009 #4} to generate allantoin, 6-aminouracil, and triuret, respectively. Recently acute increases in uric acid have been shown to lead to a mobilization of endothelial progenitor cells in mice {Patschan, 2007 #5}. We wanted to investigate the mechanism. A key chemokine for endothelial progenitor cells, stromal derived factor (SDF)-1, is regulated by the activity of CD26, a cell surface dipeptidyl peptidase which degrades SDF-1 {Christopherson, 2004 #6}. Previously CD26/dipeptidyl peptidase (DPP)IV was shown to cleave and inactivate SDF-1 blocking its ability to activate its receptor CXCR4 {Ohtsuki, 1998 #7;Proost, 1998 #8;Christopherson, 2002 #9}. We hypothesized that EPC mobilization was initiated by uric acid’s effect on CD26/DPPIV.
CD26/DPPIV is a 110 kDa membrane bound protein that belongs to the prolyl oligopeptidase family, which includes fibroblast activation protein α, DPP8, and DPP9 {Chen, 2003 #10;Abbott, 2000 #11;Olsen, 2002 #12}, and has been associated with diseases such as diabetes mellitus {Wiedeman, 2003 #13;Burcelin, 1999 #14}, obesity {Yasuda, 2004 #15}, tumor growth {Wesley, 2004 #16;Bauvois, 2004 #17;Khin, 2003 #18;Pethiyagoda, 2000 #19}, and HIV {Shioda, 1998 #20;Morimoto, 1994 #21}. CD26/DPPIV is found on the cell surface of most tissues such as gastrointestinal tract, kidney, liver, lymphocytes, and endothelial cells and also exists as a soluble form in serum {Weber, 2004 #22;Durinx, 2000 #23;Iwaki-Egawa, 1998 #24}. The soluble form of CD26/DPPIV is believed to be derived from endothelial cells, epithelial cells, and leukocytes. Substrates of CD26/DPPIV include chemokines, peptide hormones and neuropeptides {Mentlein, 1999 #25}.
While CD26/DPPIV is associated with a large number of disease states, so are alterations in uric acid levels. As an antioxidant, studies indicate an elevated level of uric acid is associated with a reducing the risk of Parkinson’s disease {de Lau, 2005 #26} while a low level is associated with an increased risk of Alzheimer’s disease {Rinaldi, 2003 #27}. Despite its beneficial properties as an antioxidant, increasing evidence suggests an association of high levels of uric acid with hypertension {Johnson, 2005 #28;Cannon, 1966 #29}, cardiovascular disease {Johnson, 2003 #30}, kidney disease {Kang, 2002 #31;Siu, 2006 #32;Talaat, 2007 #33}, and the metabolic syndrome {Nakagawa, 2006 #34}. Khosla et al have demonstrated that uric acid induces endothelial dysfunction by decreasing the bioavailability of nitric oxide in endothelial cells {Nakagawa, 2006 #35;Khosla, 2005 #36}. Since endothelial dysfunction is common in patients with cardiovascular and kidney disease, this provides a potential mechanism in explaining the role of uric acid in the induction of hypertension and cardiovascular disease. Although if uric acid can lead to an increase in endothelial progenitor cell number, a potential endothelial repair mechanism, then the net effect of an elevation of uric acid may be more complicated than just a uric acid level and this may explain why the risk of end-organ damage is altered in different patient population groups. In this study we demonstrate that uric acid’s effect on the biologic activity on an enzyme critical in controlling the metabolic state, CD26/DPPIV, requires interaction with peroxynitrite and formation of triuret.
MATERIAL AND METHODS
Materials
All tissue culture reagents were obtained from Cambrex (Walkersville, MD). Recombinant human CD26/DPPIV was obtained from R & D (Minneapolis, MN) systems. The sodium hypochlorite used in the production of hypochlorous acid was obtained from Fischer Scientific (Pittsburgh, PA). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated.
Cell Culture
HUVEC obtained from Clonetics™ (San Diego, CA) were cultured at 37°C in 5% CO2 in Endothelial Basal Media (EBM) supplemented with 10% fetal bovine serum and a SingleQuot™ that included human recombinant epidermal growth factor, basic human fibroblast growth factor with heparin, vascular endothelial growth factor, ascorbic acid, hydrocortisone, human recombinant insulin-like growth factor, heparin, and 1 × antibiotic-antimycotic from Gibco-Invitrogen (Carlsbad, CA). Cells were utilized between passage 2 and 6 for these experiments.
Preparation of uric acid, 6-aminouracil, triuret, and allantoin
Uric acid (50 mg/dl), triuret (3.33 mM), and allantoin (1.48 mM) were prepared by dissolving these compounds in NaOH (1 M) with the addition of 1X PBS. 6-aminouracil (1.48 mM) was prepared by dissolving this compound in KOH (0.3 M) with the addition of 1X PBS. The solutions were adjusted to a pH of 7.4. The concentration of sodium and potassium in the solutions and corresponding controls from NaOH 12 mM and KOH 2.67 mM, respectively.
Measurement of CD26/DPPIV activity in HUVEC and recombinant CD26/DPPIV
HUVEC, where indicated, were incubated with 40 µM MnTBAP {Paris, 1998 #37} (A.G. Scientific, San Diego, CA) for 24 hours prior to washing twice with PBS. HUVEC, where indicated, were incubated with 100 µM hypochlorous acid (prepared as previously described {Xu, 2006 #38} for 15 min, washed twice with PBS, and were kept in culture media for 30 min at 37°C. For analysis, cells were resuspended at 700 cells/µl of PBS. The cells were combined with 60 µM Gly-Pro-AMC (Bachem, King of Prussia, PA) in a 384 well microplate and cleavage of the fluorescent substrate at 37°C, was recorded every 2 minutes using a Bio-Tek FL600 Microplate Fluorescent Reader (Bio-Tek Instruments, Winooski, VT) with a 360 nm excitation filter and a 460 nm emission filter. Alternatively, 0.0625 ng/µl of CD26/DPPIV was incubated with 60 µM Gly-Pro-AMC and cleavage was recorded as above. Uric Acid (prepared as previously described {Khosla, 2005 #36} at a concentration of 15 mg/dl was added in the presence and absence of 1 mM probenecid where indicated. The control for uric acid was an identically prepared mock. In addition, cells or pure recombinant CD26/DPPIV enzyme were incubated in 2.67 mM KOH in PBS with and without 0.892 mM 6-aminouracil, PBS with and without 0.892 mM allantoin, and PBS with and without 2 mM triuret. Rate of CD26/DPPIV cleavage was determined by calculating the maximum slope and converting RFU/min to pmoles of Gly-Pro-AMC substrate cleaved/sec.
Structural Modeling
Structural modeling was performed using the interactive molecular graphics program Coot {Emsley, 2004 #39} and secondary-structure matching routine {Krissinel, 2004 #40}. Figure 4A and Figure 4B were generated using PyMOL {DeLano, 2002 #41}.
Figure 4.
Modeling of triuret in the CD26/DPPIV active site and crystal structures of CD26/DPPIV complexed with some of its known inhibitors. (A) The crystal structures of small molecule inhibitors bound in the active site of CD26/DPPIV were used as references (PDB ID’s 2G5P, 2G5T, 2G63, 2I03). The central carbonyl oxygen of triuret was first structurally aligned with the common carbonyl oxygen of each of the inhibitors using the graphics program Coot {Emsley, 2004 #39;Krissinel, 2004 #40}. Steric interactions were then minimized by allowing for rotational freedom about the C-N bond of the terminal amide groups. (B) An overlay of crystal structures of four small molecule inhibitors of CD26/DPPIV (PDB ID 2G5P (magenta), 2G5T (blue), 2G63 cyan), and 2I03 (orange).
Statistical Analysis
Statistical analysis was carried out using two-tailed, Student’s t-test, with a p value < 0.05 considered significant. The Student’s t-test was utilized since we were comparing one condition against the control in all cases.
RESULTS
Uric acid competitively inhibits HUVEC associated CD26/DPPIV activity but not human recombinant CD26/DPPIV
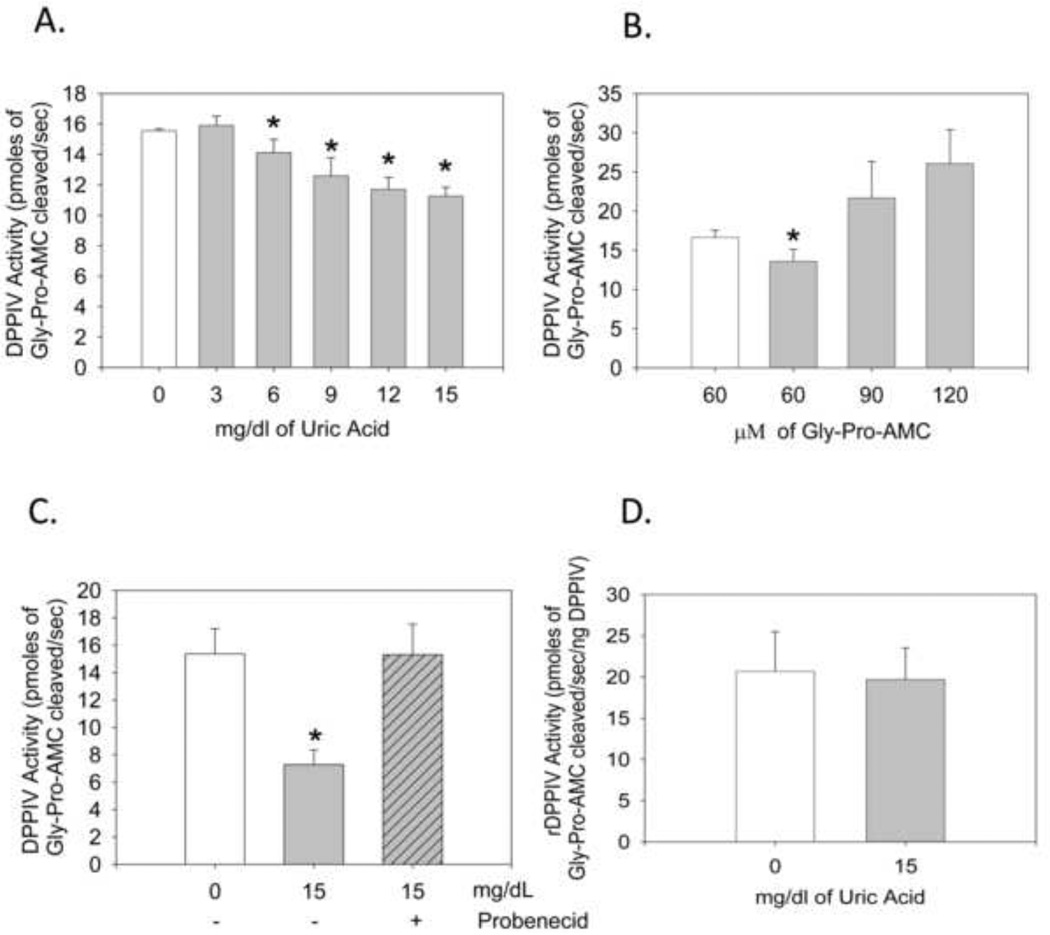
We evaluated the ability of uric acid to inhibit CD26/DPPIV by incubating HUVEC with Gly-Pro-AMC, a substrate of CD26/DPPIV that fluoresces when cleaved, in the presence and absence of uric acid. In the presence of uric acid, CD26/DPPIV activity decreased in a dose dependent manner with the greatest reduction in enzyme activity (28%) at a concentration of 15 mg/dl of uric acid (Fig. 1A). Importantly, we began to see inhibition with uric acid in the physiologic range of 6–12 mg/dl. The inhibition of uric acid appeared to be competitive, since even at 15 mg/dl it could be overcome by increasing concentrations of the Gly-Pro-AMC substrate (Fig. 1B). We were also able to block uric acid’s inhibition of CD26/DPPIV by preventing the cellular uptake of uric acid with probenecid (Fig. 1C). These data suggest that uric acid required internaliz to achieve inhibition. Consistent with this observation, the human recombinant CD26/DPPIV enzyme could not be inhibited by uric acid (Fig. 2A).
Figure 1.
Uric acid inhibition of cell bound CD26/DPPIV activity is competitive and requires cellular uptake of uric acid. Human recombinant CD26/DPPIV is not inhibited by uric acid, allantoin, or 6-aminouracil HUVEC were cultured, collected by trypsinization and then assayed for CD26/DPPIV activity. (A) HUVEC were incubated with the indicated concentration of uric acid and 60 µM Gly-Pro-AMC. Cleavage was recorded with a 360 nm excitation filter and a 460 nm emission filter. *P<0.0025 compared to 0 mg/dl uric acid. (n = 6. Error bars ± 1 s.d.) (B) HUVEC were incubated with the indicated concentration of Gly-Pro-AMC in the presence (grey bars) and absence (open bars) of 15 mg/dl uric acid. Each point is in triplicate shown ± standard deviation. *P < 0.05 compared to no uric acid. (C) 60 µM Gly-Pro-AMC was added to HUVEC in the presence or absence of 15 mg/dl of uric acid and/or probenecid (1 mM) as indicated. * P = 0.00003. (n = 5. Error bars ± 1 s.d.). (D) Uric acid (15 mg/dl) was added to human recombinant CD26/DPPIV (rCD26/DPPIV) (0.0625 ng/µl). All replicates for the 4 times the experiment has been repeated is shown (n = 9. Error bars ± 1 s.d.).
Figure 2.
Triuret can inhibit recombinant CD26/DPPIV and uric acid’s inhibition of cell bound CD26/DPPIV is dependent on the intracellular formation of triuret. (A) rCD26/DPPIV was incubated with PBS in the absence (control) or presence of allantoin (0.892 mM) or in 2.67 mM KOH in PBS in the absence (KOH) or presence of 6-aminouracil (0.892 mM), as indicated, and 60 µM Gly-Pro-AMC. Each point is in triplicate shown ± standard deviation.(B) Triuret (2 mM) was added to human recombinant CD26/DPPIV. *P = 0.001 (n = 7. Error bars ± 1 s.d.). (C) Allantoin (0.892 mM) in PBS, 6-aminouracil (0.892 mM) in KOH, and triuret (2 mM) in PBS, KOH, or PBS were added to HUVEC *P = 0.028. (n = 3. Error bars ± 1 s.d.).
Uric Acid inhibition of CD26/DPPIV activity depends on intracellular formation of triuret
Uric acid has been shown to react with superoxide {Kaur, 1990 #1}, peroxynitrite {Robinson, 2004 #3;Gersch, 2009 #4}, and nitric oxide {Gersch, 2008 #2}, generating allantoin, triuret, and 6-aminouracil, respectively,. The intracellular formation of these products could explain the requirement for uric acid uptake into cells in order to attain inhibition of cell surface CD26/DPPIV. Of the three uric acid reaction products only triuret, at a concentration of 2 mM, twice the molar ratio of 15 mg/dl of uric acid, was able to inhibit recombinant CD26/DPPIV (Fig.2B and 3A). Triuret was also able to inhibit cell associated CD26/DPPIV (Fig. 3B). Neither allantoin nor 6-aminouracil at concentrations as high as 4 mM could inhibit the cell based or recombinant CD26/DPPIV (data not shown).
Figure 3.
Uric acid’s ability to inhibit CD26/DPPIV activity is blocked with MnTBAP and enhanced with hypochlorous acid (A) HUVEC were treated with 40 µM MnTBAP for 24 hours prior to harvest. Collected cells were incubated in 60 µM Gly-Pro-AMC in the presence or absence of 15 mg/dl of uric acid as indicated.. All samples with uric acid added are shown as a percentage of pmoles of Gly-Pro-AMC cleaved/sec of control (no uric acid, same condition), normalized to 100%. * P = 0.009. (n = 5. Error bars ± 1 s.d.) (B) HUVEC were treated with 100 µM hypochlorous acid or control for 15 min, washed with PBS, and were kept in culture media at 37°C for 30 min prior to harvest. Cells were collected and analyzed as in (A). *P = 0.0026. (n = 5. Error bars ± 1 s.d.)
Uric acid’s ability to inhibit CD26/DPPIV activity is blocked with MnTBAP and enhanced with hypochlorous acid
During the course of our experiments, we found that the inhibitory capacity of uric acid on cell-bound CD26/DPPIV was dependent on passage number and cell density (data not shown). To address this issue and investigate further the requirement of intracellular peroxynitrite for CD26/DPPIV inhibition, we treated cells with MnTBAP, a cell-permeable superoxide dismutase mimetic and peroxynitrite scavenger {Paris, 1998 #37}, as well as hypochlorous acid, a treatment that leads to an increase in peroxynitrite levels {Xu, 2006 #38}. In order to maximize the effect of MnTBAP and hypochlorous acid on peroxynitrite levels, we grew cells under conditions that enhanced or reduced uric acid’s inhibiting activity, respectively. Treating HUVEC with MnTBAP eliminated the ability of uric acid to inhibit CD26/DPPIV (Fig. 3C); while treating HUVEC with hypochlorous acid enhanced uric acid’s inhibitory activity (Fig. 3D). These results support the hypothesis that formation of triuret by an intracellular reaction of uric acid with peroxynitrite is required for CD26/DPPIV inhibition.
Crystal Structure of CD26/DPPIV and its small molecule inhibitors
To thoroughly understand the ability of triuret to inhibit CD26/DPPIV, we examined the positions of several other small molecule inhibitors of CD26/DPPIV from deposited x-ray crystal structures in the Protein Data Base (PDB) (PDB ID’s 2G5P, 2G5T, 2G63, 2I03). A common feature of each of the inhibitors is the presence of a carbonyl group in which the oxygen is buried in the active site by interacting with Tyr662, Asn710, and Glu205 (Fig. 4A). Also, a secondary amine is found in each of the inhibitors and is adjacent to the previously mentioned carbonyl. Its position is such that it is able to interact with a side chain hydroxyl and backbone carbonyl oxygen of Glu206.
Based on these commonalities, we suggest that triuret interacts with the active site serine (Ser630) via the nitrogen on one of the terminal amide groups (Fig. 4B). Further interactions are mediated by the carbonyl oxygen at the molecule’s center and involve the side chains of Glu205 and Asn710. The amide group distal to Ser630 interacts with the backbone carbonyl oxygen of Glu205. As this reaction is reversible, higher concentrations of the Gly-Pro-AMC substrate in a reaction mixture would result in a lesser amount of bound triuret, consistent with our results.
DISCUSSION
In our study, we demonstrated that uric acid reversibly inhibited only HUVEC associated CD26/DPPIV activity and not human recombinant CD26/DPPIV and that cellular uptake of uric acid was necessary in order to inhibit cell associated CD26/DPPIV activity. Since the active site is known to be extracellular, this appeared to contradict our data that uric acid inhibition of CD26/DPPIV was competitive. To reconcile this, we studied the effect of reaction products of uric acid on CD26/DPPIV activity and demonstrated that triuret, the product of uric acid reaction with peroxynitrite, is able to inhibit both the human recombinant and cell associated CD26/DPPIV. This introduces a novel feature to the biologic activity of uric acid, its ability to lead to a reversible inhibition of an enzyme and that its biologic activity may be dependent on the redox state of cells.
It appears that uric acid requires interaction with peroxynitrite and formation of triuret in order to inhibit CD26/DPPIV. Therefore, the extent to which CD26/DPPIV is inhibited is dependent on the amount of peroxynitrite present in the cell that can react with uric acid to generate triuret. This suggests that the functions of CD26/DPPIV substrates may be regulated by the presence of uric acid and peroxynitrite. One possible example where this may be of importance is with regard to neuropeptide Y1–36(NPY1–36), a peptide that contributes to atherosclerosis {Zukowska, 2005 #42}. NPY1–36 is found in the central nervous system and the autonomic nervous system, is a growth factor for endothelial and vascular smooth muscle cells, and is also a substrate of CD26/DPPIV. NPY1–36 has the ability to activate five receptors (Y1–Y5). Among the five receptors, Y1 has been shown to promote atherosclerosis {Zukowska, 2005 #42}. When CD26/DPPIV cleaves NPY1–36, it cleaves two amino acids from the amino terminal producing NPY3–36, a peptide that has higher affinity for Y2–Y5 receptors and a decreased affinity for the Y1 receptors {Zukowska, 2005 #42;Ghersi, 2001 #43}. NPY3–36 binding to the Y2 receptors is the mechanism that mediates the majority of its pro-migratory activity {Zukowska, 2005 #42}. Under conditions of high uric acid and high intracellular peroxynitrite levels, one can speculate that CD26/DPPIV would be inhibited and cleavage of NPY1–36 would be reduced. By inhibiting CD26/DPPIV, levels of NPY1–36 are increased leading to activation of the atherosclerotic promoting Y1 receptors {Zukowska, 2005 #42}. Interestingly, groups that have elevated levels of uric acid such as patients with metabolic syndrome {Choi, 2007 #44;Choi, 2007 #45} and African Americans {Fang, 2000 #46} also are at increased risk for atherosclerosis.
Despite the association of hyperuricemia with hypertension and cardiovascular disease, a study demonstrated that an acute increase in uric acid is associated with acute ischemic kidney injury and is necessary for endothelial progenitor cell (EPC) mobilization {Patschan, 2007 #5}. It is possible that our results provide a mechanism for their finding. CD26/DPPIV is found on the surface of EPCs {Christopherson, 2002 #9} and cleaves {Ohtsuki, 1998 #7;Proost, 1998 #8;Christopherson, 2002 #9} the key chemokine responsible for EPC mobilization, SDF-1. Thus, elevated levels of uric acid would reduce CD26/DPPIV activity and effectively increase the amount of intact SDF-1 presented to the EPCs, possibly resulting in increased mobilization of EPCs.
If CD26/DPPIV in vivo behaves as suggested by our experiments, the degree of inhibition of CD26/DPPIV would not just be dependent on an individual’s serum and intracellular uric acid level, but also dependent on the level of intracellular peroxynitrite. To fully understand the biologic effects of uric acid, it may be necessary to determine the amount of triuret, allantoin, and 6-aminouracil in the blood, reflecting uric acid’s reaction with peroxynitrite, superoxide, and nitric oxide in the serum, respectively.
CONCLUSION
We demonstrate for the first time that uric acid inhibits CD26/DPPIV, an enzyme with diverse effects on metabolism and endothelial function. This inhibitory effect is dependent on redox state of cells and formation of intracellular triuret. This might partially explain the seemingly paradoxical association of high levels uric acid, which is thought to be an anti-oxidant, with metabolic diseases and endothelial dysfunction.
Research Highlights.
Uric acid inhibits the action of CD26/dipeptidyl peptidase (DPP)IV
The inhibition of CD26/DPPIV by uric acid requires cellular uptake and production of intracellular triuret.
Increased oxidative stress enhances the production of triuret from uric acid and inhibition of CD26/DPPIV.
This is the first time that uric acid or a uric acid reaction product has been shown to affect enzymatic activity
This opens up novel avenues of research in the role of uric acid in the development of clinically important diseases.
ACKNOWLEDGMENTS
This work was supported by NIH grants R01 HL79352, T32DK007518 and Gatorade Funds to the Division of Nephrology Hypertension & Transplantation, University of Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kaur H, Halliwell B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem Biol Interact. 1990;73:235–247. doi: 10.1016/0009-2797(90)90006-9. [DOI] [PubMed] [Google Scholar]
- 2.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27:967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson KM, Morre JT, Beckman JS. Triuret: a novel product of peroxynitrite-mediated oxidation of urate. Arch Biochem Biophys. 2004;423:213–217. doi: 10.1016/j.abb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Gersch C, Palii SP, Imaram W, Kim KM, Karumanchi SA, Angerhofer A, Johnson RJ, Henderson GN. Reactions of peroxynitrite with uric acid: formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress. Nucleosides Nucleotides Nucleic Acids. 2009;28:118–149. doi: 10.1080/15257770902736400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patschan D, Patschan S, Gobe GG, Chintala S, Goligorsky MS. Uric Acid Heralds Ischemic Tissue Injury to Mobilize Endothelial Progenitor Cells. J Am Soc Nephrol. 2007;18:1516–1524. doi: 10.1681/ASN.2006070759. [DOI] [PubMed] [Google Scholar]
- 6.Christopherson KW, II, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of Hematopoietic Stem Cell Homing and Engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsuki T, Hosono O, Kobayashi H, Munakata Y, Souta A, Shioda T, Morimoto C. Negative regulation of the anti-human immunodeficiency virus and chemotactic activity of human stromal cell-derived factor 1[alpha] by CD26/dipeptidyl peptidase IV. FEBS Letters. 1998;431:236–240. doi: 10.1016/s0014-5793(98)00763-7. [DOI] [PubMed] [Google Scholar]
- 8.Proost P, Struyf S, Schols D, Durinx C, Wuyts A, Lenaerts JP, De Clercq E, De Meester I, Van Damme J. Processing by CD26/dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell-derived factor-1alpha. FEBS Lett. 1998;432:73–76. doi: 10.1016/s0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- 9.Christopherson KW, II, Hangoc G, Broxmeyer HE. Cell Surface Peptidase CD26/Dipeptidylpeptidase IV Regulates CXCL12/Stromal Cell-Derived Factor-1{alpha}-Mediated Chemotaxis of Human Cord Blood CD34+ Progenitor Cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 10.Chen WT, Kelly T, Ghersi G. DPPIV, seprase, and related serine peptidases in multiple cellular functions. Curr Top Dev Biol. 2003;54:207–232. doi: 10.1016/s0070-2153(03)54010-8. [DOI] [PubMed] [Google Scholar]
- 11.Abbott CA, Yu DM, Woollatt E, Sutherland GR, McCaughan GW, Gorrell MD. Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8. Eur J Biochem. 2000;267:6140–6150. doi: 10.1046/j.1432-1327.2000.01617.x. [DOI] [PubMed] [Google Scholar]
- 12.Olsen C, Wagtmann N. Identification and characterization of human DPP9, a novel homologue of dipeptidyl peptidase IV. Gene. 2002;299:185–193. doi: 10.1016/s0378-1119(02)01059-4. [DOI] [PubMed] [Google Scholar]
- 13.Wiedeman PE, Trevillyan JM. Dipeptidyl peptidase IV inhibitors for the treatment of impaired glucose tolerance and type 2 diabetes. Curr Opin Investig Drugs. 2003;4:412–420. [PubMed] [Google Scholar]
- 14.Burcelin R, Dolci W, Thorens B. Long-lasting antidiabetic effect of a dipeptidyl peptidase IV-resistant analog of glucagon-like peptide-1. Metabolism. 1999;48:252–258. doi: 10.1016/s0026-0495(99)90043-4. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda N, Inoue T, Nagakura T, Yamazaki K, Kira K, Saeki T, Tanaka I. Metformin Causes Reduction of Food Intake and Body Weight Gain and Improvement of Glucose Intolerance in Combination with Dipeptidyl Peptidase IV Inhibitor in Zucker fa/fa Rats. J Pharmacol Exp Ther. 2004;310:614–619. doi: 10.1124/jpet.103.064964. [DOI] [PubMed] [Google Scholar]
- 16.Wesley UV, Tiwari S, Houghton AN. Role for dipeptidyl peptidase IV in tumor suppression of human non small cell lung carcinoma cells. Int J Cancer. 2004;109:855–866. doi: 10.1002/ijc.20091. [DOI] [PubMed] [Google Scholar]
- 17.Bauvois B. Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis? Oncogene. 2004;23:317–329. doi: 10.1038/sj.onc.1207124. [DOI] [PubMed] [Google Scholar]
- 18.Khin EE, Kikkawa F, Ino K, Kajiyama H, Suzuki T, Shibata K, Tamakoshi K, Nagasaka T, Mizutani S. Dipeptidyl peptidase IV expression in endometrial endometrioid adenocarcinoma and its inverse correlation with tumor grade. Am J Obstet Gynecol. 2003;188:670–676. doi: 10.1067/mob.2003.169. [DOI] [PubMed] [Google Scholar]
- 19.Pethiyagoda CL, Welch DR, Fleming TP. Dipeptidyl peptidase IV (DPPIV) inhibits cellular invasion of melanoma cells. Clin Exp Metastasis. 2000;18:391–400. doi: 10.1023/a:1010930918055. [DOI] [PubMed] [Google Scholar]
- 20.Shioda T, Kato H, Ohnishi Y, Tashiro K, Ikegawa M, Nakayama EE, Hu H, Kato A, Sakai Y, Liu H, Honjo T, Nomoto A, Iwamoto A, Morimoto C, Nagai Y. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor 1alpha (SDF-1alpha) and SDF-1beta are abolished by CD26/dipeptidyl peptidase IV-mediated cleavage. Proc Natl Acad Sci U S A. 1998;95:6331–6336. doi: 10.1073/pnas.95.11.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto C, Lord CI, Zhang C, Duke-Cohan JS, Letvin NL, Schlossman SF. Role of CD26/dipeptidyl peptidase IV in human immunodeficiency virus type 1 infection and apoptosis. Proc Natl Acad Sci U S A. 1994;91:9960–9964. doi: 10.1073/pnas.91.21.9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber AE. Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem. 2004;47:4135–4141. doi: 10.1021/jm030628v. [DOI] [PubMed] [Google Scholar]
- 23.Durinx C, Lambeir AM, Bosmans E, Falmagne JB, Berghmans R, Haemers A, Scharpe S, De Meester I. Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is responsible for the release of X-Pro dipeptides. Eur J Biochem. 2000;267:5608–5613. doi: 10.1046/j.1432-1327.2000.01634.x. [DOI] [PubMed] [Google Scholar]
- 24.Iwaki-Egawa S, Watanabe Y, Kikuya Y, Fujimoto Y. Dipeptidyl peptidase IV from human serum: purification, characterization, and N-terminal amino acid sequence. J Biochem (Tokyo) 1998;124:428–433. doi: 10.1093/oxfordjournals.jbchem.a022130. [DOI] [PubMed] [Google Scholar]
- 25.Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 26.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 27.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RJ, Feig DI, Herrera-Acosta J, Kang DH. Resurrection of uric acid as a causal risk factor in essential hypertension. Hypertension. 2005;45:18–20. doi: 10.1161/01.HYP.0000150785.39055.e8. [DOI] [PubMed] [Google Scholar]
- 29.Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275:457–464. doi: 10.1056/NEJM196609012750902. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 31.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 32.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27:435–440. doi: 10.1159/000105142. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa T, Kang DH, Feig D, Sanchez-Lozada LG, Srinivas TR, Sautin Y, Ejaz AA, Segal M, Johnson RJ. Unearthing uric acid: an ancient factor with recently found significance in renal and cardiovascular disease. Kidney Int. 2006;69:1722–1725. doi: 10.1038/sj.ki.5000391. [DOI] [PubMed] [Google Scholar]
- 36.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 37.Paris D, Parker TA, Town T, Suo Z, Fang C, Humphrey J, Crawford F, Mullan M. Role of peroxynitrite in the vasoactive and cytotoxic effects of Alzheimer's beta-amyloid1-40 peptide. Exp Neurol. 1998;152:116–122. doi: 10.1006/exnr.1998.6828. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 39.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 40.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 41.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; San Carlos, CA: 2002. [Google Scholar]
- 42.Zukowska Z. Atherosclerosis and angiogenesis: what do nerves have to do with it? Pharmacol Rep. 2005;57(Suppl):229–234. [PubMed] [Google Scholar]
- 43.Ghersi G, Chen W, Lee EW, Zukowska Z. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides. 2001;22:453–458. doi: 10.1016/s0196-9781(01)00340-0. [DOI] [PubMed] [Google Scholar]
- 44.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442–447. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 45.Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57:109–115. doi: 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 46.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]