Abstract
We describe an approach to cataract phacoemulsification that uses the carouseling technique within the capsular bag. This is made possible by a newly designed phacoemulsification tip with 3 unique modifications: a 20-degree right bend in the tip, a semicircular opening, and a third irrigation port. These 3 features facilitate the carouseling technique of phacoemulsification without expressing the lens into the anterior chamber. The method decreases corneal endothelial injury by maximizing the distance between the delivered thermal energy and the corneal endothelium. The preoperative and postoperative pachymetry and endothelial cell counts in the first 8 patients treated using this technique are reported.
Cataract surgery has evolved rapidly since Kelman's invention of phacoemulsification in 1967.1 With the later introduction of the specular microscope by Maurice2 to assess corneal endothelium, a new understanding of the relationship between phacoemulsification and corneal endothelial damage has arisen.3 This has resulted in a wave of research and innovation in cataract extraction techniques to decrease corneal endothelial damage and optimize efficiency.
The current understanding of the mechanism of endothelial damage is based on several theories. These include thermal and free radical injury (both related to duration and power of ultrasound), corneal distortion, and endothelial contact with nuclear fragments.4–6 Various techniques have tried to minimize ultrasound energy and distortion of the cornea via instrument manipulation as well as to establish new parameters that would decrease nuclear fragment contact with the endothelium.
Zetterström and Laurell7 performed phacoemulsification in the posterior chamber and thus decreased the risk for endothelial damage by maximizing the distance from the corneal endothelium. This decreases the risk for contact injury form nuclear fragments and thermal injury from phacoemulsification. The concept of proximity-induced damage is also supported by the finding that shorter eyes have a significantly higher risk for endothelial cell loss.6
Carouseling, a new phacoemulsification technique, is advantageous because it minimizes instrument movement within the eye, decreasing corneal distortion, and offers a smooth fluid approach to removing cataracts. Traditional carouseling involves hydroexpression of one edge of the nucleus into the anterior chamber followed by phacoemulsification from the equatorial edge. This can result in endothelial injury from the thermal energy close to the cornea as well as an equatorial groove that makes holding purchase on the lens more difficult. We propose an approach to phacoemulsification that allows cataract extraction using the carousel technique in the posterior chamber endocapsularly—carouseling in the bag.
Endocapsular carouseling is made possible by a newly designed phaco tip (Ambati D-tip [Microsurgical Technology]) with 3 key advances: The tip is bent 20 degrees to the right and has a semicircular (reverse D-shaped) opening and a third irrigation port on the sleeve oriented behind the bevel (Figure 1). The first modification, the 20-degree bend to the right, enables the tip to enter the peripheral right quadrant of the lens capsule, parallel to the floor and planar with the lens, while the bevel is angled toward the left (Figure 2).
Figure 1.
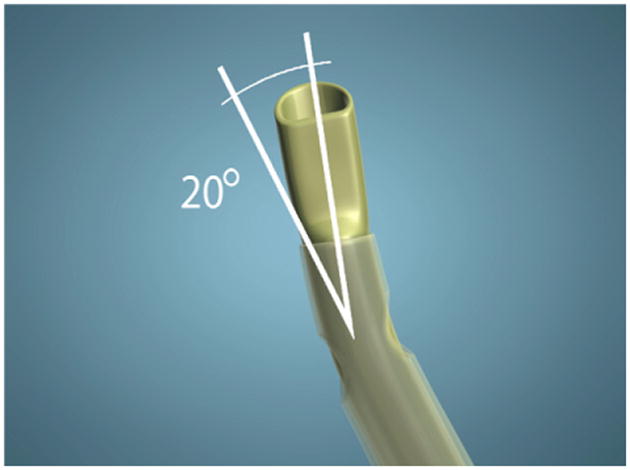
The newly designed phaco tip.
Figure 2.
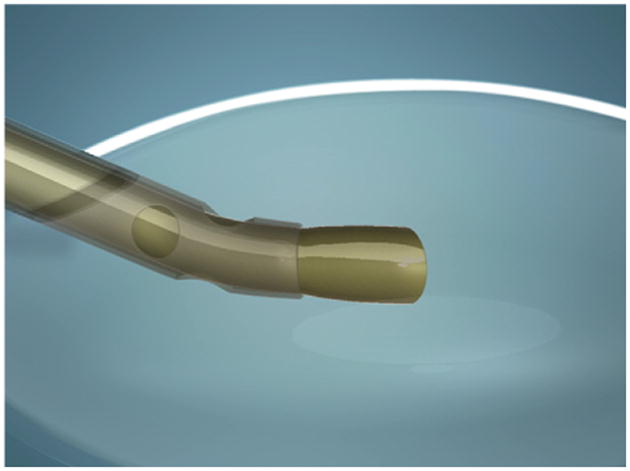
Once inserted in the right peripheral quadrant, the 20-degree bend aligns the tip so it is planar to the lens.
The bevel is configured with a D-tip or semicircular profile, preventing the formation of an equatorial groove, which can occur with conventional circular tips. Rounded corners at the vertices of the semicircular orifice enhance safety (Figure 3). Preventing the equatorial groove enables continuous purchase of the lens throughout the complete carouseling process. An additional component of the D-profile tip is a flat distal shaft, which entails reverse flare configuration, avoiding clogging that is present with conventional flared tips.
Figure 3.
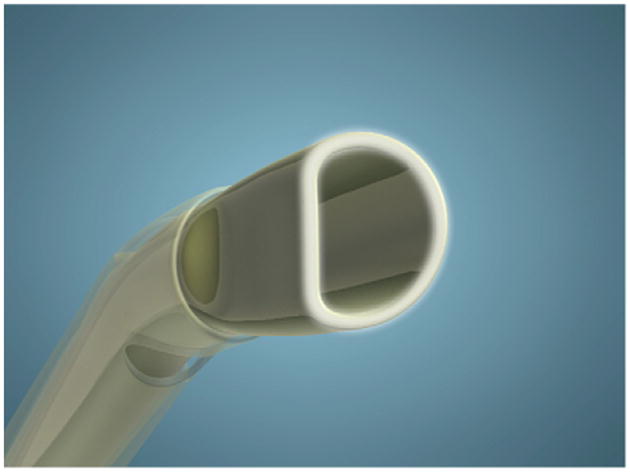
The D configuration of the tip.
In addition to the 2 irrigation side ports that maintain the chamber, the third irrigation port sets up a third infusion current that keeps the peripheral capsule inflated and stages a laminar countercurrent. Thus, the current travels counterclockwise while the cataract is aspirated clockwise (Figure 4). Having a current flow opposite the peripheral lens enhances surface shear, enabling more rapid phacoemulsification.
Figure 4.
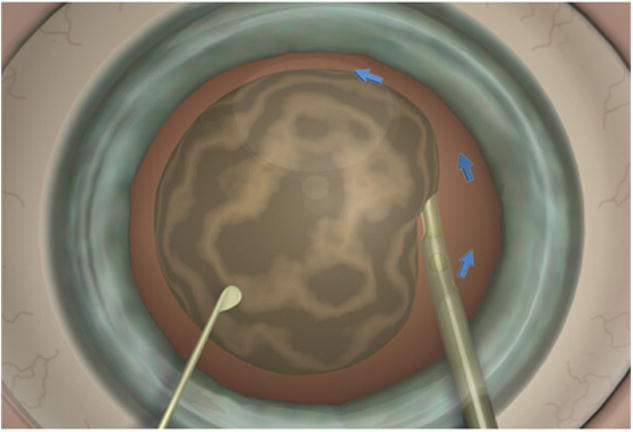
The blue arrows indicate the current created by the third port. The cataract is pulled into the phaco tip, thus rotating clockwise against the counterclockwise current created by the additional irrigation port.
Surgical Technique
The general surgical technique is accomplished by inserting the tip bevel into the eye through a 2.2 mm incision. With the bevel oriented downward, the tip is maneuvered into the right quadrant of the lens capsule to access the peripheral cataract. The third irrigation port not only keeps the peripheral lens capsule inflated, but also induces recursive hydrodelineation of peripheral lens lamellae. Peripheral placement of the tip is vital to obtain the full value of this technique and avoid an epinuclear bowl. The bevel is rotated to face left, and aspiration is engaged with a limited need for ultrasound energy.
Once proper apposition is achieved, the cataract is removed rapidly. The left-hand instrument is primarily used to steady the eye and perform gentle rotation of the cataract as necessary. Smooth, controlled carouseling of the cataract occurs within the bag, minimizing thermal injury to the corneal endothelium while improving phacoemulsification efficiency. Staying at the edge with proper apposition allows rapid removal.
High flow settings are used throughout the procedure. Any type of ophthalmic viscosurgical device (OVD) can be used to facilitate entry to the peripheral lens capsule, especially in the initial cases performing this technique. The video (available at http://jcrsjournal.org) illustrates the technique.
Results
A retrospective analysis of a consecutive series of the first 8 phacoemulsification cases (6 patients) using the endocapsular carousel technique was performed. All surgeries were performed by the same surgeon (B.K.A) between November 2009 and January 2010.
The mean age of the 6 women was 64.3 years (range 57 to 75 years). All cataracts were graded 3+ or less. Two patients had previously diagnosed ophthalmic conditions: one with Fuchs endothelial dystrophy and the other with age-related macular degeneration. All cases were performed using an Alcon Infiniti machine and consistent settings were used: 25% power, 425 mm Hg vacuum, dynamic rise of 2, 100% OZil, 110 cm bottle height, and 40 mL/min flow. An OVD (sodium chondroitin sulfate 4%–sodium hyaluronate 1.65% [DisCoVisc]) was used to facilitate entry to the peripheral lens capsule. No intraoperative or postoperative complications occurred.
Preoperative endothelial cell count and pachymetry were compared with the 1 day and 3 month postoperative values using a paired 2-sample Student t test (Table 1).
Table 1.
Preoperative and postoperative endothelial cell count and pachymetry.
| Parameter | Preoperative | Postoperative | |
|---|---|---|---|
|
| |||
| 1 Day | 3 Months | ||
| Mean pachymetry | 572 ± 39 | 596 ± 59 | 578 ± 46 |
| % increase | — | 4.08% (P=.07) | 1.05% (P=.32) |
| Mean ECC | 2299 ± 349 | 2240 ± 324 | 2190 ± 467 |
| % ECL | — | 2.57% (P=.15) | 4.72% (P=.19) |
ECC = endothelial cell count; ECL = endothelial cell loss
Discussion
The data from the 8 cases is supportive of minimal corneal endothelial injury. Pachymetry performed 1 day postoperatively has been shown to strongly correlate with long-term endothelial damage and thus serves as a predictive measure.8 In our cases, the 2 most significant data points correspond to one another: The pachymetry on day 1 was less than a 5% increase, and the follow-up endothelial cell loss was 4.7%. The slight increase in pachymetry on day 1 was resolved by the 3-month follow-up. Schultz et al.9 determined that a 3-month period was sufficient to allow stable and complete endothelial wound healing. The endothelial cell loss in our cases is comparable to, if not lower than, that in other reported techniques, such as divide and conquer and chopping, and also improves the efficiency of cataract removal.10-14 Note that this technique is approved for cataracts graded 3 + or less only.
To better understand the impact of adding a third irrigation port, a computational fluid dynamic simulation was performed. This was accomplished by using 3 commercially available software programs (Gambit, SolidWorks, and Fluent) along with the geometric dimensions of the eye obtained from Chalam.15 The vacuum of the phaco tip was set to a “pressure outlet,” and the 3 irrigation ports were set as “pressure inlets” based on the bottle height, 110 cm, and patient height, 55 cm, and the applied vacuum set at 262.5 mm Hg (peak vacuum pressure was 425 mm Hg).
This computer simulation confirmed that by adding a third port, the fluid vectors flowed in an organized counterclockwise direction. Fluid stream lines, which are lines tangent to the velocity vector, are shown in Figure 5; they can be seen traveling 270 degrees around the peripheral capsule before being drawn to the vacuum. With standard side ports only, velocity vectors throughout the capsular bag are disorganized (Figure 6). The addition of a third port induces a peripheral current with velocity vectors organized within the capsule (Figure 7).
Figure 5.
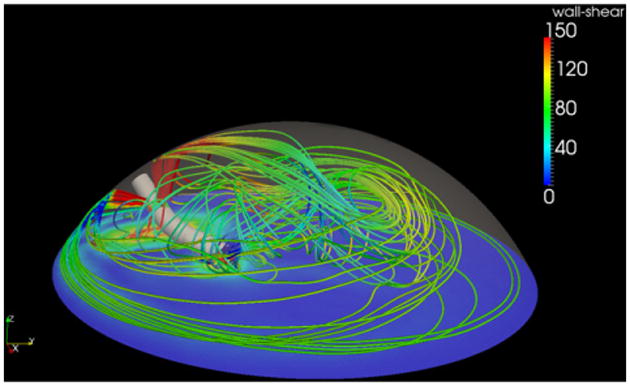
Stream lines created from the new 3-port irrigation system.
Figure 6.
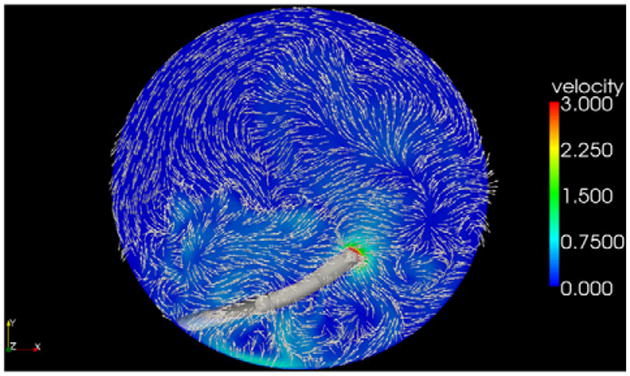
Velocity vectors 1.0 mm above the posterior capsule of a standard 2-port irrigation system.
Figure 7.
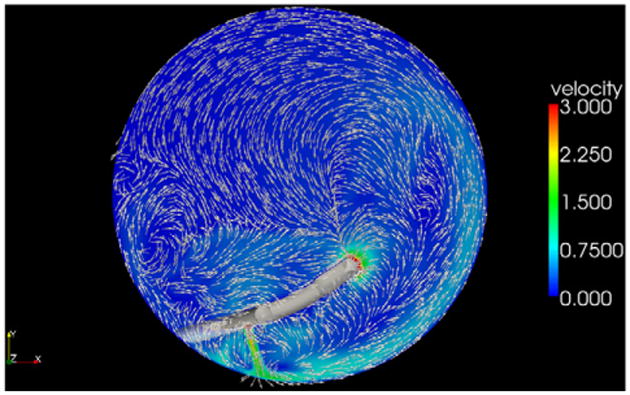
Velocity vectors 1.0 mm above the posterior capsule with the addition of a third port. The peripheral current (aqua) is seen traveling counterclockwise.
The significance of a peripheral countercurrent is that it contributes to cataract removal by creating an additional force on the cataract in the form of shear stress. When a fluid moves across a solid surface, it exerts a force called shear stress on the solid. This concept is well studied in blood flow and the shear stress on the vascular endothelium.16 For our simulated probe location and boundary conditions, the peak velocity of the peripheral current was 1.3 m/s, which induced a maximal shear stress of 32.5 Pascals on the equatorial lens surface. For comparison, this is almost 15 times the peakwall stress of the carotid arteryinanormal 60-year-old man.17 Having this additional shear stress aids in cataract degradation and thus reduces the amount of ultrasound energy required to emulsify the lens.
Although this shear stress helps remove the cataract, it does not jeopardize the integrity of the capsule. The shear stress on the capsule (rather than the lens), or wall shear stress, is decreased by the addition of a third port. With the standard 2-port irrigation system, the side port that faces down creates a wall shear stress on the posterior capsule. By adding a third port, our simulation showed a decrease in wall shear stress created by the side ports due to the peripheral current that redistributes the force throughout the capsule. Since the 2 side ports are used to stabilize the eye and do not aid in removing the cataract, this reduction in wall shear stress could reduce adverse effects, if any, caused by the stagnating bottom jet on the posterior capsule.
In conclusion, staying beneath the anterior capsule during cataract removal minimizes thermal injury to the cornea. The presence of a third port keeps the peripheral capsule inflated, enhancing safety. Carouseling in the bag is dependent on the newly designed tip and allows phacoemulsification to be performed in a smooth and predictable fashion with minimal movements required by the instruments. The technique therefore enables higher efficiency for the surgeon and improved safeguards for patient safety.
Supplementary Material
Footnotes
Financial Disclosure: G.J. Jardine, G.C. Wong, J.R. Elsnab, and B.K. Gale have no financial or proprietary interest in any material or method mentioned. Additional disclosure is found in the footnotes.
Additional financial disclosure: B. Ambati developed the instrument described in this article with Microsurgical Technologies and has a royalty interest.
Presented at the ASCRS Symposium on Cataract, IOL and Refractive Surgery, Boston, Massachusetts, USA, April 2010.
References
- 1.Kelman CD. Phaco-emulsification and aspiration; a new technique of cataract removal;a preliminary report. Am J Ophthalmol. 1967;64:23–35. [PubMed] [Google Scholar]
- 2.Maurice DM. A scanning slit optical microscope. [Accessed October 21, 2010];Invest Ophthalmol. 1974 13:1033–1037. Available at: http://www.iovs.org/cgi/reprint/13/12/1033. [PubMed] [Google Scholar]
- 3.Olson RJ, Mamalis N, Werner L, Apple DJ. Cataract treatment in the beginning of the 21st century. Am J Ophthalmol. 2003;136:146–154. doi: 10.1016/s0002-9394(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 4.Cameron MD, Poyer JF, Aust SD. Identification of free radicals produced during phacoemulsification. J Cataract Refract Surg. 2001;27:463–470. doi: 10.1016/s0886-3350(00)00643-x. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996;22:1079–1084. doi: 10.1016/s0886-3350(96)80121-0. [DOI] [PubMed] [Google Scholar]
- 6.Walkow T, Anders N, Klebe S. Endothelial cell loss after phacoemulsification: relation to preoperative and intraoperative parameters. J Cataract Refract Surg. 2000;26:727–732. doi: 10.1016/s0886-3350(99)00462-9. [DOI] [PubMed] [Google Scholar]
- 7.Zetterström C, Laurell CG. Comparison of endothelial cell loss and phacoemulsification energy during endocapsular phacoemulsification surgery. J Cataract Refract Surg. 1995;21:55–58. doi: 10.1016/s0886-3350(13)80480-4. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg B, Jonsson M, Behndig A. Postoperative corneal swelling correlates strongly to corneal endothelial cell loss after phacoemulsification cataract surgery. Am J Ophthalmol. 2005;139:1035–1041. doi: 10.1016/j.ajo.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 9.Schultz RO, Glasser DB, Matsuda M, Yee RW, Edelhauser HF. Response of the corneal endothelium to cataract surgery. [Accessed October 21, 2010];Arch Ophthalmol. 1986 104:1164–1169. doi: 10.1001/archopht.1986.01050200070053. Available at: http://archopht.ama-assn.org/cgi/reprint/104/8/1164. [DOI] [PubMed] [Google Scholar]
- 10.Miyata K, Maruoka S, Nakahara M, Otani S, Nejima R, Samejima T, Amano S. Corneal endothelial cell protection during phacoemulsification; low- versus high-molecular-weight sodium hyaluronate. J Cataract Refract Surg. 2002;28:1557–1560. doi: 10.1016/s0886-3350(02)01540-7. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien PD, Fitzpatrick P, Kilmartin DJ, Beatty S. Risk factors for endothelial cell loss after phacoemulsification surgeryby a junior resident. J Cataract Refract Surg. 2004;30:839–843. doi: 10.1016/S0886-3350(03)00648-5. [DOI] [PubMed] [Google Scholar]
- 12.Richard J, Hoffart L, Chavane F, Ridings B, Conrath J. Corneal endothelial cell loss after cataract extraction by using ultrasound phacoemulsification versus a fluid-based system. Cornea. 2008;27:17–21. doi: 10.1097/ICO.0b013e3181583115. [DOI] [PubMed] [Google Scholar]
- 13.Storr-Paulsen A, Norregaard JC, Ahmed S, Storr-Paulsen T, Pedersen TH. Endothelial cell damage after cataract surgery: divide-and-conquer versus phaco-chop technique. J Cataract Refract Surg. 2008;34:996–1000. doi: 10.1016/j.jcrs.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Alió JL, Mulet ME, Shalaby AM, Attia WH. Phacoemulsification in the anterior chamber. J Cataract Refract Surg. 2002;28:67–75. doi: 10.1016/s0886-3350(01)01018-5. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Ophthalmology. Fundamentals and Principles of Ophthalmology, Section 2, 2008-2009. San Francisco, CA: American Academy of Ophthalmology; 2008. Basic and Clinical Science Course. [Google Scholar]
- 16.Reneman RS, Arts T, Hoeks APG. Wall shear stress – an important determinant of endothelial cell function and structure – in the arterial system in vivo; discrepancies with theory. [Accessed October 21, 2010];J Vasc Res. 2006 43:251–269. doi: 10.1159/000091648. Available at: http://content.karger.com/ProdukteDB/produkte.asp?Aktion=ShowPDF&ArtikelNr=91648&Ausgabe=231661&ProduktNr=224160&filename=91648.pdf. [DOI] [PubMed] [Google Scholar]
- 17.Samijo SK, Willigers JM, Barkhuysen R, Kitslaar PJEHM, Reneman RS, Brands PJ, Hoeks APG. Wall shear stress in the human common carotid artery as function of age and gender. [Accessed October 21, 2010];Cardiovasc Res. 1998 39:515–522. doi: 10.1016/s0008-6363(98)00074-1. Available at: http://cardiovascres.oxfordjournals.org/content/39/2/515.full.pdf+html. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


