To the Editor:
A growing body of literature demonstrates that recombinase activating gene (RAG1/2) deficiency is seen in patients with autoimmune features and variable immunodeficiency, thus expanding the phenotype beyond T-/B-/NK+ severe combined immunodeficiency (SCID) and Omenn syndrome (OS) (1, 2). Based on data from SCID newborn screening programs in the United States and Puerto Rico, the estimated incidence of SCID is approximately 1:50,000 (3). Recent data from California’s SCID newborn screening identified RAG1/2 mutations in 28.6% of SCID/OS cases, for an incidence of about 1:250,000(4). However, this may not include individuals with less severe phenotypes caused by RAG1/2 defects.
We present a family affected by compound heterozygous RAG1 mutations which resulted in a combined immunodeficiency phenotype with autoimmune cytopenias. We performed a population genetic analysis to estimate the incidence of disease caused by RAG1/2 homozygous or compound heterozygous mutations and found that the contribution of immune dysregulatory disease due to RAG1/2 mutations present in the general population may be much higher than previously estimated.
Patient 1 (P1) is the eldest of five siblings born to non-consanguineous Caucasian parents (Figure 1, II.1). Clinical and laboratory data are summarized in Table 1 and Table E1 (online repository). Briefly, P1 developed early-onset autoimmune hemolytic anemia (AIHA), recurrent viral and bacterial infections, and nephrotic syndrome. Patient 2 (P2) is the full sibling of P1 (Figure 1, II.4), born full term with an eczematous rash. She developed recurrent bacterial and viral upper respiratory infections, early-onset AIHA, and neutropenia with antineutrophil antibodies. The autoimmune cytopenias did not respond to anti-CD20 treatment (rituximab). Both siblings had significantly reduced naïve CD4+ T cell counts. Notable family history includes the siblings’ healthy mother who had false positive Rapid Plasma Reagin (RPR) antibodies and several maternal family members with multiple sclerosis.
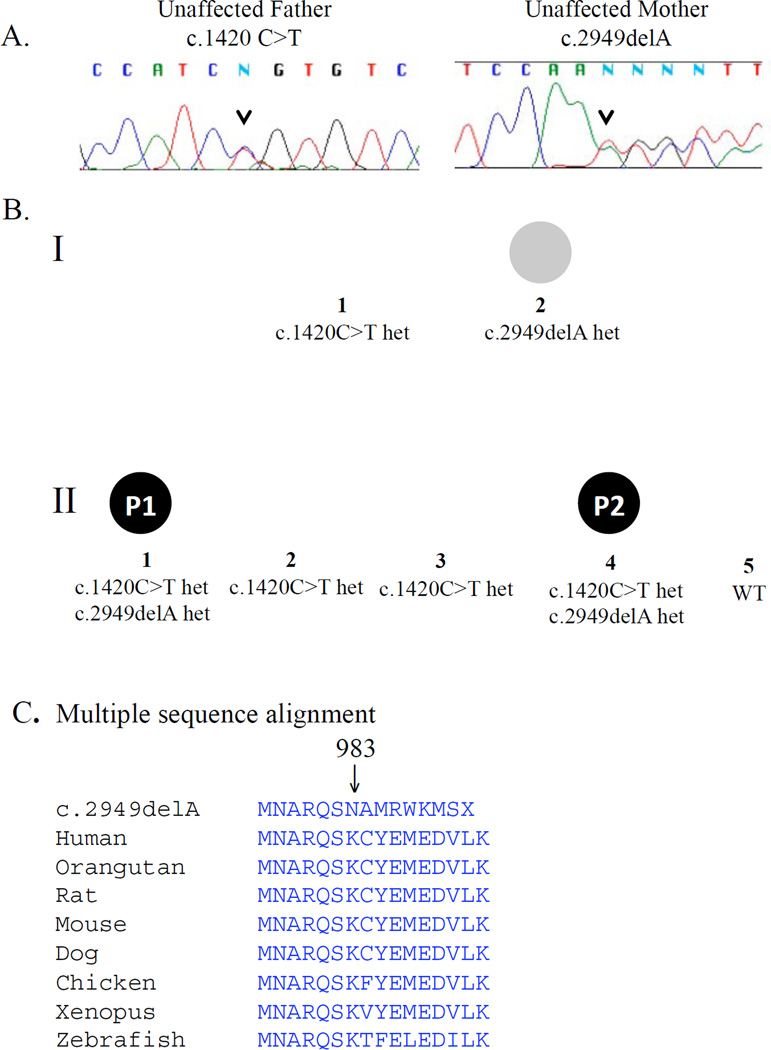
Figure 1.
A. Sanger sequencing chromatogram of parents of the affected children confirms RAG1 mutation carrier status. Arrow indicates mutation position. B. Pedigree of family with RAG1 deficiency. Patient 1(II.1) and patient 2 (II.4) were affected with autoimmune cytopenias and infections. Het = heterozygous, WT = wildtype. C. Multiple sequence alignment of the RAG1 locus with c.2949delA(p.K983NfsX9) mutation.
Table 1.
Clinical features and key laboratory data
| Patient 1 (II.1) | Patient 2 (II.4) | |
|---|---|---|
| Infections | Recurrent otitis media Pneumonias (bacterial and viral) Parvovirus aplastic crisis H1N1 Influenza A encephalitis Varicella from vaccinated family contact |
Recurrent otitis media RSV bronchiolitis Varicella disease and varicella periorbital cellulitis/keratitis from vaccinated family contact. |
| Clinical autoimmune features | Nephrotic syndrome (18 months; after childhood immunization; not biopsied but responded to systemic steroids) Autoimmune hemolytic anemia (3 years) |
Eczematous rash during infancy Neutropenia (4 months) Autoimmune hemolytic anemia (15 months) |
| Leukocyte counts (cells/µl) | Birth: ALC 2700, normal ANC 3.5 years: ALC 1500, normal ANC Eosinophils: normal | 4 months: ALC 6700, ANC 100 26 months: ALC 600, ANC 100 Eosinophils: 700 (4 months), then normal |
| Autoantibodies | Warm IgG agglutinin to RBC Lupus anticoagulant + ANCA; +anti-MPO Thyroglobulin and thyroid peroxidase Ab Anti-IFNα Ab |
Direct Coombs+ (IgG and complement) Anti-neutrophil Ab Anti-IFNα Ab |
| Pathology | Bone marrow biopsy: Expanded γδ T cell population (46% of total lymphocytes), granulocyte maturation arrest at promyelocyte stage, myeloid hypoplasia, cellular marrow with trilineage hematopoiesis, and mild increase in CD34+ myeloblasts. The B cell light chains were shifted towards lambda predominance, although the kappa:lambda ratio was within normal limits. |
Time points in parentheses indicate age of onset or age laboratory data was noted.
Ab = antibody; ALC = absolute lymphocyte count per µl; ANC = absolute neutrophil count per µl; ANCA = antineutrophil cytoplasmic antibody; IFNα = interferon-α; MPO = myeloperoxidase; RSV = respiratory syncytial virus.
P1 underwent a 10/10 HLA-matched sibling bone marrow transplant at 8 years following conditioning with busulfan, fludarabine and anti-thymocyte globulin. Graft-versus-host disease (GVHD) prophylaxis included cyclosporine and methotrexate. One year post-transplant, P1 has excellent donor chimerism. Immune globulin replacement was discontinued 16 months post-transplant. P2 received a 10/10 matched unrelated marrow hematopoietic stem cell transplant (HSCT) at 29 months following similar conditioning. She had delayed engraftment of neutrophils with persistence of anti-neutrophil antibodies. Complications have included grade 3 gastrointestinal GVHD 6 months post-transplant, pancytopenia, and concern for graft failure.
To identify an underlying genetic defect, P2 was screened for known mutations associated with SCID. No disease-causing mutations were identified in JAK3, DCLRE1C (ARTEMIS), IL7R, or RAG2. She harbored compound heterozygous mutations in exon 2 of RAG1, a genotype that was also confirmed for P1 (Figure 1). The c.1420C>T (p.Arg474Cys) mutation has previously been reported in cases of primary immunodeficiency, including SCID, Omenn syndrome and CD4+ T cell lymphopenia (Table E2, online repository). The c.2949delA (p.Lys983AsnfsX9) mutation is novel. Sanger sequencing of the remaining family members identified each parent as a RAG1 mutation carrier (Figure 1). The c.2949delA mutation is located at the carboxy-terminus of RAG1, at a RAG2 interaction site within the catalytic core (5, 6). A homozygous mutation at nearby amino acid position Q981P has been described by de Villartay et al.(7) with clinical features very similar to P1 and P2.
Serum samples from healthy and affected family members were probed against an autoantibody protein microarray. IgG/IgM autoantibodies were increased in P1 and P2, and surprisingly, also in their mother (Figure E1 and Tables E3–E6, online repository). Furthermore, serum was tested for anticytokine antibodies that have been reported among patients with thymoma or autoimmune polyendocrinopathy, candidiasis, ectodermal dystrophy (APECED)(8, 9). Interestingly, our two affected patients also produce anti-interferon-α (IFNα) autoantibodies that were able to neutralize IFNα-induction of STAT1 phosphorylation in normal peripheral blood mononuclear cells, and may comprise a phenotypic signature for certain RAG-deficient patients (Figure E2, online repository).
We assessed the European population carrier rate of RAG1/2 variants that have been identified in primary immunodeficiency diseases (PIDs; for methods, see Online Repository Text). Of more than 100 disease-causing RAG1 variants in the Human Gene Mutation Database, only 2 were present in 379 individuals of European ancestry in the 1000 Genomes database (Table E7, online repository). Seven and three unrelated individuals were heterozygous for the c.1346G>A and c.3016A>G disease-associated RAG1 variants, respectively. Therefore, the predicted population frequency based on Hardy-Weinberg equilibrium is 1:5746 individuals of European descent who are homozygous or compound heterozygous, much higher than what is expected for RAG1/2 SCID or OS cases. The single RAG2 disease-causing variant present in 1000 Genomes is in linkage disequilibrium with the c.3016A>G variant. Thus, the RAG1/2 carrier rate does not change. Our population genetic estimate indicates that RAG1 mutations likely contribute to many more undiagnosed cases of combined immunodeficiency, autoimmune cytopenias, and/or organ-specific autoimmune disease, although it is possible there is incomplete penetrance of these genotypes. California has a diverse and large Hispanic demographic and there may be differences when comparing estimates to a predominantly European population. Our estimate does not account for very rare RAG1 mutations not present in the 1000 Genomes database. Because limited data are available for functional activity in RAG1 variants identified in patients with PIDs, their disease-causing role cannot be easily established.
In this case report, compound heterozygous RAG1 mutations resulted in a combined immunodeficiency phenotype with autoimmune cytopenias and multiple autoantibodies, including anti-IFNα antibodies as have been reported in APECED or thymoma patients. The mechanism for generation of these autoantibodies is unclear, but may depend on an underdeveloped thymus with decreased AIRE expression. The mother has a history of false-positive RPR and demonstrated presence of elevated levels of autoantibodies that was not seen in the c.1420C>T male carriers. Our findings suggest that the c.2949delA variant, even in heterozygous state, may contribute to generation of autoantibodies in RAG1-related disease.
In patients with autoimmune cytopenias, lymphopenia and moderate-to-severe infections, screening for RAG1/2 mutations may well be worthwhile to identify molecular pathogenesis and treatment options. In our cohort, the presence of mild lymphopenia, autoimmune cytopenias, and a skewed naïve:memory T cell ratio led to the diagnosis of RAG1 deficiency and subsequent HSCT. Earlier identification of a monogenic immune disorder and prompt HSCT during infancy might have spared progressive autoimmune complications. These findings provide additional data that RAG has important implications in both immunodeficiency and autoimmune diseases.
Supplementary Material
Acknowledgments
Declaration of sources of funding for the research reported in the manuscript:
This work was partly supported by the National Institute of Allergy and Infectious Diseases, NIH grant 5P01AI076210-04 (L.D.N.), National Institute of Allergy and Infectious Diseases, NIH U54AI082973 (L.D.N.), Manton Foundation (L.D.N.), the March of Dimes grant 6-FY10-282 and the Jeffrey Modell Foundation (to L.D.N.), and by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH (L.B.R. and S.K.B.). J.E.W. has NIH funding for evaluation of immune deficiencies (1K08AI103035-01). L.B.J. is supported by NIH grant GM59290 and the Utah Genome Project. DNA extraction was performed by the University of Utah Center for Clinical & Translational Science (CCTS) Translational Technologies & Resources (TTR) core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: Drs. Walter and Notarangelo have received federal funding and support from private foundations.
References
- 1.Avila EM, Uzel G, Hsu A, Milner JD, Turner ML, Pittaluga S, et al. Highly variable clinical phenotypes of hypomorphic RAG1 mutations. Pediatrics. 2010 Nov;126(5):e1248–e1252. doi: 10.1542/peds.2009-3171. [DOI] [PubMed] [Google Scholar]
- 2.Niehues T, Perez-Becker R, Schuetz C. More than just SCID--the phenotypic range of combined immunodeficiencies associated with mutations in the recombinase activating genes (RAG) 1 and 2. Clin Immunol. 2010 May;135(2):183–192. doi: 10.1016/j.clim.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Buckley RH. The long quest for neonatal screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2012 Mar;129(3):597–604. doi: 10.1016/j.jaci.2011.12.964. quiz 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan A, Church JA, Cowan MJ, Agarwal R, Kapoor N, Kohn DB, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: Results of the first 2 years. J Allergy Clin Immunol. 2013 Jul;132(1):140–150. doi: 10.1016/j.jaci.2013.04.024. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobacchi C, Marrella V, Rucci F, Vezzoni P, Villa A. RAG-dependent primary immunodeficiencies. Hum Mutat. 2006 Dec;27(12):1174–1184. doi: 10.1002/humu.20408. [DOI] [PubMed] [Google Scholar]
- 6.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol. 2011 Apr;11(4):251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 7.de Villartay JP, Lim A, Al-Mousa H, Dupont S, Dechanet-Merville J, Coumau-Gatbois E, et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest. 2005 Nov;115(11):3291–3299. doi: 10.1172/JCI25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burbelo PD, Browne SK, Sampaio EP, Giaccone G, Zaman R, Kristosturyan E, et al. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood. 2010 Dec 2;116(23):4848–4858. doi: 10.1182/blood-2010-05-286161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meager A, Visvalingam K, Peterson P, Moll K, Murumagi A, Krohn K, et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006 Jul;3(7):e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.