Abstract
Few therapeutic strategies exist for hematologic malignancies relapsing postallogeneic hematopoietic cell transplant. We present outcomes on 35 patients with nonchronic myelogenous leukemia (CML) hematologic malignancies, the majority having acute myelogenous leukemia (AML) or myelodysplastic syndromes/myeloproliferative disorders (MDS/MPD) (n = 22) receiving lymphodepleting chemotherapy followed by donor lymphocyte infusion (DLI) at 2 T cell dose levels (0.5 and 1.0 × 108 CD3/kg). Forty-nine percent of patients achieved complete remission (CR), with a median duration of remission of 6 months (range: 2-71+). CR rates were similar between the 2 groups. The incidence of acute graft-versus-host disease (aGVHD) of any grade was 49%. We saw a higher incidence of grade II-IV aGVHD with a rate of 66% using the higher dose DLI (grade III, 33% and grade 4, 20%) versus only 25% (10% grade III-IV) with the lower dose DLI (P = .06). Overall survival at 1 and 2 years was 30% (95% confidence interval [CI], 16%-45%) and 19% (95% CI, 8%-34%); however, for those achieving CR, 1- and 2-year survival was improved at 44% (95% CI 20%-66%) and 28% (95% CI, 8%-52%) (P = .03), respectively. These results demonstrate that DLI after lymphodepleting chemotherapy for relapsed hematologic malignancies results in frequent CRs. The lower DLI dose regimen improved the tolerability of this therapeutic approach, with modest rates of severe aGVHD.
Keywords: Lymphodepleting chemotherapy, Relapse, Allogeneic cell transplantation, Donor lymphocyte infusion, DLI
Introduction
Relapse of underlying hematologic malignancy following allogeneic hematopoietic cell transplant (HCT) remains a major obstacle for long-term disease-free survival [1]. Therapeutic options for patients relapsing with nonchronic myelogenous leukemia (CML) disease postallogeneic HCT are limited and often unsuccessful. The literature describing responses to donor lymphocyte infusion (DLI) postallogeneic HCT varies depending on the underlying hematologic malignancy, remission status at time of DLI, and use of disease-specific chemotherapy to reduce the tumor burden before DLI infusion [2-6]. The initial use of DLI in patients with CML produced optimism that DLI alone would be a successful therapy for relapsed hematologic malignancies given the high (70+%) complete remission (CR) rates [7-9]. However, responses to DLI in other hematologic malignancies have been less successful. DLI studies with acute myelogenous leukemia (AML) patients, with the majority receiving AML-specific reinduction chemotherapy before DLI yield CR rates ranging from 28% to 65% [4,5,10,11]. The largest of these series showed improved 2-year survival in those with favorable cytogenetics and those in remission at the time of DLI (56%), thus nearing but not reaching the success with DLI for relapsed CML [4]. The discrepant results between CML and AML/myelodysplastic syndrome (MDS) responses are potentially related to the underlying biology and kinetics of disease or the differential responsiveness to a graft-versus-leukemia effect between chronic and acute leukemias. In lymphoid malignancies, CR rates following DLI range from 42% to 76% depending on the underlying disease, but most reports describe less consistent use of pre-DLI chemotherapy [2,3,12].
An alternative to disease-specific chemotherapy is the use of lymphodepleting chemotherapy immediately followed by DLI in order to alter the immunologic milieu by potentially increasing access to cytokines and decreasing numbers of T regulatory cells and myeloid-derived suppressor cells that may inhibit DLI function. Initially described by Dudley et al. [13] in the setting of refractory, heavily pretreated melanoma, lymphodepleting chemotherapy followed by adoptive transfer of tumor specific autologous lymphocytes was associated with significant responses. At the University of Minnesota we utilized the approach of lymphodepleting chemotherapy before DLI in relapsed hematologic malignancies and previously reported our 2004 to 2006 [14] experience. Our initial lymphodepleting regimen included a combination of fludarabine and cyclophosphomide therapy before DLI at a dose of 1 × 108 CD3+ cells/kg. We observed substantial rates of CR in the initial 15 patients; however, this came at the expense of high rates of grade III-IV acute graft-versus-host disease (aGVHD), which in some cases was the cause of death. We report here updated outcomes on all 35 patients that include an additional 20 patients treated with the same lymphodepleting conditioning before a reduced dose of DLI of 0.5 × 108 CD3+ cells/kg. We show that both regimens are effective at inducing remissions, but that the lower T cell dose DLI is associated with less severe aGVHD.
Patients and Methods
Patients with relapsed hematologic malignancy postsibling or unrelated donor HCT were eligible for enrollment. Patients were required to be within 1 year of identification of relapse with at least 10% donor marrow DNA by chimerism or FISH cytogenetics. The same allogeneic transplant donor from transplant was used for DLI. Patients were required to have adequate organ function including a creatinine ≤2.0 mg/dL, liver function tests <5 times the upper limit of normal, a left ventricular cardiac ejection fraction of >40%, pulmonary functions >50% of predicted with testing required only if symptomatic or prior known impairment, and chest radiograph with no evidence of active infection. Patients were required to be off immunosuppressive agents for at least 3 days before DLI infusion and have no evidence of active GVHD. Karnofsky performance status was required to be ≥60%. Patients with active central nervous system leukemia, active fungal infections, and human immunodeficiency virus positivity were excluded.
Donor lymphocytes were collected by lymphopheresis the morning of planned DLI (sibling donors) and at outside institutions according to procedures set by the National Marrow Donor Program before the day of DLI to allow for transport of the cells to the our center (adult unrelated donors). Donors received no priming medications or priming agents before lymphopheresis [14].
Before DLI infusion, patients received a lymphodepleting regimen of intravenous (i.v.) fludarabine 25 mg/m2 for 5 consecutive days (−6 to −2) and cyclophosphamide 60 mg/kg i.v. over 2 hours once on day −5 with 2-mercaptoethane sulfonate and hyperhydration support. The DLI infusion was given on day 0. Fifteen patients received DLI with a T cell dose of 1 × 108 CD3+ cells/kg, as previously reported [14]. Because of the encouraging CR rates (53%) but high rates of severe aGVHD (53% grade III and IV) in the initial DLI dose cohort, the DLI T cell dose was reduced to 0.5 × 108 CD3+ cells/kg for 20 subsequent patients.
Response assessments to chemotherapy and DLI, with bone marrow biopsy and or CT scan imaging if indicated, were completed at 1 and 3 months post-DLI infusion. Repeat assessments for response, survival, and toxicity continued at 6 months and 1 year, with ongoing yearly follow-up for 5 years postinfusion for surviving patients.
Patients
The median age of the 35 patients was 51 with equal gender representation. Fifty-four percent (n = 19) of the chemotherapy and DLI occurred between 2004 and 2006, with the remainder between 2007 and 2009. The majority of patients during both time periods and DLI dose levels had either AML or myelodysplastic syndromes/myeloproliferative disorders (MDS/MPD) (63%, n = 22). Median time from HCT to relapse for the entire cohort was 211 days (range: 80-3151); however, there was a slight difference in time from HCT to relapse between the 2 DLI dose cohorts. Those receiving the higher T cell dose of 1 × 108 CD3+ cells/kg had a slightly shorter median time from HCT to relapse of 161 days (range: 80-721), whereas those receiving the 0.5 × 108 CD3+ cells/kg T cell dose had a median time from HCT to relapse of 248 days (range: 131-3151) (P = .03). Data regarding date of immunosuppression (IS) cessation before DLI was available in 33 patients. IS was discontinued a median of 66 days before DLI; however, the range was wide at 14-2014 because of a number of late posttransplant relapses. Median follow-up among survivors for the entire cohort of patients is 2.3 years (range: 0.3-6.2). Patient and transplant characteristics are described in Table 1.
Table 1. Patient Characteristics.
| Factors | Total |
|---|---|
| Total | 35 |
| Year of DLI | |
| 2004-2006 | 19 |
| 2007-2009 | 16 |
| Age at transplant | |
| Median (range), IQR | 51 (1-66), (44-58) |
| DLI dose | |
| 1 × 108 CD 3+/kg | 15 |
| 0.5 × 108 CD 3+/kg | 20 |
| Gender | |
| Male | 18 |
| Female | 17 |
| Donor type | |
| Sibling | 30 |
| URD | 5 |
| Disease | |
| AML | 15 (43%) |
| MDS/MPD | 7 (20%) |
| NHL/Hodgkins | 5 (14%) |
| CLL | 3 (8.5%) |
| Multiple myeloma | 2 (5.5%) |
| ALL, prolymphocytic leukemia | 3 (9%) |
| JMML | |
| Disease status at | |
| CR* | 3 (8.5%) |
| Marrow only | 22 (63%) |
| EM disease | 7 (20%) |
| EM + marrow | 3 (8.5%) |
| Months from HCT to relapse (range) | 7 (2.7-105) |
| Follow-up among survivors | |
| Median (range) in years | 2.3 (0.3-6.2) |
NHL indicates non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; ALL, acute lymphocytic leukemia; JMML, juvenile myelomonocytic leukemia; URD, unrelated donor; IQR, interquartile range; EM, extramedullary.
All 3 patients who entered the trial in CR were AML patients who received reinduction chemotherapy before enrollment on this trial.
All study procedures, patient samples, and data collection occurred after obtaining informed consent using methods approved by the University of Minnesota institutional review board and registered at clinical trials.gov as NCT00167180.
Statistics
Patients with non-CML diagnoses were included in this analysis. Comparison of demographics, patient characteristics, and toxicities across dose cohorts were assessed by the chi-square test, Fisher's exact test or the Wilcoxon test where appropriate [15]. Primary endpoints of the study were to evaluate safety of the lymphodepleting preparative regimen when combined with DLI in relapsed non-CML patients post HCT and to test whether lymphodepleting chemotherapy improved the efficacy of DLI. Cumulative incidence was used to estimate aGVHD, treating non-GVHD death as a competing risk [16]. Overall survival (OS) was estimated using the Kaplan-Meier method [17]. Responses were defined as best response achieved with classifications of no response/dead, partial response (PR), or complete response (CR). Duration of CR was defined as the time from documented CR post-DLI until relapse. Patients who relapsed and then went on to obtain CR after receiving additional alternative therapy were still defined as relapse with respect to the chemo-DLI intervention. Response was statistically compared across categories by Fisher's exact test. Time to relapse post-HCT (<6 months = early and ≥6 months = late), site of disease before chemotherapy and DLI (marrow, extramedullary, or both), and site of disease relapse post-DLI were also evaluated for impact on outcomes of remission and subsequent relapse. For the 1 patient who received a second course of chemotherapy and DLI, analysis of response and duration was determined following the first course.
Results
Response
Seventeen of the 35 patients (49%) obtained a CR following the lymphodepleting chemotherapy and immediate DLI. CR rates were similar when comparing DLI dose cohorts, patients with early versus late post-transplant relapses, comparing AML/MDS versus other hematologic malignancies, or comparing site of initial post-HCT disease relapse. CR rates between the high and low DLI dose cohorts were similar at 53% and 45%, respectively (P = .89). Rates of CR were not influenced by the time of relapse posttransplantation (early <6 months versus late ≥6 months). Of the 12 patients relapsing early, 58% (n = 7) achieved CR compared with 43% (n = 10 of 23) for those with later relapse. However, important to note is that the majority of early relapse patients received the high-dose DLI while the majority of later relapse patients received the lower dose DLI. Additionally, type of hematologic malignancy did not impact CR rates with 50% (n = 11) of AML/MDS patients compared with 46% (n = 6) of patients with other hematologic malignancies obtaining CR (P = .97). Last, site of disease relapse post-HCT (extramedullary, bone marrow, or both) had no impact on CR rates (P = .40).
The median duration of CR was 6 months (range: 2-71+). A total of 5 patients remained in CR for 12 months or greater with 3 patients relapsing at 12, 17, and 66 months and 2 patients (1 with multiple myeloma, and 1 with chronic lumphocytic leukemia) in ongoing CR at 24 and 71 months, respectively. Both patients in ongoing CR received a peripheral blood stem cell transplant from their male sibling. Both developed relatively early aGVHD at 44 and 35 days that transitioned to chronic GVHD (cGVHD) that required continued IS at last follow-up.
Relapse Postchemo-DLI-Induced CR
Of those 17 patients achieving a complete remission postchemotherapy and DLI, 9 subsequently relapsed. The incidence of relapse for the entire group at 3, 6, and 12 months were 12% (0%-27%, 95% confidence interval [CI]), 36% (12%-60%, 95% CI), and 56% (29%-83%, 95% CI), respectively. Although CR rates were similar between the early and post-HCT relapse cohorts, there was a trend toward improved duration of CR in the patients relapsing ≥6 months posttransplantation. Specifically, those patients who relapsed <6 months posttransplantation had a median duration of CR of 76 days (range: 20-298) compared with a median duration of CR of 132 days (range: 100-231) for those who relapsed ≥6 months posttransplant (P = .30). Relapse rates for patients with AML/MDS were similar to those with other hematologic malignancies; however, the tempo to relapse varied slightly between the groups. At 3 months, the incidence of relapse for AML/MDS patients was 9% (0%-25%, 95% CI) compared with 17% (0%-44%, 95% CI) for other hematologic malignancies. By 6 months the incidence of relapse in the AML/MDS cohort was 39% (9%-69%, 95% CI) compared with 50% (23%-77%, 95% CI) in the other hematologic malignancy cohort. However, by 12 months, no additional patients in the other hematologic malignancy cohort relapsed, whereas the incidence increased in the AML/MDS cohort to 60% (27%-93%, 95% CI).
GVHD
The incidence of aGVHD of any grade following DLI was 49% with a median time to development of 21 days (range: 9-92). There was no apparent correlation between time from IS cessation to DLI and time to development of aGVHD with early aGVHD developing even in patients with a late posttransplant relapse who had been off IS for years.
Severity and incidence of aGVHD differed between the DLI dose cohorts (P = .06). Following the higher DLI dose, the incidence of aGVHD grade II-IV was 66% (grade III, 33% and grade 4, 20%). Following the reduced dose DLI, grade II-IV aGVHD developed in only 25% (grade III-IV, 10%) (Figure 1).
Figure 1.
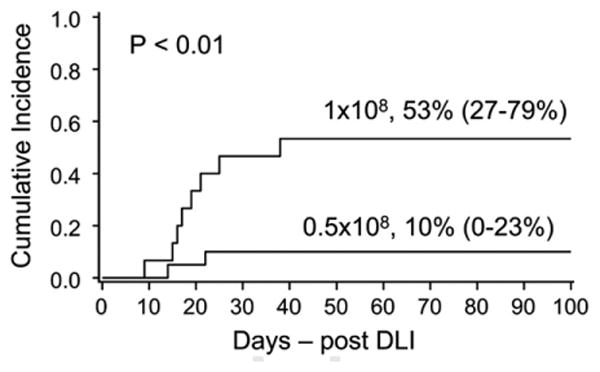
Grade III-IV aGVHD by DLI dose. Rates of severe aGHVD grades III-IV based on DLI dose: significantly higher rates in those patients receiving higher dose DLI.
Acute GVHD was associated with a trend toward more frequent CR. Sixty-five percent of those with aGVHD obtained a CR versus only 33% with no GVHD (P = .06). The median duration of CR was similar with and without aGVHD (6 months [range: 2-71+] and 4.5 months (range: 2-66]), respectively.
The incidence of cGVHD for those patients surviving 100 days post-DLI was 18%. The 2 patients who remain alive and free of underlying hematologic malignancy have ongoing evidence of cGVHD.
Toxicity
Toxicity was significantly increased in the patients receiving DLI at a dose of 1 × 108. As noted above, a substantially higher rate of grade II-IV aGVHD was observed in the higher dose DLI cohort. Reported grade III-IV toxicities, not including GVHD or death, were also increased in the higher dose DLI cohort with a total of 10 reported toxicities including diarrhea (n = 5), rash (n = 2), liver dysfunction (n = 1), pancreatic abnormalities (n = 1), and other miscellaneous GI issues (n = 1). Grade III-IV toxicities were fewer in the lower dose DLI cohort totaling 6 including diarrhea (n = 2), rash (n = 1), liver dysfunction (n = 1), miscellaneous gastrointestinal issues (n = 1), and dermatologic abnormalities (n = 1).
Immune Reconstitution
Mononuclear cell subsets (CD14+, CD19+, CD3+, CD56+/CD3−, CD56+/CD3+) were quantified in the 2 DLI dose cohorts at baseline, day +14, and day +28 post-DLI and were similar at all time points. Percent Ki67 in lymphocyte subsets (CD3+/4+, CD3+/8+, and natural killer [NK] cells) was measured as a surrogate biomarker for in vivo proliferation in the low and high DLI cohorts. Compared with the higher dose DLI patients, lower dose DLI patients had approximately half of the CD3+/CD4+ and CD3+/CD8+ lymphocytes proliferating but differences were not significant because of the small sample size. Interestingly, proliferation of NK cells was slightly greater in the lower dose DLI cohort, suggesting that NK cells and T cells may compete in a lymphodeplete environment (data not shown). Given the similar CR rates between the 2 DLI dose cohorts, the slight difference in T and NK cell proliferation between the 2 cohorts did not impact outcome.
Survival
OS at 1 and 2 years was 30% (95% CI, 16%-45%) and 19% (95% CI, 8%-34%), respectively. Achievement of CR impacted survival with 1- and 2-year OS improved to 44% (95% CI 20%-66%) and 28% (95% CI, 8%-52%) (P = .03), respectively (Figure 2). Timing of initial relapse post HCT, interestingly, did not correlate with differences in survival. OS at 1 year was 25% (6%-50%, 95% CI) and 32% (15%-52%, 95% CI) for the early versus later relapses, respectively (P = .74).
Figure 2.
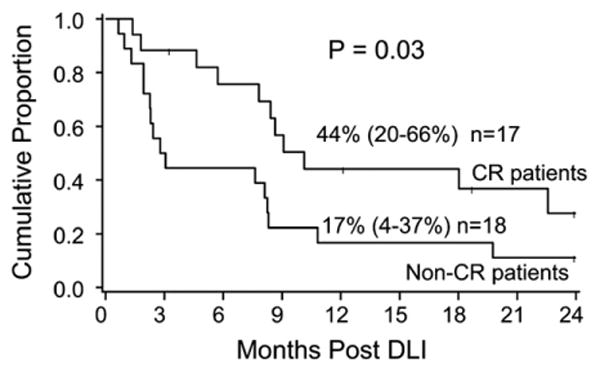
One-year survival by response. One-year OS based on response to DLI. Patients achieving a complete remission had substantially improved 1-year OS.
The causes of death were GVHD (n = 7), relapsed disease (n = 16), and new malignancy (n = 1). Acute GVHD-related death was more frequent in the higher dose DLI group (n = 6 versus 1).
Discussion
Relapsed disease after allogeneic HCT for hematologic malignancy remains a challenge with limited therapeutic options. We have shown that DLI after lymphodepleting chemotherapy is permissive of in vivo lymphocyte expansion [14] resulting in frequent CR and promising survival. The initial approach with higher dose DLI was hampered by relatively high rates of severe aGVHD (grade III-IV) and grade II-IV toxicities but lower dose DLI produced only modest rates of aGVHD without compromise of response.
Posttransplant therapeutic immune modulation for relapsed hematologic malignancy is changing. The initial success of DLI alone in CML has been less effective in other hematologic malignancies. Most recent efforts include debulking chemotherapy followed by DLI typically utilizing disease specific chemotherapy before DLI [4,6,10,11]. We tested a standardized lymphodepleting chemotherapy approach before DLI based on the premise of altering the immunologic milieu to maximize the response to DLI. As others have shown, lymphodepleting chemotherapy serves to increase access to lymphoid stimulating cytokines and to decrease the numbers of regulatory T cells and myeloid-derived suppressor cells that may inhibit DLI function [18]. Compared with earlier reports, we observed promising CR rates and encouraging survival in CR patients [4,5,10]. However, in contrast to other series [4,5] our CR rates or OS were not adversely impacted by early relapse posttransplantation (defined as <6 months). In close analysis of the cohort of patients within the early versus late post-HCT relapse groups, the majority of patients in the early post-HCT relapse cohort received the higher dose DLI and the majority of the patients in the later relapse cohort received the lower dose DLI. Thus, the lack of difference in CR rates or OS may be a reflection of the interplay between time of posttransplant relapse and DLI T cell dose. However, given the similar outcomes despite timing of posttransplant relapse, it could still be argued that the combination of lymphodepleting chemotherapy followed by DLI may have the ability to overcome the poor risk feature of early post-HCT relapse. Although there remain only 2 patients in sustained remissions, an additional 3 patients remained in CR for 12 months or greater, suggesting that the lymphodepleting chemotherapy and DLI therapeutic platform is a valid approach for this high-risk group of patients.
Acute GVHD following DLI is a potentially life-threatening complication that can threaten the success of the intervention, even when CR is obtained. Reduction in the DLI CD3+ dose produced significantly less frequent and less severe aGVHD without compromising remission rates. Regardless of DLI T cell dose, aGVHD was associated with improved CR rates. Interestingly, our patients developed aGVHD relatively early post-DLI regardless of the duration of time between prior IS cessation and DLI infusion. These findings suggest that the lymphodepleting chemotherapy may alter the cytokine milieu to permit the development of early aGVHD. The development of aGVHD and cGVHD were associated with improved CR rates and long-term disease-free survival, suggesting the importance of GVHD for success of the therapy.
The immune reconstitution studies showed similar lymphocyte subsets between the 2 cohorts and slightly higher CD3+ proliferation at day +14 in the higher dose cohort and NK cell proliferation in the lower dose DLI cohort. However, these differences did not appear to impact the CR rates and are not able to explain the absence or presence of aGVHD development. The association of GVHD with long-term response suggests that additional interventions to promote controlled GVHD may improve outcome. Additional therapeutic manipulations to prolong remission duration could include serial, consolidative DLI infusions, possibly coupled with tumor sensitizing maneuvers such as tumor specific vaccines or antitumor antibodies.
Our study has several limitations including small sample size and heterogeneous patient population. Additionally, we did not perform bone marrow assessments or disease restaging scans between the chemotherapy and the DLI. This limits our ability to definitively interpret which component of therapy (the chemotherapy or the DLI) had more of an impact on remission rates. Despite that, the association of improved outcome with GVHD onset would suggest that the DLI was the primary determinant of success. Future studies to measure T regulatory cells and myeloid-derived suppressor cells pre- and postlymphodepleting chemotherapy are warranted and may help understand the biology of these responses. Despite these limitations, our study provides insight into the impact of lymphodepleting chemotherapy followed immediately by DLI to treat post-HCT relapse. The importance of GVHD development in successful outcomes suggests that the lymphodepleting chemotherapy enhances DLI to overcome the poor risk feature of early post-HCT relapse. This platform can lead to additional interventions that may prolong response, but the challenge is to enhance antitumor immunity without severe GVHD. In summary, we show that lymphodepleting chemotherapy followed by DLI for patients with advanced hematologic malignancies relapsing postallogeneic HCT can be effective therapy, but further interventions to extend post-DLI remissions such as repeat DLI, vaccine therapies, or immune modulation with histone deacetylase inhibitors to augment the immune impact will be important to further improve efficacy.
Acknowledgments
Drs. Miller, Blazar, Burns, Verneris, and Weisdorf developed the clinical protocol. Todd Defor completed all statistical analysis. Dr. Warlick reviewed clinical outcomes and wrote the initial manuscript. Drs. Miller, Blazar, Burns, Verneris, Weisdorf, and Ustun critically reviewed and approved the manuscript.
Footnotes
Financial disclosure: The authors have no financial disclosures to declare.
References
- 1.Pavletic SZ, Kumar S, Mohty M, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following allogeneic cell transplantation. Biol Blood Marrow Transplant. 2010;16:871–890. doi: 10.1016/j.bbmt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop MR, Dean RM, Steinberg SM, et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol. 2008;19:1935–1940. doi: 10.1093/annonc/mdn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloor AJ, Thomson K, Chowdhry N, et al. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:50–58. doi: 10.1016/j.bbmt.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 5.Levine JE, Braun T, Penza SL, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002;20:405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 6.Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev. 2010;36:528–538. doi: 10.1016/j.ctrv.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 8.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 9.Shiobara S, Nakao S, Ueda M, et al. Donor leukocyte infusion for Japanese patients with relapsed leukemia after allogeneic bone marrow transplantation: lower incidence of acute graft-versus-host disease and improved outcome. Bone Marrow Transplant. 2000;26:769–774. doi: 10.1038/sj.bmt.1702596. [DOI] [PubMed] [Google Scholar]
- 10.Choi SJ, Lee JH, Lee JH, et al. Treatment of relapsed acute myeloid leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a high incidence of isolated extramedullary relapse. Leukemia. 2004;18:1789–1797. doi: 10.1038/sj.leu.2403523. [DOI] [PubMed] [Google Scholar]
- 11.Inamoto Y, Fefer A, Sandmaier BM, et al. A phase I/II study of chemotherapy followed by donor lymphocyte infusion plus interleukin-2 for relapsed acute leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1308–1315. doi: 10.1016/j.bbmt.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peggs KS, Sureda A, Qian W, et al. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol. 2007;139:70–80. doi: 10.1111/j.1365-2141.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 13.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JS, Weisdorf DJ, Burns LJ, et al. Lymphodepletion followed by donor lymphocyte infusion (DLI) causes significantly more acute graft-versus-host disease than DLI alone. Blood. 2007;110:2761–2763. doi: 10.1182/blood-2007-05-090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snedecor G, Cochran W. Statistical Methods. 8th. Ames, IA: Iowa State University Press; 1989. [Google Scholar]
- 16.Lin DY. Non-parametric inference for cumulative incidence functions in competing risk studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1995;53:457–481. [Google Scholar]
- 18.Wrzesinski C, Paulos CM, Kaiser A, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]


