Abstract
Umbilical cord blood (UCB) has increased access to hematopoietic-cell transplantation (HCT) for patients without HLA-matched sibling donors (MSD). We compared outcomes of HCT using MSD (N=38) or UCB (N=60) among older patients (age ≥55 years) with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS). All patients received a reduced intensity regimen consisting of cyclophosphamide, fludarabine and 200 cGy total body irradiation. Median age at HCT was 63 years for MSD and 61 years for UCB recipients. Among UCB recipients, 95% received two UCB units and 88% received 1–2 locus HLA mismatched units to optimize cell dose. Overall survival at 3-years was 37% for MSD and 31% for UCB recipients (P=0.21). On multivariate analysis, donor source (MSD vs. UCB) did not impact risks of overall survival, leukemia-free survival, relapse or treatment-related mortality. UCB is feasible as an alternative donor source for RIC HCT among older patients with AML and MDS who do not have a suitable MSD.
Keywords: Hematopoietic cell transplantation, Older patients, Umbilical cord blood, Acute myeloid leukemia, Myelodysplastic syndromes, Reduced intensity conditioning regimens
INTRODUCTION
Acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) occur most commonly in older patients. For many older patients with high-risk AML and MDS, allogeneic hematopoietic-cell transplantation (HCT) offers the best chance of long-term survival. HCT has traditionally been underutilized in older patients because of the perceived higher risks of transplant complications. A recent large analysis reported comparable outcomes of HCT using reduced intensity conditioning (RIC) regimens and either related or unrelated donors among older patients with AML and MDS, indicating that age alone is not a contraindication to HCT.(1)
Availability of a suitable donor is a major barrier to successful HCT in older patients. Unrelated umbilical cord blood (UCB) is increasingly accepted as an alternative donor source for patients without an HLA identical matched sibling donor (MSD).(2–7) Although smaller studies have highlighted the feasibility of UCB for HCT in older patients, its use for treatment of AML and MDS has not been well described.(8, 9) We hypothesized that for older patients with AML and MDS lacking a MSD, UCB could lead to comparable survival, thus extending the availability of HCT for patients who would otherwise be ineligible because of donor availability. We compared the safety and efficacy of RIC HCT using either MSD or UCB in patients ≥ 55 years.
MATERIALS AND METHODS
Data were collected prospectively for 98 consecutive patients aged ≥ 55 years who received RIC HCT using either MSD or UCB for AML or MDS between 2001 and 2009. All received HCT using RIC because of their older age. Our cohort included 38 MSD HCT recipients and 60 patients with no HLA matched related donors who received UCB HCT.
All MSD grafts were 6/6 HLA matched (at HLA-A, B and DRB1) and used a minimum cell dose of 3 × 106 CD34+ cells/kg; all patients received filgrastim mobilized peripheral blood grafts. Using UCB selection criteria that we have previously published,(3, 5, 10) UCB grafts were matched at 4–6 of 6 HLA-A,-B (antigen level) and -DRB1 (allele level) to the recipient, and in patients receiving two UCB units, to each other. Fifty-six (95%) UCB HCT recipients received two UCB units and 53 (88%) received at least 1–2 HLA mismatched units.
Pre-transplantation comorbidities were scored retrospectively for all patients using the HCT-specific comorbidity index (HCT CI) described by Sorror et al,(11) and were categorized as low-risk (score 0), intermediate-risk (score 1–2) and high-risk (score ≥3).
The RIC regimen for all patients consisted of cyclophosphamide (50 mg/kg intravenously on day −6), fludarabine (40 mg/m2 intravenously daily from days −6 through −2) and total body irradiation (200 cGy on day −1). Equine anti-thymocyte globulin (ATG) 15 mg/kg intravenously every 12 hours for six doses was added to a subgroup of patients who had not received chemotherapy within 3 months of HCT or a previous autologous transplant (n=46). All patients received GVHD prophylaxis with cyclosporine (days −3 to +180) and mycophenolate mofetil (days −3 to +30). Filgrastim was administered to all patients from day +1 until the absolute neutrophil count was more than 2.5 × 109/L for two days. Treatment protocols were approved by the University of Minnesota institutional review board, registered at clinicaltrials.gov and all patients gave informed consent prior to HCT.
Donor chimerism was determined serially on marrow and/or blood samples on days +21–28, +60, +100, 6 months and then annually after HCT. Chimerism analysis was performed using quantitative PCR of informative polymorphic variable-number tandem repeat (VNTR) or short tandem repeat (STR) regions in recipient and donor.(10)
The primary endpoint was probability of overall survival (OS). Secondary study endpoints included probability of leukemia-free survival (LFS) and cumulative incidences of acute and chronic graft-versus-host disease (GVHD), relapse, treatment related mortality (TRM), and neutrophil engraftment. LFS was defined as survival in continuous complete remission (CR). TRM was defined as death following HCT without disease progression or relapse. Standard clinical criteria were used to diagnose and grade GVHD.(12, 13)
Comparison of patient and transplant characteristics was performed using chi-square, Fisher’s exact or Wilcoxon’s rank sum test as appropriate. Cumulative incidence estimates of TRM and GVHD considered relapse as a competing risk.(14) The Kaplan-Meier method was used to plot survival curves for OS and LFS.(15) Multivariable Cox regression analyses were performed for OS and LFS(15), and multivariable Fine-Gray regression analyses for relapse and TRM.(16) All multivariate models included donor type (MSD vs. UCB) and were adjusted for disease status (AML in CR1 vs. AML not in CR1 vs. MDS) and HCT-CI score (low vs. intermediate vs. high). Event times were measured from date of transplantation to date of death or last contact. Analysis was performed with follow up through August 2010.
All p-values were two sided. Analyses were performed in SAS 9.2 (Cary, North Carolina, USA). Thirty seven patients included in this analysis have been reported in a previous analysis of RIC UCB HCT in older patients from our center.(8)
RESULTS
Table 1 describes demographic characteristics of our cohort. The median follow up of survivors was 2.8 years (range, 1–6 years). Among 70 patients with AML, all were in CR at the time of HCT; the majority received HCT in CR1 (59%); and few were in CR2 (26%) or CR3+ (16%). Cytogenetic risk at diagnosis was good, intermediate and poor in 3%, 53% and 43% AML patients, respectively.(17) Among MDS patients (N=28), 32% had good, 21% had intermediate and 46% had poor cytogenetic risk disease at diagnosis.(18) There was no difference in cytogenetic risk or disease status among MSD or UCB recipients. Thirteen patients with AML had a preceding diagnosis of MDS (MSD-3, UCB-10).
Table 1.
Patient, disease and transplant characteristics
| Characteristics | MSD (n=38) | UCB (n=60) | P-value |
|---|---|---|---|
| Median age, years (range) | 63 (56–70) | 61 (55–69) | 0.35 |
| Gender, male | 29 (76%) | 43 (72%) | 0.61 |
| Median weight, kilograms (range) | 88 (57–125) | 85 (54–122) | 0.13 |
| Year of transplant | 0.86 | ||
| 2001–2005 | 12 (26%) | 20 (33%) | |
| 2006–2009 | 26 (68%) | 40 (67%) | |
| Diagnosis | 0.72 | ||
| AML in CR1 | 14 (37%) | 27 (45%) | |
| AML not in CR1 | 12 (32%) | 17 (28%) | |
| MDS | 12 (32%) | 16 (27%) | |
| CMV serostatus | 0.56 | ||
| Recipient negative | 13 (34%) | 24 (40%) | |
| Recipient positive | 25 (66%) | 36 (60%) | |
| Prior HCT1 | 0 (0%) | 4 (7%) | 0.15 |
| Median time from diagnosis to HCT, months (range) | 7 (2–41) | 6 (2–76) | 0.60 |
| HCT-specific comorbidity index score | 0.16 | ||
| 0 | 8 (21%) | 18 (30%) | |
| 1–2 | 15 (39%) | 13 (22%) | |
| ≥ 3 | 15 (39%) | 29 (48%) | |
| ATG used in conditioning | 12 (32%) | 34 (57%) | 0.02 |
| HLA compatibility2 | <0.01 | ||
| 6/6 antigen match | 38 (100%) | 7 (12%) | |
| 5/6 antigen match | 0 | 22 (36%) | |
| 4/6 antigen match | 0 | 31 (51%) | |
| Median total nucleated cell dose, ×108 NC/kg (range) | 9.3 (5.0–21.3) | 0.4 (0.2–0.7) | <0.01 |
| Median follow up, months (range) | 24 (12–36) | 25 (10–36) | 0.87 |
MSD – matched sibling donor; UCB – unrelated umbilical cord blood donor; AML – acute myeloid leukemia; MDS – myelodysplastic syndromes; CR – complete remission; CMV – cytomegalovirus; HCT – hematopoietic cell transplantation; ATG – anti-thymocyte globulin; HLA – human leukocyte antigen; NC – nucleated cell
All four patients had received prior autologous HCT
Worst HLA match for patients undergoing UCB transplantation using two UCB units (N=56)
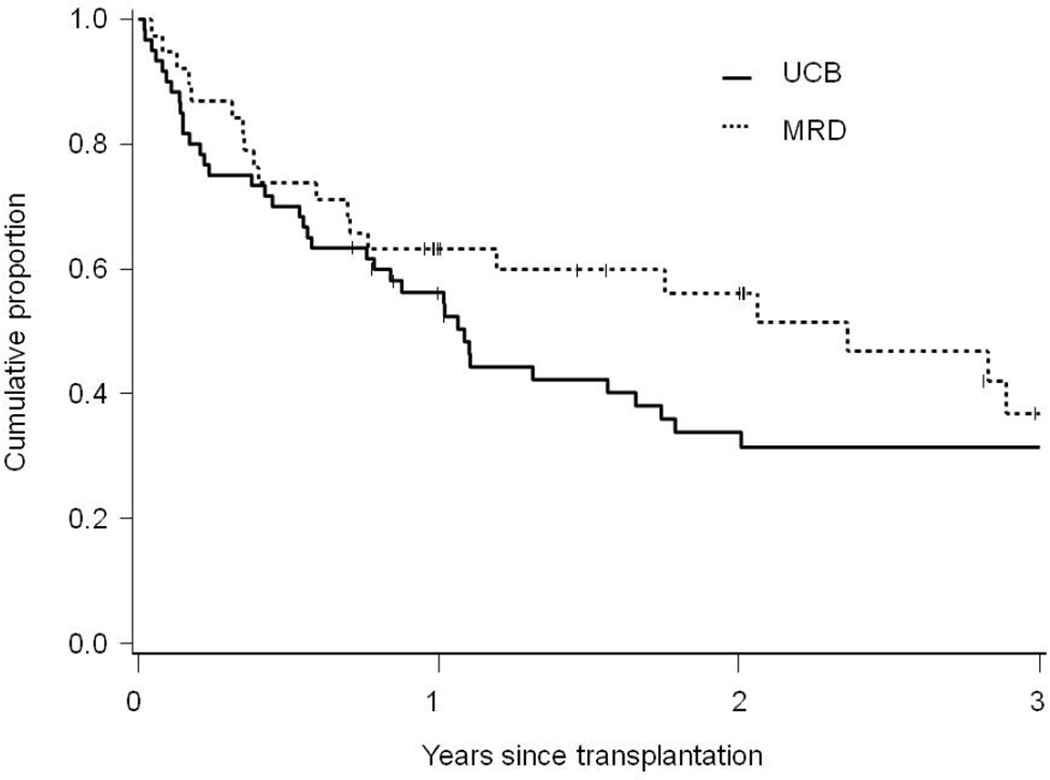
In univariate analyses, outcomes of MSD and UCB HCT were similar (Table 2 and Figure 1). In multivariate analyses, donor source had no impact on risks of OS, LFS, relapse or TRM. Compared to MSD, the relative risks for UCB recipients for OS were 1.3 (95% confidence intervals, 0.8–2.3, P=0.34), for LFS were 1.2 (0.7–2.1, P=0.44), for relapse were 0.7 (0.3–1.3, P=0.23) and for TRM were 1.2 (0.6–2.7, P=0.61) (Table 3). HCT-CI score did not impact risks of OS, LFS or relapse, but was associated with higher risks of TRM. Compared to patients with low-risk HCT-CI score, the relative risk of TRM for those with intermediate-risk score was 1.2 (0.3–5.3, P=0.79) and for those with high-risk score was 4.0 (1.2–13.3, P=0.03).
Table 2.
Outcomes following RIC HCT for AML and MDS in older patients
| Outcome | MSD (n=38)3 | UCB (n=60)3 | P-value |
|---|---|---|---|
| Overall survival at 3-years | 37% (19–55%) | 31% (19–44%) | 0.21 |
| Leukemia-free survival at 3-years | 34% (17–52%) | 22% (12–35%) | 0.23 |
| Relapse at 2-years1 | 34% (17–50%) | 47% (33–62%) | 0.19 |
| Treatment-related mortality at 2-years1 | 25% (11–40%) | 25% (13–37%) | 0.82 |
| Neutrophil engraftment at day 421,2 | 100% | 85% (70–100%) | <0.01 |
| Acute GVHD (grades 2–4) at day 1001 | 38% (22–55%) | 45% (31– 58%) | 0.19 |
| Acute GVHD (grades 3–4) at day 1001 | 26% (11–41%) | 21% (9– 33%) | 0.95 |
| Chronic GVHD at 2 years1 | 61% (40–82%) | 33% (20–46%) | 0.04 |
MSD – matched sibling donor; UCB – unrelated umbilical cord blood donor; GVHD – graft-versus-host disease
Cumulative incidence estimates
ANC engraftment was defined as first of three consecutive days with ANC >0.5 × 109/L
Estimates in brackets are 95% confidence intervals
Figure 1.
Overall survival following reduced-intensity conditioning hematopoietic-cell transplantation using umbilical cord blood (UCB) or HLA-matched sibling donors (MSD) among older patients (55–70 years) with acute myeloid leukemia or myelodysplastic syndromes
Table 3.
Multivariate analysis for outcomes of following RIC HCT for AML and MDS in older patients
| Outcome | Relative risk for UCB (95% confidence intervals)1 |
P-value |
|---|---|---|
| Overall survival | 1.31 (0.75–2.29) | 0.34 |
| Leukemia-free survival | 1.23 (0.73–2.09) | 0.44 |
| Relapse | 0.66 (0.33–1.30) | 0.23 |
| Treatment related mortality | 1.22 (0.56–2.67) | 0.61 |
Referent – matched sibling donors
In unadjusted analyses, graft source had no impact on the cumulative incidence of acute grades 2–4 or grades 3–4 GVHD. Consistent with our previous observations, UCB HCT recipients had a lower cumulative incidence of neutrophil engraftment by day 42 post-transplantation (85% vs. 100% for MSD, P<0.01). UCB HCT recipients had a lower incidence of chronic GVHD at 2-years post-transplant (61% vs. 33% for MSD, P=0.04). Among 21 MSD HCT recipients with chronic GVHD, 76% presented as classic and 24% as overlap syndrome; according to National Institutes of Health criteria,(19) chronic GVHD severity was mild in 10%, moderate in 76% and severe in 14% of patients. Among 20 UCB HCT recipients with chronic GVHD, presentation was as classic syndrome in 40% and as overlap syndrome in 60% of patients; 10% had mild, 80% had moderate and 10% had severe chronic GVHD.
DISCUSSION
Our study highlights the feasibility of using 4–6/6 HLA matched UCB as a donor source for RIC HCT among patients with AML or MDS aged 55–70 years who do not have a MSD. Together, use of RIC and UCB extends the availability of transplant therapy to older patients previously excluded on the basis of age and lack of a suitable MSD.
UCB recipients had a significantly lower cumulative incidence of chronic GVHD (33% vs. 61%). Given the relatively small sample size, we did not consider chronic GVHD in multivariate models. A greater proportion of UCB recipients received ATG as a part of their conditioning regimen, which may in part explain their decreased risk of chronic GVHD.(20, 21) We and others have previously reported the lower risks of chronic GVHD with UCB compared to other donor sources.(8, 22–24) This may greatly reduce the morbidity and any continuing medical disability among UCB recipients, particularly compared to the higher risks of chronic GVHD that accompany volunteer unrelated donor HCT.(22, 23)
Cell dose is a critical determinant of engraftment and transplant outcomes following UCB transplantation. We have previously reported on the safety and efficacy of double unit UCB transplantation in both the myeloablative and RIC settings.(2, 3, 10) In this study, the majority (95%) of UCB recipients received two UCB units with acceptable outcomes, supporting the use of this approach in older patients. Furthermore, a recent analysis from our institution suggests that the transplantation of two partially HLA-matched UCB units may be associated with enhanced graft-versus-leukemia activity and lower risks of leukemia relapse compared to single unit UCB HCT.(25)
We did not have a sufficient number of unrelated donor HCT recipients for comparison with UCB transplantation in our study; only 5 patients older than 55 years received a RIC HCT using an unrelated donor for AML or MDS at our institution during the study period. Since UCB is primarily being investigated as an alternative graft source for patients who do not have a sibling donor, studies comparing its outcomes with unrelated donor transplantation in older patients are still needed. The rapid availability of UCB and the lower risks of chronic GVHD despite using HLA mismatched units are some advantages that make UCB an attractive alternative donor source over unrelated donors among older patients with high-risk AML and MDS. Irrespective of the pros and cons of both donor sources, increasing availability of both unrelated donors and UCB is expected to make allogeneic HCT available as a treatment option to a greater number of older patients who were previously not offered HCT because of a lack of suitable donor.
The relatively small sample size is another shortcoming of our study. Our findings have to be confirmed in analyses that include larger cohorts of patients through controlled clinical trials or registry based analyses, especially with matched unrelated donor and haplo-identical transplant comparison groups. The prospective selection criteria for transplantation, use of a homogeneous conditioning and GVHD prophylaxis regimen and use of common supportive care and followup protocols are strengths of our study.
In conclusion, our study shows the feasibility of HLA mismatched UCB as an alternative graft source for older patients with AML and MDS who do not have a MSD and require allogeneic HCT.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
None of the authors have any relevant financial conflicts of interest to disclose.
REFERENCES
- 1.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010 Apr 10;28(11):1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005 Feb 1;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007 Jun 14; doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004 Nov 25;351(22):2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 5.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006 Oct;18(5):571–575. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006 May 1;107(9):3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- 7.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004 Nov 25;351(22):2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS, Brunstein CG, Tomblyn M, Thomas AJ, Miller JS, Arora M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008 Mar;14(3):282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Laughlin MJ. Extending cord blood transplant to adults: dealing with problems and results overall. Semin Hematol. 2010 Jan;47(1):86–96. doi: 10.1053/j.seminhematol.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003 Sep 1;102(5):1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 11.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005 Oct 15;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991 Jul;28(3):250–259. [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995 Jun;15(6):825–828. [PubMed] [Google Scholar]
- 14.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997 Apr 30;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000 Dec 15;96(13):4075–4083. [PubMed] [Google Scholar]
- 18.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997 Mar 15;89(6):2079–2088. [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005 Dec;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006 May;12(5):560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Nakai K, Mineishi S, Kami M, Saito T, Hori A, Kojima R, et al. Antithymocyte globulin affects the occurrence of acute and chronic graft-versus-host disease after a reduced-intensity conditioning regimen by modulating mixed chimerism induction and immune reconstitution. Transplantation. 2003 Jun 27;75(12):2135–2143. doi: 10.1097/01.TP.0000066453.32263.F7. [DOI] [PubMed] [Google Scholar]
- 22.Arora M, Nagaraj S, Wagner JE, Barker JN, Brunstein CG, Burns LJ, et al. Chronic graft-versus-host disease (cGVHD) following unrelated donor hematopoietic stem cell transplantation (HSCT): higher response rate in recipients of unrelated donor (URD) umbilical cord blood (UCB) Biol Blood Marrow Transplant. 2007 Oct;13(10):1145–1152. doi: 10.1016/j.bbmt.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. The lancet oncology. 2010 Jul;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha V, Wagner JE, Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000 Jun 22;342(25):1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 25.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009 Nov 5;114(19):4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]