Abstract
Corneal neovascularization can lead to a devastating disease process that involves the breakdown of the limbal barrier and the formation of blood vessels in the cornea, leading to severe visual impairment. This review discusses the delicate balance between antiangiogenic and angiogenic factors that govern the antiangiogenic privilege of the cornea. Current treatment methods, clinical trials, and future prospects in the management of corneal neovascularization also are discussed.
The cornea exhibits the unique quality of being essentially devoid of both blood and lymphatic vessels. This avascularity is an active process that has been termed corneal angiogenic privilege. Its integrity is maintained by a series of cellular factors and mechanisms. When these are compromised because of disease, the end result may be invasion of vessels into the clear cornea, a pathologic feature termed corneal neovascularization.
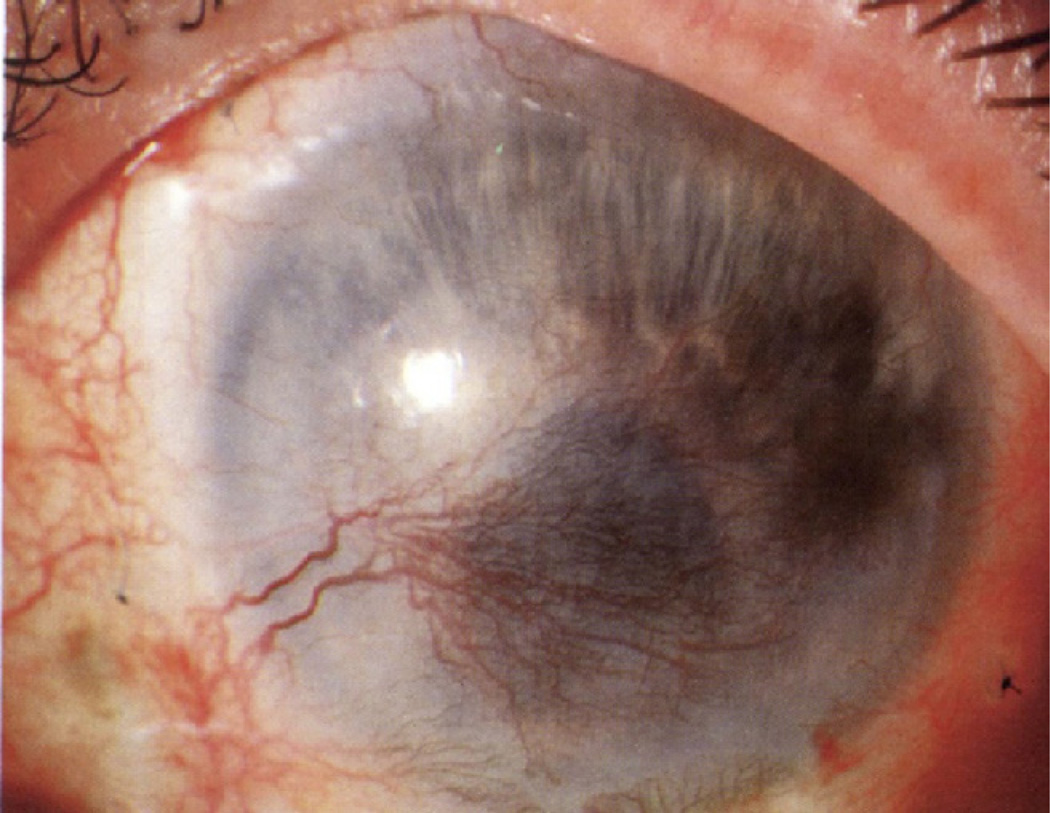
Corneal neovascularization can result in a devastating disease process that can lead to marked vision impairment and eventual loss. Corneal neovascularization develops in an estimated 1.4 million patients every year, and 12% of these cases are associated with decreased visual acuity.1 Blood vessel invasion into the cornea alters corneal anatomic features and produces scleralization of the transparent layer, obscuring the visual axis. This process may be incompatible with acceptable visual acuity and is associated with the leading causes of corneal blindness both worldwide (trachoma) and in industrialized nations (herpetic keratitis; e.g., Fig 1).1
Figure 1.
Photograph showing severe corneal neovascularization resulting from herpes simplex virus keratitis.
Neovascularization induces tissue scarring, lipid deposition, stromal hemorrhage, and corneal edema, severely altering visual acuity. In addition to reducing visual acuity, vascularity introduces circulating immune cells, reducing the immune privilege of the cornea and the graft survival of a subsequent penetrating keratoplasty. There are many causes of corneal neovascularization (Table 1). Inflammatory diseases such as severe conjunctivitis, blepharitis, cic-atricial pemphigoid, or Stevens–Johnson syndrome will cause corneal neovascularization. Infectious disease such as viral herpes zoster virus or herpes simplex virus; bacterial pathogens such as Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas species, Chlamydia species, and syphilis; and fungal pathogens such as Candida species, Fusarium species, and Aspergillus species; or nematodes such as Onchocerca volvulus can also cause corneal neovascularization. Neurotrophic damage occurring from varicella zoster and herpes simplex keratitis also can lead to ulcers, melting, and perforation, and eventually to corneal neovascularization. Ischemia and cellular degeneration with the accompanying loss of limbal stem cell barrier and zone of avascularity can also cause corneal neovascularization. In addition, extended contact lens wear, alkali burns, and acid burns are major contributors to corneal neovascularization.1
Table 1.
Clinical Conditions Associated with Corneal Neovascularization
| Infectious | Herpes simplex keratitis |
| Herpes zoster keratitis | |
| Syphilis | |
| Pseudomonas | |
| Chlamydia trachomatis | |
| Candidiasis | |
| Fusarium | |
| Aspergillosis | |
| Onchocerciasis | |
| Inflammatory | Graft rejection |
| Acne rosacea | |
| Stevens-Johnson syndrome | |
| Graft-versus-host disease | |
| Pemphigoid | |
| Atopic conjunctivitis | |
| Trauma | Alkali burns |
| Contact lens | |
| Ulceration | |
| Degenerative | Terrien marginal degeneration |
| Pterygium | |
| Aniridia |
Because a wide variety of insults lead to the common pathway of angiogenesis in developing corneal neovascularization, this allows a ready target for therapeutic intervention that is effective for many causes. This review discusses the pathogenesis, the current therapies, and future directions in the management of corneal neovascularization.
Pathogenesis
The avascularity of the cornea is the result of a delicate balance of angiogenic and antiangiogenic factors (Table 2). Circumstances such as injury, inflammation, or other pathologic features favor angiogenic factors, tipping the scale and enabling vessel formation. Briefly, the roles of the antiangiogenic and angiogenic mediators involved in the cornea avascularity are described to understand better the principles underlying current pharmacotherapy.
Table 2.
Angiogenic and Antiangiogenic Factors in Corneal Neovascularization
| Angiogenic molecules | VEGF | CTGF |
| sVEGFR-1, 2, 3 | IL-1, −8 | |
| FGF | MCP-1 | |
| PlGF | Leptin | |
| TGF-α, TGF-β | Integrins (αV β3) | |
| IGF | Angiogenin | |
| PDGF | TXA-2, COX-2 | |
| MMPs | NO | |
| HGF/SF | PAF | |
| TNF-α | ||
| Antiangiogenic molecules | PEDF | IL-18 |
| Endostatin | Arestin | |
| Angiostatin | Canstatin | |
| PRL | Tumstatin | |
| TIMPs | TNF-α | |
| TSP-1, TSP-2 | MMPs | |
| IL-4, IL-12, IL-13, | IFN-γ |
COX-2 = cyclooxygenase-2; CTGF = connective tissue growth factor; FGF = fibroblast growth factor; HGF/SF = Hepatocyte growth factor/ scatter factor; IFN = interferon; IGF = insulin-like growth factor; IL = interleukin; MCP = monocyte chemotactic protein; MMP = matrix metalloproteinase; NO = nitrous oxide; PAF = platelet activating factor; PDGF = platelet derived growth factor; PEDF = pigment epithelium derived growth factor; PIGF = Phosphatidylinositol-glycan biosynthesis class F protein; PRL = prolactin; TGF = transforming growth factor; TIMP = tumor inhibitor of metalloproteinase; TNF = tumor necrosis factor; TSP = total serum protein; TXA = thromboxane A2; VEGF = vascular endothelial growth factor.
Several factors have been identified in the cornea that maintain avascularity: thrombospondins 1 and 2, endostatin, angiostatin, pigment epithelium-derived factor, and matrix metalloproteinases (MMPs). Thrombospondins are a class of proteins found in the extracellular matrix (ECM) that counteract vessel growth by multiple mechanisms. Thrombospondin 1 and thrombospondin 2 act through a pathway that induces vascular endothelial cell apoptosis and also are thought to limit the availability of other growth factors through interactions with the ECM. Angiostatin is a 38-kDa proteolytic fragment of plasminogen found in the corneal epithelium. Endostatin is a 20-kDa proteolytic fragment of collagen XVIII found in the cornea, retina, and lens. Although angiostatin causes endothelial cell cycle arrest in G2, endostatin stops at cycle G1; both cause vascular endothelial cell apoptosis. Pigment epithelial-derived factor has both antiangiogenic and neurotrophic properties. Thrombospondins, angiostatin, endostatin, and pigment epithelium-derived factor all play an integral role in maintaining ocular privilege.
Matrix metalloproteinases are a group of zinc-dependent endopeptidases that engage in ECM remodeling and angiogenesis; however, MMPs have the ability to be angiogenic or antiangiogenic under different conditions.1 Matrix metalloproteinases are involved in the proteolytic cleavage of proenzymes and, as a result, produce antiangiogenic fragments discussed earlier in the cornea. In the human cornea, 8 of the 24 known MMPs are expressed. These include collagenase I and II, gelatinase A and B, stromelysin, matri-lysin, and membrane-type MMP.
Angiogenesis is mediated by several different factors: basic fibroblast growth factor, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) in the cornea. Platelet-derived growth factor induces pericyte recruitment and supports vascular maturation. Basic fibroblast growth factor is a member of the fibroblast growth factor (FGF) family that interacts with FGF receptors (FGFRs; e.g., FGFR-1, FGFR-2, FGFR-3, FGFR-4) and heparin-sulfate proteoglycans located in the ECM2. The FGF–heparin-sulfate proteoglycan interaction is an integral part of angiogenesis regulation. Fibroblast growth factors induce a signaling cascade via the mitogen-activated protein kinase pathway and protein kinase C (PKC), effectively inducing ECM degradation and angiogenesis via VEGF.1
Vascular endothelial growth factor commonly is considered the most prominent angiogenic factor, and other angiogenic molecules may act indirectly via VEGF signaling. The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E, which are involved in the growth of blood and lymphatic vessels. Vascular endothelial growth factor-A is considered the major factor involved in hemangiogenesis and is subdivided into various isoforms. Vascular endothelial growth factor-C and VEGF-D also have been implicated in playing a substantial role in lymphangiogenesis. VEGF receptors (VEGFRs) are responsible for counterbalancing the angiogenic role of VEGF. The corneal epithelium expresses a soluble VEGF receptor-1 (sflt-1), which acts as a physiologic decoy receptor for VEGF-A, sequestering and inactivating its angiogenic potential.2 Vascular endothelial growth factor receptor-2 and VEGFR-3 acts in a similar manner, ultimately acting as a so-called sink for lymphangiogenic molecules VEGF-C and VEGF-D.3,4
Chemokines are important in mediating the development of corneal angiogenesis. The absence of chemokine (C-C motif) receptor (CCR2) and CCR5 in mice inhibited development of injury-induced corneal neovascularization.5,6
Data
Current, indications for antiangiogenesis therapy in cornea include: (1) corneal neovascularization, (2) aggressive corneal neovascularization in recurrent pterygia, (3) corneal neovascularization in patients with limbal stem cell deficiency, (4) corneal neovascularization and iris neovascularization causing neovascular glaucoma, and (5) corneal transplant rejection.
In addition to treating the underlying source, the ideal treatment not only would target destruction of corneal vessels, but also would restore the zone of angiogenic privilege, preventing further vascularization. Several treatments have been explored. One method is direct vessel occlusion via cautery, topical ascorbic acid, or cryotherapy. Other options include photodynamic therapy, immunomodulators, and restoration of the ocular surface. Although most therapies are associated with moderate improvement, the most successful therapies are subconjunctival corticosteroids and VEGF inhibitors that are aimed directly at inhibiting angiogenesis.
Photodynamic Therapy
Photodynamic therapy (PDT) has been used widely in treatment of choroidal neovascularization and multiple surface cancers. It also has been reported to regress corneal neovascularization safely and successfully in mice, rats, rabbits, and humans, but several sessions may be required. Photodynamic therapy destroys endothelial cells and vascular basement membrane via generation of reactive oxygen species and cascading oxidative damage, leading to vessel thrombosis and architectural remodeling. It also has minimal local and systemic effects, making it an alternative treatment option when multiple sessions are needed.7
Brooks et al7 described the first case of PDT in a patient with lipid keratopathy secondary to corneal neovascularization after penetrating keratoplasty. After PDT, his vision improved from 20/200 to 20/30 and regression was noted even after 6 weeks of treatment. Treatment of corneal neovascularization with a dihematoporphyrin ether photosensitizer and argon laser therapy was reported by Sheppard et al.8 Immediate reduction after 72 hours was noted in all patients (n = 7), and 6 of 7 patients continued to show documented vascular reduction 6 months after treatment. Common side effect with the use of laser therapy is phototoxicity.
Currently, there are 2 clinical trials assessing the efficacy of PDT. As a follow-up to the above study, Sheppard continues to evaluate the safety and efficacy dihematoporphyrin derivative-enabled PDT in treating corneal neovascularization. The study is a randomized, placebo-controlled design with 3 treatment arms. Two arms study administration and dosage differences of dihematoporphyrin, whereas the third arm is a placebo control. Patients will be followed for 90 days. Yoon, from Chonnam, Korea, is also investigating the efficacy of verteporfin (6 mg/mm2)-enabled PDT in the treatment of corneal neovascularization. This open-label, uncontrolled study is assessing 18 eyes that are refractory to prednisolone acetate 1%. Efficacy is being evaluated by pretreatment and posttreatment anterior segment photographs and best-corrected visual acuity.9
Immunomodulation
Interleukin-1 Receptor Antagonists
Interleukin (IL)-1 is a proinflammatory molecule produced by various cells, such as neutrophils, macrophages, and fibroblasts, that induce the expression of growth factors, chemokines, and adhesion molecules that lead to neovascularization. Murine experimental models of corneal neovascularization can be induced via administration of IL-1; therefore, IL receptor antagonism was theorized to treat corneal neovascularization. Interleukin-1ra is a soluble receptor antagonist that binds to IL-1 and induces a protein sink.10 It binds with similar affinity to IL-1α and IL-1β neutralizing intracellular signaling that is thought to act via downstream activation of VEGF and inducible nitric oxide synthase. In a recent study, Lu et al11 has shown that endogenous IL-1ra is a potent antiangiogenic factor in alkali-induced corneal neovascularization and has possible therapeutic application as a topical pharmacologic agent. Interleukin-1ra inhibits inflammatory cell infiltration and thus promotes corneal transplant survival by reducing allosensitization.
Dana12 at the Massachusetts Eye and Ear Infirmary is currently conducting a study on the safety and efficacy of topical IL-1ra in a randomized, double-blind, crossover study. Patients are assigned to a treatment arm of custom-made topical IL-1ra 3 times daily for 6 weeks or topical placebo. At 6 weeks, the cohorts crossover for an additional 6 weeks of treatment. Efficacy will be assessed by measurement of neovascular area, area of invasion, and vessel caliber.12
Cyclosporine
Cyclosporine has been reported to decrease corneal vascularity in various animal models.13 LUX Biosciences is assessing the efficacy of a cyclosporine-impregnated silicone subconjunctival implant (LX 201) in preventing allograft rejection and corneal neovascularization. This is a phase III, multicenter, randomized, double-blind, placebo-controlled study designed with 3 arms. Arm I will consist of a 0.5×0.08×0.04-inch subconjunctival implant with 30% cyclosporine. Arm II will consist of a 0.75×0.08×0.04-inch subconjunctival implant with 30% cyclosporine. Patients in arm III will receive a 0.75×0.08×0.04-inch subconjunctival placebo implant.14
Ocular Surface Restoration
Restoring the eye’s natural defenses is one way of preventing or aiding the regression of corneal neovascularization. This can be done with amniotic membrane, conjunctival, or limbal transplantation. Previously, autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane was reported to be successful in restoring a clear corneal surface in the rabbit model.15 With cultivated limbal epithelial transplantation (CLET), those with limbal cell deficiency who previously were contraindicated for penetrating keratoplasty now can be treated successsfully.16 Currently, 3 clinical trials registered with the National Institutes of Health are investigating CLET. Breaching the limbal stem cell sflt-1 barrier allows an avenue for conjunctival epithelium and vessels to grow onto and into the cornea. The hope is that a CLET will reconstitute the barrier defenses, keeping conjunctival epithelium and vasculature outside the limbal zone.
Zakaria17 from Belgium is currently investigating the safety and efficacy of CLET. This phase I/II open-label study is analyzing patients with limbal stem cell deficiency grade IIa–IIb. They will undergo autologous transplantation in unilateral corneal neovascularization; living donor tissue and cadaveric donor tissue will be used in the case of bilateral disease. After the tissue expansion reaches 12 mm in diameter, patient corneas are debrided and stem cells are glued onto the cornea. Patients will be followed up for 1 year and efficacy will be assessed by the amount of new epithelialization, by reduction of conjunctivalization and vascularity, and by visual acuity.17
Similarly, Aghdami18 in Iran is assessing the safety and efficacy of allografted limbal stem cells in a phase I/II trial that is open label and uncontrolled. Limbal stem cells are harvested via small excisions of limbal conjunctiva, expanded, and then placed onto an amniotic membrane graft. The membrane-backed graft then is used for surface reconstruction in patients with limbal stem cell deficiency. Ten patients will be followed up for 1 year, and efficacy will be established by evaluation of corneal epithelial stability and integrity.18
Another study headed by Raffiee19 in Iran is designed to assess the safety and efficacy of amniotic membrane transplantation is the setting of ocular burn. This phase II/III study is designed as a randomized, controlled evaluation of conventional medical therapy with amniotic membrane transplantation against conventional medical therapy alone. Ninety patients with grade 2 through 4 chemical burns enrolled within 2 weeks of the burn will be included. After standard therapy and wound debridement, patients randomized to an amniotic membrane transplantation will have the device in place for 7 to 14 days. In addition to corneal neovascularization, researchers will use visual acuity, pain, symblepharon formation, and epithelial defect healing assessed by digital photography as primary outcome measures for 1 year.19
An alternative method of preventing corneal neovascularization is the restoration of the cornea’s own defenses and the integrity of the corneal epithelium, which has endogenous angioinhibitory characteristics. Various animal studies have shown topical insulin and insulin-like growth factor to be effective in hastening re-epithelialization in various disease states.20 Chen in Taiwan is studying corneal neovascularization comparing topical insulin in combination with standard therapy versus standard therapy alone in a randomized, single-blind safety and efficacy phase I study. Eighteen patients who underwent epithelial debridement or penetrating keratoplasty were enrolled into the study. Patients are randomized to receive corticosteroids, antibiotics, and mydriatics (standard regimen) or topical insulin (100 U/ml 1 drop every 2 hours) in conjunction with the standard regimen. Patients will be followed for 6 months, and corneal neovascularization, corneal re-epithelialization, corneal ulcer, corneal melt, and recurrent erosions will be measured.21
Steroids
Conventional therapy for corneal neovascularization relies on steroids such as hydrocortisone, dexamethasone, and prednisone, as well as triamcinolone acetonide, all of which are thought to act by suppressing the migration, activation, and recruitment of macrophages, mast cells, cytokines, and other types of inflammatory cells that release heparins and growth factors promoting angiogenesis.
However, several concerns are present with the use of steroids. Side effects include the development of cataract and glaucoma and the increased risk of infection.22 Long-term use is another issue. A recent study on triamcinolone acetonide showed that the therapeutic levels were maintained in the cornea at least 2 weeks after the administration of subconjunctival injection. Although treatments with triamcinolone have not shown any toxicity, there is a potential risk of systemic absorption and risk of infection with long-term use in the eye, and further clinical trials are necessary to assess this issue.
Vascular Endothelial Growth Factor Inhibition
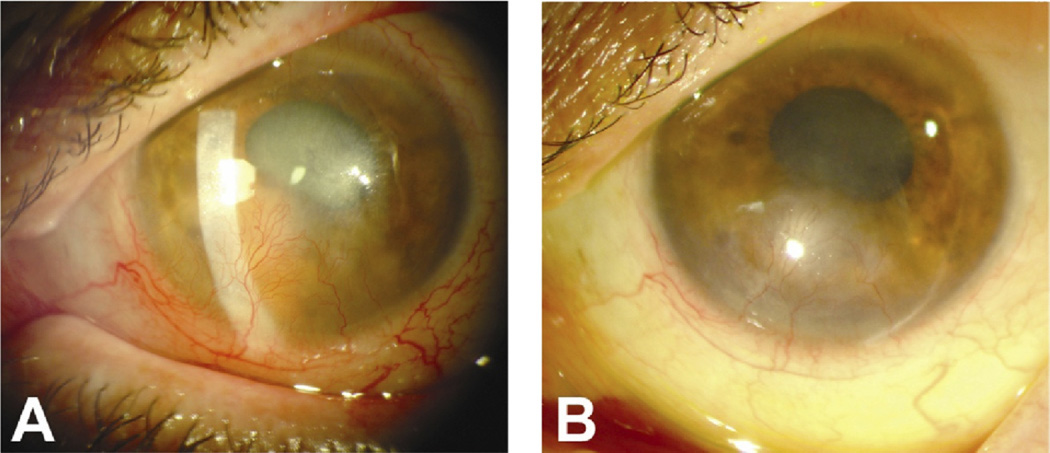
Bevacizumab (Avastin; Genetech, South San Franscisco, CA) is a recombinant humanized monoclonal immunoglobulin G1 antibody directed against all isoforms of VEGF-A. The antibody binds to and deactivates VEGF-A, which results in the inhibition of abnormal blood vessel formation and decreased vascular permeability. Bevacizumab is commercially available and the Food and Drug Administration approved it for the treatment of metastatic colorectal cancer. Since mid 2005, it has been used off label for a number of ocular diseases (e.g., Fig 2).
Figure 2.
A, Photograph showing corneal neovascularization resulting from neurotrophic keratopathy. B, Photograph of same eye after 5 monthly injections of bevacizumab.
Bevacizumab is available as a topical or subconjunctival injection, and there is conflicting evidence regarding its efficacy. Koeing et al20 has shown that use of off-label topical bevacizumab eye drops results in a 61% mean reduction in vascularized area during treatment, along with a 24% mean reduction in vessel diameter. Topical administration can be effective only if the drug can penetrate the corneal epithelium, and bevacizumab typically is considered ineffective because of its molecular weight, 149 kDa, which is too large to penetrate. Although the epithelium over the neovascularization is defective and results in incompetent barrier function, its absorption is limited because of the clearance through tear outflow. Subconjunctival administration of bevacizumab has better delivery, but carries the unfortunate possibility of causing thinning or erosive changes to the conjunctiva, sclera, and epithelium with prolonged use. Although short-term use of topical and subconjunctival bevacizumab has been proven to show positive results, more long-term evaluation of the effects of VEGF inhibition is necessary.
Dana12 at the Massachusetts Eye and Ear Infirmary is studying the effects of topical bevacizumab (1.0%) on corneal neovascularization. The study is designed as an open-label, single-group format including 20 patients. Topical therapy is initiated twice daily for a 3-week duration, and patients will be followed-up for 6 months after treatment. Efficacy will be monitored by assessing regression of corneal neovascularization as documented by computerized photographic images; safety will be measured by comprehensive examination. Similarly, Bower is implementing a safety and efficacy study at Walter Reed Medical Center, testing topical bevacizumab for the treatment of corneal neovascularization. The study has an open-label, single-group design. Patients clinically stable for 3 to 6 months who had more than 2 mm of corneal vascular invasion onto the cornea were included in the study. Kim from Korea23 is also recruiting patients into an open-label study assessing the safety of topical bevacizumab. His specific aim is to assess corneal thinning with the topical medication.23
Currently, several studies are assessing the safety and efficacy of subconjunctival VEGF inhibitors. Yoo from Bascom Palmer is recruiting 10 patients for a study analyzing the safety of subconjunctival ranibizumab use in patients with neovascular pterygia as a pretreatment before pterygia excision. Patients will be given either a subcon-junctival injection of 0.5 mg Ranibizumab 3 days before surgery or on the day of surgery. This is a phase I, parallel-assignment study that examines corneal and conjunctival wound healing, wound dehiscence, and anterior chamber concentration of drug after subconjunctival Ranibizumab delivery. Chen from Taiwan is also assessing the efficacy of subconjunctival bevacizumab and changes in dosing. Designed as an open-label single-group study, patients with neovascularity and lipid keratopathy extending more than 2 mm from the limbus were enrolled. Patients were injected with 1.25 to 2.5 mg of bevacizumab on a monthly basis for 3 months. If corneal neovascularization has not regressed, treatment continues for an additional 3 months. Visual acuity and the extent of lipid infiltrate will be assessed. In Brazil, Nazaralla is conducting a phase IV study assessing the safety and efficacy of subconjunctival bevacizumab in treating corneal neovascularization refractory to other treatments. After a 1.25-mg subconjunctival bevacizumab injection, patients will be followed-up for 2 months; efficacy will be assessed by improvement on anterior segment photographs. Going a step further, Hsiao in Taiwan is comparing topical 1.0% bevacizumab 4 times daily with subconjunctival injection 2.5 mg bevacizumab and monitoring efficacy for 6 months. This will give better insight into the long-term effects of topical and subconjunctival administration of bevacizumab.24
Although anti-VEGF therapy is efficient at preventing corneal neovascularization, there are desirable VEGF functions that bevacizumab may prevent, such as formation of collateral vessels, control of vascular tone, corneal nerve regeneration, and wound healing. It is important to note that long-term neutralization of VEGF may have unintended local or systemic consequences. Delivery models should be explored, because even small doses of anti-VEGF drugs are noted to cause hypertension, proteinuria, and various cardiovascular events. Vascular endothelial growth factors antibodies have a variety of side effects; therefore, patients of childbearing potential or patients with renal disease, with end-stage liver disease, abnormality in anticoagulation, a history of thrombotic event, hypertension, active infectious disease, or a history of glaucoma are contraindi-cated and have been excluded from the studies described previously.
Combination Therapy
Although mainstay therapy currently comprises steroids and VEGF inhibitors, some combination approaches have been investigated with promising results. Topically administered combinations of triamcinolone acetonide 10 µg/ml with low molecular weight heparin 10 mg/ml or with doxycycline 10 mg/ml have synergistic effects that contribute to efficient suppression of corneal neovascularization. Heparins affect angiogenesis by modulating expression of angiogenic growth factors and inhibitors; low molecular weight heparins seem to diminish the binding of these growth factors to their receptors. Using topically administered doxycycline also has been shown to have added antiangiogenic effects.22,25
Researchers also have examined the influence of delivery method combinations on visual acuity. Everardo in Mexico is currently performing a randomized phase II/III trial comparing subconjunctival bevacizumab (0.1 ml) with subconjunctival combination bevacizumab (0.1 ml) and triamcinolone (0.1 ml). Dana26 is studying the safety and efficacy of topical ranibizumab coupled with subconjunctival ranibizumab with an open-label, phase I trial of 10 patients. Visual acuity, ocular examination, physical examination, and corneal photographs will be monitored over 16 weeks.26
Recently, Gerten et al27 showed marked reduction in corneal neovascularization with bevacizumab in conjunction with argon laser therapy. Argon laser-induced coagulation provides a potential treatment by closing pathologic blood vessels in the cornea, and bevacizumab acts to prevent new angiogenesis. The theory is that by combining various methods of control, the different mechanisms that sustain corneal neovascularization will be targeted and that this therapy will be more efficacious in preventing further progression. Also, the treatment may allow us to use lower doses.
Translational Implications
Endogenous Inhibition
The intact inner and outer cornea has endogenous antiangiogenic characteristics harboring angiostatin, endostatin, thrombospondins, and pigment epithelium-derived factor. The use of a recombinant adenoassociated viral vector carrying endogenous genes is a strategy to treat corneal neovascularization. In a recent study, subconjunctival injection of recombinant endostatin–adenoassociated virus was used to examine the inhibition of silver nitrate-induced corneal neovascularization in mice. Gene expression in corneal tissue was observed as early as 4 days after transfer and lasted stably for more than 8 months with minimal immune reaction. Immunohistochemistry staining of CD 31 and endostatin showed that the treatment significantly inhibits corneal neovascularization.28 Adenoassociated virus is capable of directly delivering genes to the ocular surface epithelium by way of subconjunctival injection and is able to sustain high levels of gene expression in vivo to inhibit angiogenesis.
Anti-inflammation
Resolvins and lipoxins are lipid mediators that are generated from polyunsaturated fatty acids and are anti-inflammatory and immunoregulatory agents. Resolvin D1, resolvin E1, and aspirin-triggered A4 recently were shown to downregulate significantly the expression of angiogenic growth factors, their receptors, neutrophils, and macrophages by suppression of IL-1β. Studies administering these mediators have resulted in significant reduction of hemangiogenesis in the cornea in the murine corneal neovascularization model. These lipid mediators present another potential mechanism for controlling corneal neovascularization.29 Other alternative medical therapies also have been shown to inhibit inflammatory factors. Propolis, a resinous material found in beeswax; epigallactocatechin-3-gallate, found in green tea; Resveratrol, a polyphenol compound in red wine; and vitamin D all have shown anti-inflammatory and antiangiogenic properties.30–33
Tyrosine Kinase Receptor Inhibition
Vascular endothelial growth factor, FGF, and PDGF and their cognate receptor tyrosine kinases are strongly implicated in corneal neovascularization. Tyrosine kinases play a pivotal role in intracellular signal transduction and regulate crucial processes such as proliferation, migration, and survival of endothelial cells. Therefore, blocking receptor tyrosine kinases and nonreceptor cytoplasmic tyrosine kinases represents a rational approach to targeting corneal neovascularization.
Currently, there are several multikinase inhibitors that are small-molecule inhibitors of VEGFR-1, VEGFR-2, and related receptor tyrosine kinases such as PDGFR. Combined activity against VEGF and PDGF signaling transduction is beneficial because both growth factors are involved in angiogenesis. Inhibitors of PDGFRs have been shown to produce pericyte loosening or detachment from endothelial cells of tumor vessels. In addition, blood vessels lacking pericytes are more susceptible to VEGF deprivation. Therefore, inhibitors targeting both VEGFR and PDGFR can contribute to the regression of actively proliferating endothelial cells in multiple models of ocular neovascularization.34 Midostaurin (PKC412) is an orally administered small multikinase tyrosine inhibitor that binds to the intracellular enzymatically active domain of VEGFR, PDGF, and stem cell factor and prevents phosphorylation and activation of the VEGF cascade. It was shown to inhibit choroidal neovascularization.35 Another drug, Ruboxistaurin (Eli Lilly and Co., Indianapolis, IN), has similar antiangiogenic effects in vivo and in vitro via the suppression of ERK1/2 and Akt in the retina and has potential use in the cornea as well.36 TG100801 is another multikinase tyrosine inhibitor of predominantly VEGFR-2 and other protein kinases that regulate angiogenesis. It is a topically administered prodrug delivered as an eye drop that is readily converted to the active compound in the eye. Thus far, it exhibits excellent ocular pharmacokinetics and poor systemic circulation. Currently, it is in clinical trials for the treatment of AMD and has potential application for corneal neovascularization.37
Rob04
Roundabout proteins are guidance receptors that maintain vascular integrity. Slit is a ligand that is expressed in angiogenic tissue that activates rob04.38 Recently, the slit2–rob04 pathway was elucidated. Activation by rob04 receptors by slit2 has been shown to inhibit VEGF-165 induced migration, tube formation, and permeability in vitro and has been shown to block Src kinase activation. Slit treatment and overexpression of rob04 receptor present another potential therapy to stabilize vasculature in corneal neovascularization. Thus far, adenoviral infection of rob04 has been shown to have antiangiogenic effects, decreasing basal migration and kinase phosphorylation in both retinopathy of prematurity and choroidal neovascularization models. Stabilization of the mature vascular bed by activation of the rob04 pathway may have a broad therapeutic potential to suppress corneal neovascularization in the future.
Vascular Endothelial Growth Factor Inhibition
VEGFTrap
VEGFTrap (aflibercept) is a soluble decoy receptor that binds to all isoforms of VEGFA and other members of the VEGF family and prevents the ligand-induced activation of its receptor. Aflibercept contains the second immunoglobulin domain of VEGFR-1 and the third immunoglobulin domain of VEGFR-2 bound to the Fc portion of human immunoglobulin G1. The affinity of VEGFTrap for VEGF seems to be superior to that of bevacizumab. Lockhart et al39 reported the first phase I trial of intravenous aflibercept in humans. Studies of the subcutaneous administration of this agent also have been reported; however, only the intravenous form has moved forward to phase II and III studies in humans for the treatment of neovascular age-related macular degeneration. Recently, it has gained interest for the inhibition of corneal neovascularization. Oliveira et al40 only recently showed that VEGFTrap suppresses basic FGF-induced corneal neovascularization in the murine model, and it seems to be a potential therapeutic avenue.
Specific Vascular Endothelial Growth Factor Receptors
As previously described, VEGFR-1, VEGFR-2, and VEGFR-3 each play an integral role hemangiogenesis and lymphangiogenesis, respectively. They exist as soluble receptors in the intact corneal epithelium, acting as so-called physiologic sinks to angiogenic factors to maintain avascularity in the cornea.
Hemangiogenesis and Vascular Endothelial Growth Factor Receptor-1
Studies by Ambati et al found the necessity of sflt-1 expression in the cornea to maintain the avascularity of the cornea. They also found that mice possessing corn1 and pax6+/− mutations with spontaneously vascularized corneas are deficient in sflt-1. In addition to trapping VEGF-A, sflt-1 also can heterodimerize with mbflt-1 and VEGFR-2. Several groups have shown positive results using adenoassociated viral-mediated expression of sflt-1 in the prevention of diabetic retinopathy, ischemia-induced proliferative retinopathy, and choroidal neovascularization in the murine and monkey model.41,42 Adenoassociated viral-mediated secretion gene therapy is nontoxic, and sflt-1 levels are maintained in the eye more than 1 year after injection.43,44 This may become a potential therapy in the treatment of corneal neovascularization as well.
Lymphangiogenesis, Vascular Endothelial Growth Factor Receptor-2, and Vascular Endothelial Growth Factor Receptor-3
Soluble VEGFR-2 is involved in the regulation of lymphangiogenesis. Albuquerque et al3 showed that VEGFR-2 is necessary for corneal alymphaticity and inhibits developmental and reparative lymphangiogenesis by blocking the function of VEGF-C. In mice deficient in VEGFR-2, there is invasion of lymphatic vessels into the cornea and hyperplasia of lymphatics in the skin. Vascular endothelial growth factor receptor-2 sufficiently enhances allograft survival, despite not inhibiting hemangiogenesis in mice, and may have therapeutic effects in the treatment of transplant rejection without causing the potential adverse effects of nonspecific antiangiogenic therapy.
Cursiefen et al4 have demonstrated the role of VEGFR-3 and its role in corneal lymphangiogenesis. In their study, mild cautery, which is known to cause an inflammatory response but not a neovascular response, was applied to mice that were VEGFR-3 deficient, and these were compared with wild-type mice. The results of this study showed a high angiogenic response in VEGFR-3–deficient mice, with minimal response in normal mice. Two monoclonal antibodies to VEGFR-3 have been explored, AFL4 and mF4–31C1; use of both has shown that blocking VEGFR-3 receptor signaling prevents normal lymphangiogenesis and VEGF-C–induced lymphangiogenesis in the mouse cornea, without an effect on hemangiogenesis or existing lymphatic vessels.45 Lymphangiogenesis is emerging as an important area of interest because its contributions to corneal neovascularization are not as clear, but likely play a key role in corneal transplant rejection.
These findings regarding VEGFR-1, VEGFR-2, and VEGFR-3 have major therapeutic implications. By adding or restoring these receptors, we can prevent corneal neovascularization. They also could be engineered to be overex-pressed on transplanted corneas to inhibit posttransplantation angiogenesis. Other possibilities include cultured corneal epithelium that produces a receptor that can be applied to common nonhealing corneal ulcers to promote wound healing. Flt-1 subunits can also be delivered via plasmids encapsulated in nanoparticles. Thus far, studies have shown that in vivo delivery of plasmids expressing flt-1 intraceptors inhibit injury-induced upregulation of VEGF and corneal neovascularization in murine models. Albumin nanoparticles are a potential method of drug delivery; they are nontoxic to the cornea and can express intraceptors for extended periods and are effective at suppressing corneal neovascularization in murine models.46
Interference Ribonucleic Acid
Small interfering RNA (siRNA) represents a fairly new technology that works by silencing the cellular machinery by sending a duplicate copy into the cell. The eye’s unique isolation from the rest of the body makes it an ideal candidate for siRNA therapies. Thus far, interference Ribonucleic acid (RNAi) has been shown to suppress hypoxia-induced VEGF in human corneal epithelial cells in vitro and regres-sion of alkali-injury–induced murine corneal neovascularization.47 However, delivery of siRNA to target cells remains a problem, because naked siRNAs do not penetrate cell membranes and can induce off-target effects via activation of surface toll-like receptor (TLR3).48,49
The cornea bears an antiangiogenic privilege, allowing for the maximal entry of light and visual acuity. This antiangiogenic privilege is maintained by a fine balance between antiangiogenic and angiogenic factors in the cornea. Any alterations in this balance resulting from chemical, mechanical, degenerative, or infectious insults can lead to the development of corneal neovascularization, compromising the optical performance of the cornea. This review has outlined the major pathophysiologic processes that govern corneal neovascularization and current treatment methods. The most common therapies currently used are steroids and the VEGF-A inhibitor bevacizumab (under broad study in several trials). We are now seeing a trend toward developing more specific VEGF inhibition with sflt-1, VEGFR-2, and VEGFR-3. Novel delivery methods such as gene therapy via nanoparticles are a burgeoning area of interest.38 Considering the complex interplay of the pathogenesis of corneal neovascularization, a multifaceted approach to preventing corneal neovascularization is needed, perhaps using several of the mentioned treatments. With the advent of these newer therapies, we may be able to achieve corneal clarity and to find an effective way to allow corneal transplantation or refractive surgery in those patients who previously were considered at high risk for rejection and graft failure.
Footnotes
Financial Disclosure(s): The author(s) have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103:11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambati BK, Joussen AM, Kuziel WA, et al. Inhibition of corneal neovascularization by genetic ablation of CCR2. Cornea. 2003;22:465–467. doi: 10.1097/00003226-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Ambati BK, Anand A, Joussen AM, et al. Sustained inhibition of corneal neovascularization by genetic ablation of CCR5. Invest Ophthalmol Vis Sci. 2003;44:590–593. doi: 10.1167/iovs.02-0685. [DOI] [PubMed] [Google Scholar]
- 7.Brooks BJ, Ambati BK, Marcus DM, Ratanasit A. Photodynamic therapy for corneal neovascularisation and lipid degeneration. Br J Ophthalmol. 2004;88:840. doi: 10.1136/bjo.2003.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheppard JD, Jr, Epstein RJ, Lattanzio FA, Jr, et al. Argon laser photodynamic therapy of human corneal neovascularization after intravenous administration of dihematoporphyrin ether. Am J Ophthalmol. 2006;141:524–529. doi: 10.1016/j.ajo.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Yoon KC. principal investigator. Photodynamic Therapy with Verteporfin for Corneal Neovascularization. [Accessed October 7, 2010];ClinicalTrials.gov Identifier NCT00471406. Available at: http://clinicaltrials.gov/ct2/show/NCT00471406?term=corneal+neovascularization&rank=8.
- 10.Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- 11.Lu P, Li L, Liu G, et al. Enhanced experimental corneal neovascularization along with aberrant angiogenic factor expression in the absence of IL-1 receptor antagonist. Invest Ophthalmol Vis Sci. 2009;50:4761–4768. doi: 10.1167/iovs.08-2732. [DOI] [PubMed] [Google Scholar]
- 12.Dana R. principal investigator. Topical Avastin for Treatment of Corneal Neovascularization. [Accessed September 30, 2010];ClinicalTrials.gov Identifier NCT00559936. Available at: http://clinicaltrials.gov/ct2/show/NCT00559936.
- 13.Bourges JL, Lallemand F, Agla E, et al. Evaluation of a topical cyclosporine A prodrug on corneal graft rejection in rats. Mol Vis. 2006;12:1461–1466. [PubMed] [Google Scholar]
- 14.Lux Biosciences, principal investigator. Study to Assess LX201 for Prevention of Corneal Allograft Rejection or Graft Failure in Subjects Who Have Experienced One or More Rejection Episodes Following Penetrating Keratoplasty. [Accessed on October 6, 2010];ClinicalTrials.gov Identifier NCT00447642. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00447642?term=corneal+neovascularization&rank=16.
- 15.Ono K, Yokoo S, Mimura T, et al. Autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane in a rabbit model. Mol Vis. 2007;13:1138–1143. [PMC free article] [PubMed] [Google Scholar]
- 16.Kawashima M, Kawakita T, Satake Y, et al. Phenotypic study after cultivated limbal epithelial transplantation for limbal stem cell deficiency. Arch Ophthalmol. 2007;125:1337–1344. doi: 10.1001/archopht.125.10.1337. [DOI] [PubMed] [Google Scholar]
- 17.Zakaria N. [Accessed October 6, 2010];principal investigator. Cultivated Stem Cell Transplantation for the Treatment of Limbal Stem Cell Deficiency (LECT) ClinicalTrials.gov Identifier NCT00845117. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00845117?term=corneal+neovascularization&rank=20.
- 18.Aghdami N. Autologous Transplantation of Cultivated Limbal Stem Cells on Amniotic Membrane in Limbal Stem Cell Deficiency (LSD) Patients. [Accessed October 7, 2010];ClinicalTrials.gov Identifier NCT00736307. Available at: http://www.nextbio.com/b/search/individualtrial.nb?id=NCT00736307.
- 19.Raffiee AB. The Role of Amniotic Membrane Transplantation in Ocular Chemical Burns. [Accessed September 7, 2010];ClinicalTrials.gov Identifier NCT00370812. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00370812?term=corneal+neovascularization&rank=18.
- 20.Yamada N, Matsuda R, Morishige N, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92:896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- 21.Chen WL. principal investigator. Topical Autologous Insulin Application for the Treatment of Corneal Epithelium Defect After Ocular Surgeries. [Accessed October 7, 2010];ClinicalTrials.gov Identifier NCT01031888. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01031888?term=corneal+neovascularization&rank=14.
- 22.Aydin E, Kivilcim M, Peyman GA, et al. Inhibition of experimental angiogenesis of cornea by various doses of doxycycline and combination of triamcinolone acetonide with low-molecular-weight heparin and doxycycline. Cornea. 2008;27:446–453. doi: 10.1097/ICO.0b013e3181605ff9. [DOI] [PubMed] [Google Scholar]
- 23.Kim TI. principal investigator. Corneal Thinning During Topical Bevacizumab Therapy. [Accessed October 8, 2010];ClinicalTrials.gov identifier NCT00515684. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00515684?term=corneal+neovascularization&rank=12.
- 24.Hsiao CH. principal investigator. Bevacizumab for the treatment of corneal neovascularization. [Accessed September 30, 2010];ClinicalTrials.gov identifier NCT00992849. Available at: http://clinicaltrials.gov/ct2/show/NCT00992849.
- 25.Murata M, Shimizu S, Horiuchi S, Taira M. Inhibitory effect of triamcinolone acetonide on corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2006;244:205–209. doi: 10.1007/s00417-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 26.Dana R. principal investigator. Effectiveness and Safety of Subconjunctival and Topical Ranibizumab for Treatment of Corneal Neovascularization. [Accessed September 8, 2010];ClinicalTrials.gov Identifier NCT00681889. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00681889?term=corneal+neovascularization&rank=7.
- 27.Gerten G. Bevacizumab (Avastin) and argon laser to treat neovascularization in corneal transplant surgery. Cornea. 2008;27:1195–1199. doi: 10.1097/ICO.0b013e318180e50f. [DOI] [PubMed] [Google Scholar]
- 28.Lai LJ, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14:313–322. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- 29.Jin Y, Arita M, Zhang Q, et al. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keshavarz M, Mostafaie A, Mansouri K, et al. Inhibition of corneal neovascularization with propolis extract. Arch Med Res. 2009;40:59–61. doi: 10.1016/j.arcmed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Sen T, Moulik S, Dutta A, et al. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009;84:194–204. doi: 10.1016/j.lfs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Gagliano N, Moscheni C, Torri C, et al. Effect of resveratrol on matrix metalloproteinase-2 (MMP-2) and Secreted Protein Acidic and Rich in Cysteine (SPARC) on human cultured glioblastoma cells. Biomed Pharmacother. 2005;59:359–364. doi: 10.1016/j.biopha.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Riachy R, Vandewalle B, Moerman E, et al. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11:151–159. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- 34.Wang FE, Shi G, Niesman MR, et al. Receptor tyrosine kinase inhibitors AG013764 and AG013711 reduce choroidal neovascularization in rat eye. Exp Eye Res. 2007;84:922–933. doi: 10.1016/j.exer.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saishin Y, Silva RL, Callahan K, et al. Periocular injection of microspheres containing PKC412 inhibits choroidal neovascularization in a porcine model. Invest Ophthalmol Vis Sci. 2003;44:4989–4993. doi: 10.1167/iovs.03-0600. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura S, Chikaraishi Y, Tsuruma K, et al. Ruboxistaurin, a PKCbeta inhibitor, inhibits retinal neovascularization via suppression of phosphorylation of ERK1/2 and Akt. Exp Eye Res. 2009;90:137–145. doi: 10.1016/j.exer.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Palanki MS, Akiyama H, Campochiaro P, et al. Development of prodrug 4-chloro-3-(5-methyl-3-{[4-(2-pyrrolidin-1-ylethoxy)phenyl]amino}-1,2,4-be nzotriazin-7-yl)phenyl ben-zoate (TG100801): a topically administered therapeutic candidate in clinical trials for the treatment of age-related macular degeneration. J Med Chem. 2008;51:1546–1559. doi: 10.1021/jm7011276. [DOI] [PubMed] [Google Scholar]
- 38.Jones CA, London NR, Chen H, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockhart AC, Rothenberg ML, Dupont J, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–214. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira HB, Sakimoto T, Javier JA, et al. VEGF Trap(R1R2) suppresses experimental corneal angiogenesis. Eur J Ophthalmol. 2010;20:48–54. doi: 10.1177/112067211002000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bainbridge JW, Mistry A, De Alwis M, et al. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther. 2002;9:320–326. doi: 10.1038/sj.gt.3301680. [DOI] [PubMed] [Google Scholar]
- 42.Ideno J, Mizukami H, Kakehashi A, et al. Prevention of diabetic retinopathy by intraocular soluble flt-1 gene transfer in a spontaneously diabetic rat model. Int J Mol Med. 2007;19:75–79. [PubMed] [Google Scholar]
- 43.Lai CM, Shen WY, Brankov M, et al. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol Ther. 2005;12:659–668. doi: 10.1016/j.ymthe.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Igarashi T, Miyake K, Masuda I, et al. Adeno-associated vector (type 8)-mediated expression of soluble Flt-1 efficiently inhibits neovascularization in a murine choroidal neovascularization model. Hum Gene Ther. 2010;21:631–637. doi: 10.1089/hum.2009.153. [DOI] [PubMed] [Google Scholar]
- 45.Bock F, Onderka J, Dietrich T, et al. Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol. 2008;246:115–119. doi: 10.1007/s00417-007-0683-5. [DOI] [PubMed] [Google Scholar]
- 46.Jani PD, Singh N, Jenkins C, et al. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–2036. doi: 10.1167/iovs.06-0853. [DOI] [PubMed] [Google Scholar]
- 47.Singh N, Higgins E, Amin S, et al. Unique homologous siRNA blocks hypoxia-induced VEGF upregulation in human corneal cells and inhibits and regresses murine corneal neovascularization. Cornea. 2007;26:65–72. doi: 10.1097/ICO.0b013e31802b4201. [DOI] [PubMed] [Google Scholar]
- 48.Kleinman ME, Yamada K, Takeda A, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho W, Albuquerque RJ, Kleinman ME. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci U S A. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]