Abstract
Sciatic nerve tissue was obtained from the gluteus maximus muscle segment of normal human cadavers and amniotic membrane tissue was obtained from healthy human puerperant placentas. Both tissues were analyzed for their stress relaxation and creep properties to determine suitability for transplantation applications. Human amniotic membrane and sciatic nerve tissues had similar tendencies for stress relaxation and creep properties. The stress value of the amniotic membrane stress relaxation group decreased to a greater extent compared with the sciatic nerve stress relaxation group. Similarly, the stress value of the amniotic membrane creep group increased to a greater extent compared with the sciatic nerve creep group. The stress relaxation curve for human amniotic membrane and sciatic nerve showed a logarithm correlation, while the creep curve showed an exponential correlation. These data indicate that amniotic membrane tissue has better stress relaxation and creep properties compared with sciatic nerve tissue.
Keywords: neural regeneration, basic research, cadaver, human, sciatic nerve, amniotic membrane, stress relaxation, creep, viscoelasticity, stress, biomechanics, nerve tissue engineering, neuroregeneration
Research Highlights
-
(1)
Human amniotic membrane has been widely applied in the repair of peripheral nerve injury. This study further verifies that human amniotic membrane has a similar tendency for stress relaxation and creep properties compared with sciatic nerve, indicating that it has good stress relaxation and creep properties for transplantation applications.
-
(2)
The stress relaxation curve of human amniotic membrane and sciatic nerve has a logarithmic relationship, while the creep curve has an exponential relationship.
INTRODUCTION
When used as a scaffold for tissue engineering purposes, amniotic membrane plays an important role in neural regeneration[1,2]. Increasing evidence regarding the biological properties of human amniotic membrane and peripheral nerve tissues, as well as human amniotic membrane transplantation for repair of peripheral nerve injury, shows that amniotic membrane has wide applications, convenient use, good biocompatibility and low antigenicity[3,4,5,6,7,8,9,10,11,12,13,14]. Human amniotic membrane transplantation for peripheral nerve regeneration can inhibit nerve fibrosis and prevent scar formation. The amniotic membrane likely forms an enclosed regeneration chamber to isolate the nerve from surrounding tissue, thus reducing invasion of fibrous tissue and inflammatory cells and effectively preventing formation of anastomotic scar tissue. In addition, amniotic membrane matrix contributes to the inhibition of fibrosis and stimulates fibroblasts to produce collagen and extracellular matrix components. As a result, amniotic membrane has been widely applied in the field of neuroscience research. Mligiliche et al[14] prepared nerve conduits of different diameters for repair of sciatic nerve defects. This was achieved using connective tissue matrix sheets from amniotic membrane after removal of epithelial cells. They found a catheter of 1–2 mm diameter had the best repair effect.
Furthermore, Mohammad et al[15] demonstrated that de-epithelized amniotic membrane bridging was similar to autologous nerve grafting, but better than using a silica gel tube, for guiding neural regeneration in 1.0 cm rat sciatic nerve defects.
Ma and colleagues[16] conducted a uniaxial tensile test of the upper trunk brachial plexus in 30 fresh fetal cadavers. Experimental results showed there was no significant difference in the branchial plexus tensile mechanical properties between male and female fetuses. However, the 7 200-second stress relaxation measurement of the fetal brachial plexus was greater in younger compared with older fetuses. Creep measurements showed no significant differences. After the sciatic nerve injury models were treated with autologous nerve anastomosis and human amnion anastomosis, the stress relaxation and creep properties were assessed. Results showed that allogeneic human amnion anastomosis in sciatic nerve injury models had good rheological properties, conducive to the injured nerve anastomosis and functional reconstruction. A conclusion can therefore be drawn that human amniotic membrane is the preferred material for nerve transplantation. However, the stress relaxation and creep characteristics of normal, human sciatic nerve and amniotic membrane are seldom reported.
To further develop human amniotic membrane transplantation for repair of sciatic nerve injury, it is essential to understand the stress relaxation and creep properties of normal human sciatic nerve and amniotic membrane.
In this study, we analyzed and compared the stress relaxation and creep properties of normal human sciatic nerve and amniotic membrane tissues. This was carried out in a broader attempt to explore the feasibility of using human amniotic membrane transplantation for repairing sciatic nerve injury, and to provide data on the viscoelasticity properties of human amniotic membrane.
RESULTS
Quantitative analysis of normal human sciatic nerve and amniotic membrane specimens
A total of 32 human amniotic membrane samples were randomly divided into two groups: human amniotic membrane stress relaxation group and human amniotic membrane creep group (n=16 for each group). A total of 32 normal cadaver sciatic nerve samples were randomly assigned into two groups: normal sciatic nerve stress relaxation group and normal sciatic nerve creep group (n = 16 for each group). One sciatic nerve and one human amniotic membrane sample were selected from each group before and after stress relaxation and creep experiments for ultrastructural tissue analysis using scanning electron microscopy. All 64 specimens were involved in the final analysis.
Stress relaxation curve of normal human sciatic nerve and amniotic membrane specimens
A total of 100 data sets of the two tissue groups from the 7 200-second stress relaxation experiments were collected across 18 time points to generate a computerized curve fit. Stress decreased rapidly up to 600 seconds and slowly after 600 seconds; therefore, the time points selected included 0, 60, 120, 180, 300, 420, 600, 900, 1 200, 1 500, 1 800, 2 400, 3 000, 3 600, 4 800, 5 400, 6 600 and 7 200 seconds (Figure 1).
Figure 1.
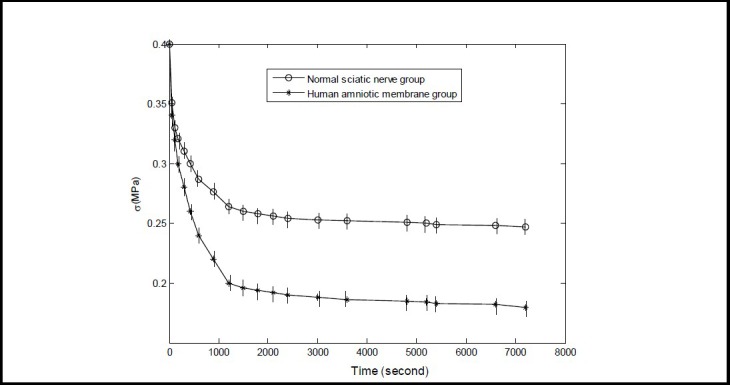
Stress relaxation curve of normal human sciatic nerve and amnion tissues.
The Y- and X-axis represent stress and time, respectively. Data are expressed as mean ± SD, n = 15. The 7 200- second stress value in the normal sciatic nerve and amniotic membrane groups decreased by 0.152 and 0.22 MPa, respectively, compared with the 0 second value (P < 0.05; one-way analysis of variance and Sceffe method). The stress value decreased to a greater extent in the amniotic membrane group compared with the sciatic nerve group (P < 0.05; paired t-test).
As shown in Figure 1, stress for each sample decreased rapidly up to 600 seconds, then decreased slowly, and plateaued to 7 200 seconds. The stress relaxation curve of the two tissue groups displayed a logarithmic relationship. The 7 200-second stress value decreased to a greater extent in the human amniotic membrane group compared with the sciatic nerve group (P < 0.05).
Normalized stress relaxation function curve of normal human sciatic nerve and amniotic membrane specimens
The normalized stress relaxation function of the two tissue groups at the 18 time points was subjected to computerized curve fitting. The mean and standard deviation at each time point are shown in (Figure 2).
Figure 2.
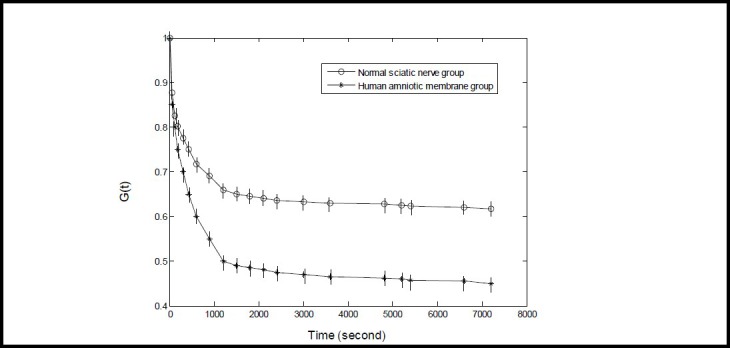
Normalized stress relaxation function curve of normal human sciatic nerve and amnion tissues.
The Y-axis G(t) represents the normalized stress relaxation function (G(t) zero dimension) and the X-axis represents time. The normalized stress relaxation function was calculated using the formula of G(t)= σ(t)/ σ(0), in which G(t) is the normalized stress relaxation function, σ(0) is the stress value at 0 second, and σ(t) is the stress value at a given time point. The normalized stress relaxation function value at 0 second was defined as 1. Data are expressed as mean ± SD, n = 15. The 7 200 second stress relaxation function value decreased to a greater extent in the amniotic membrane group compared with the sciatic nerve group (P < 0.05; paired t-test).
As shown in Figure 2, the 7 200 second stress relaxation function value decreased to a greater extent for the amniotic membrane group compared with the sciatic nerve group (P < 0.05). The normalized stress relaxation function curve values dropped over time, and maintained a basic level at 7 200 seconds. The stress relaxation curve for the two tissue groups displayed a logarithmic relationship (Figure 2).
Establishment of normalized stress relaxation function of normal human sciatic nerve and amniotic membrane specimens
As shown in Figure 1, the stress relaxation curves displayed a logarithmic relationship. According to calculations reported by Ma et al[19], we suppose that:
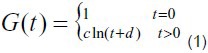
in which, c and d are the undetermined coefficients,
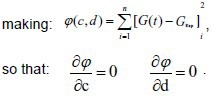
Finally, the canonical equation:
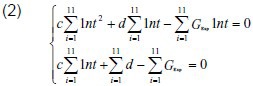
Experimental data were input into formula (2) to calculate the c and d values of the two groups. The calculated c and d values were then input into formula (1) to obtain the normalized stress relaxation function equation of the two groups, as follows:
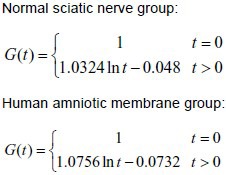
Creep curve of normal human sciatic nerve and amniotic membrane specimens
A total of 100 data sets from the two tissue groups from the 7 200-second creep experiment were collected at 18 time points to generate a computerized curve fit. Stress decreased rapidly up to 600 seconds and slowly after 600 seconds; therefore, the time points were selected to include 0, 60, 120, 180, 300, 420, 600, 900, 1 200, 1 500, 1 800, 2 400, 3 000, 3 600, 4 800, 5 400, 6 600 and 7 200 seconds (Figure 3).
Figure 3.
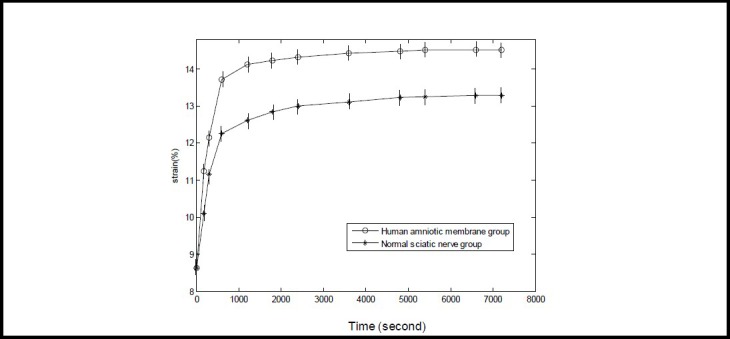
Creep curve of normal sciatic nerve and human amniotic membrane specimens.
The Y-axis represents strain (%; zero dimension) calculated from the experimental data by computer fitting, and the X-axis represents time. Data are expressed as mean ± SD, n = 15.
The 7 200-second strain of normal sciatic nerve and amniotic membrane groups increased by 4.66% and 5.88%, respectively, when compared with 0 second (P < 0.05; one-way analysis of variance and Sceffe method). The 7 200-second strain increase value was greater in the human amniotic membrane group compared with the sciatic nerve group (P < 0.05; paired t-test).
As shown in Figure 3, strain for each specimen increased rapidly up to 600 seconds, then increased slowly, and plateaued to 7 200 seconds. The creep curve of the two tissue groups displayed an exponential relationship. The 7 200-second strain value increased to a greater extent in the amniotic membrane group compared with the sciatic nerve group (P < 0.05).
Normalized creep function curve of normal human sciatic nerve and amniotic membrane specimens
As shown in Figure 4, the normalized creep function value of each specimen at 0 second was defined as 1. The 7 200-second creep function value was greater for the amniotic membrane group compared with the sciatic nerve group (P < 0.05). The normalized creep function curve of the two groups displayed an exponential relationship.
Figure 4.
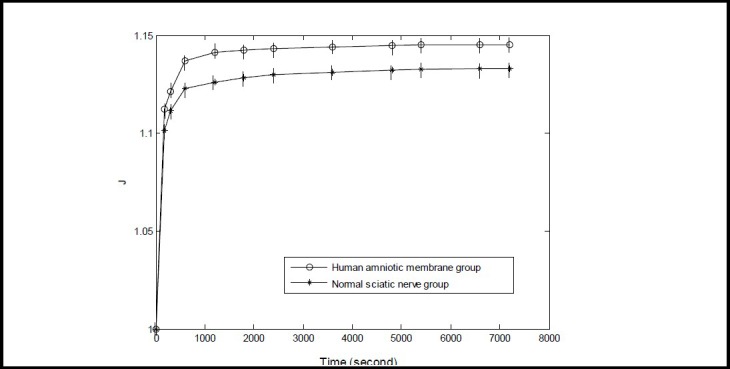
Normalized creep function curve of normal human sciatic nerve and amniotic membrane specimens.
The Y-axis represents the normalized creep function (zero dimension, greater than 1) and the X-axis represents time. The normalized creep function was calculated according to the formula of J(t)=L(t)/L(0), in which L(t) is the normalized creep function, L(0) is the length of specimen at 0 second, and L(t) is the length of specimen at a given time point. Data are expressed as mean ± SD, n = 15. The 7 200-second creep function value was greater in the amniotic membrane group compared with the sciatic nerve group (P < 0.05; paired t-test).
Establishment of normalized creep function equation
As shown in Figure 3, the creep curves changed exponentially. According to Ma et al[19], we suppose that:
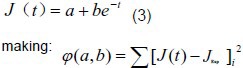
Finally, the canonical equation:
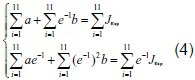
Experimental data were input into formula (4) to calculate the a and b values of the two groups. The calculated a and b values were then input into formula (3) to obtain the normalized creep function equation of the two groups as follows:

Scanning electron microscopy to observe the ultrastructure of human sciatic nerve and amniotic membrane cross-sections
Using scanning electron microscopy, normal sciatic nerve fibers were found to be arranged in rows, axons were surrounded by a myelin sheath and connective tissue-formed endoneurium encased the myelin and axons on the nerve fiber surface (Figure 5A). After conducting stress relaxation and creep experiments, the ultrastructural component arrangements were comparable, with no obvious changes (Figure 5B). Human amniotic fiber alignment was organized and tight, and fiber structure was unchanged after stress relaxation and creep experiments (Figure 6).
Figure 5.
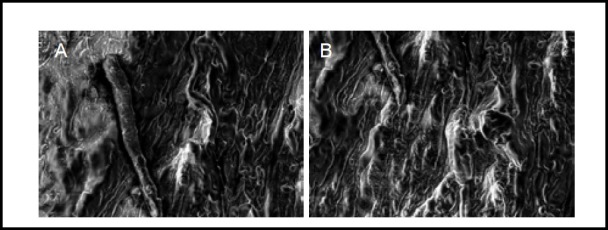
Scanning electron microscopy images showing the ultrastructure of normal human sciatic nerve before and after stress relaxation and creep experiments (× 2 000).
Normal sciatic nerve (A): Fibers were arranged in rows, axons and other components were clearly seen. Sciatic nerve after stress relaxation and creep experiments (B): Fibers were arranged in rows, axons and other components were still clearly visible, with no obvious change.
Figure 6.
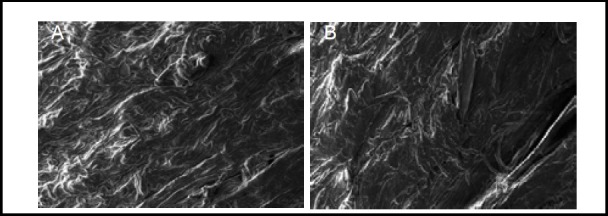
Scanning electron microscopy images showing the ultrastructure of normal human amniotic membrane before and after stress relaxation and creep experiments (× 2 000).
Normal amniotic membrane (A) and after stress relaxation and creep experiments (B). Human amniotic fibers in both groups were neatly arranged, and fiber structure remained unchanged.
DISCUSSION
Scanning electron microscopy imaging showed that for both sciatic nerve and amniotic tissues, there were no obvious changes in tissue structure after stress relaxation and creep experiments were performed.
We believe this was because the constant strain in the stress relaxation experiment and constant stress in the creep experiment were small and lower than the physiological limit. The stress relaxation experiment showed that the 7 200-second stress value decreased to a greater extent in the amniotic membrane group compared with the sciatic nerve group. The creep experiment showed that the 7 200-second strain value increased to a greater extent in the amniotic membrane group compared with the sciatic nerve group. The stress relaxation and creep curves were similar between the two tissue groups.
The stress relaxation and creep characteristics of human amniotic membrane play an important role in determining anastomotic tension and elongation after transplantation into the sciatic nerve. Under constant strain, the decreased stress and relaxation values of human amniotic membrane are conducive to reducing anastomotic tension after transplantation into the sciatic nerve. Under constant physiological load elongation, transplanted human amniotic membrane can limit anastomotic opening and shift after sciatic nerve injury, and assist in promoting sciatic nerve regeneration and functional restoration. A previous study reported the stress relaxation and creep viscoelastic properties of fetal brachial plexus nerve[16]. However, the longitudinal incision used in this study likely damaged the collagen and elastic fibers in the brachial plexus, so cannot fully reflect the viscoelastic properties. In our study, human adult fresh cadaver sciatic nerve tissue and fetal brachial plexus tissue were found to have different viscoelastic properties. Human amniotic membrane and sciatic nerve are soft tissues, in which creep is under constant stress and stress relaxation is under constant strain, allowing the tissues to carry out their physiological functions. It is therefore necessary to understand the stress relaxation and creep properties of human amniotic membrane and sciatic nerve tissues, and quantitative analysis is required for clinical peripheral nerve transplantation approaches. This method is not only suitable for human sciatic nerve and amniotic membrane transplantation, but also is applied to human peripheral nerve and identical tissues in other species. Our experimental findings provide graphical curve stress relaxation and creep data on normal human sciatic nerve and amniotic membrane. Furthermore, we have shown changes in stress, strain and time after human amniotic membrane and autogenous nerve transplantation in clinical treatment of sciatic nerve injury.
This study showed that the 7 200-second stress reduction and strain increase values of sciatic nerve and amniotic membrane tissues were greater compared with injured sciatic nerve bridging nerve anastomosis and human amnion anastomosis. This is evidence that allogeneic human amniotic membrane and autogenous sciatic nerve transplantation, after sciatic nerve injury, may adapt to changes in stress, strain and time. It is innovative to detect stress relaxation and creep characteristics of human sciatic nerve and amniotic membrane by stress, strain and time variations, and to observe the ultrastructural morphology of these tissues before and after performing stress relaxation and creep experiments.
The 7 200-second stress reduction and strain increase measurement of the human amniotic membrane group were greater compared with the sciatic nerve group, indicating that human amniotic membrane has good stress relaxation and creep properties. The experimental results prove that the viscoelasticity of human amniotic membrane is superior to sciatic nerve. The stress relaxation and creep properties of human amniotic membrane are suitable for transplantation after sciatic nerve injury. The experimental findings support the application of human amniotic membrane transplantation for sciatic and peripheral nerve injuries. Because cadaver tissue sources are limited, only the gluteus maximus segment of the sciatic nerve was harvested for experimentation. This meant we were unable to compare sciatic nerve tissue at different sites and perform a comparison of sciatic nerve specimens from identical sections. Further studies are needed to explore the viscoelasticity of other segments of the sciatic nerve and the stress relaxation and creep properties of sciatic nerve and human amniotic membrane under a variety of stress and strain states. Because of the limited number of experimental specimens and inter-donor differences of biological materials, this poses a limitation to the study.
In summary, the stress relaxation curve of human amniotic membrane and sciatic nerve exhibits a logarithmic change, whilst the creep curve exhibits an exponential change. Human amniotic membrane has similar stress relaxation and creep change tendencies to autogenous sciatic nerve, and therefore has good stress relaxation and creep properties.
MATERIALS AND METHODS
Design
An in vitro comparative observation.
Time and setting
Experiments were performed from January to May 2008 in the Mechanics Experiment Center of Jilin University, China.
Materials
Human amniotic membrane specimens were derived from 10 healthy gravida placentas following cesarean section, from the China-Japan Union Hospital of Jilin University, China, after maternal informed consent. Human amniotic membrane specimens were stored in saline solution at 4°C immediately after removal. Sciatic nerve specimens were obtained from seven fresh, normal, Chinese adult male cadavers, aged 24–29 years old, from the Department of Anatomy, Jilin University School of Medicine, China. Within 24 hours after death, gluteus maximus specimens were harvested from the bilateral sciatic nerve following cadaveric dissection and were preserved in plastic bags at −20°C.
Methods
Preparation of sciatic nerve specimens
Gluteus maximus muscle bilateral sciatic nerve segments (a total of 14 specimens, two from each cadaver) were used for the stress relaxation and creep experiments after cold storage for 1 day. Prior to experimentation, specimens were taken from the freezer and thawed at room temperature. Specimens were then cut into 31 sections, 25 mm in length and 8.8–11.2 mm in diameter, using an S-5 sterile plastic-handle operating knife (UE Medical Product Ltd., Xuyi County, Huai’an City, Jiangsu Province, China). Fifteen specimens were randomly selected as the normal sciatic nerve stress relaxation group, and another 15 as the normal sciatic nerve creep group. The remaining specimen was used for scanning electron microscopy analysis.
Preparation and intervention of human amniotic membrane
Amniotic epithelial cells were removed using an ammonia digestion method, and human amniotic membrane specimens were prepared. The specimens were randomly divided into two groups. The time from tissue removal to testing was identical and all specimens were preset prior to experimentation. The experimental speed and temperature were identical in the two groups.
Stress relaxation experiment
An automatic controlled electronic universal testing machine (Shimadzu, Tokyo, Japan) was used to control the stress and strain speed, maintain a constant stress and strain, define the experimental time and acquire the experimental data. The testing machine was equipped with a −30 to 250°C incubator to maintain a constant temperature. Specimen length and diameter were measured with a reading microscope (Changchun City Third Optical Instrument Factory, Changchun, Jilin Province, China). Length was recorded as 25 mm and diameter as 8.6–11.2 mm. Experiments were performed under simulated normal human body temperature at 36.5 ± 0.5°C. Each sample was placed within the clamper and received strain at a speed of 50%/min. When stress reached 0.4 MPa, the strain value of the sciatic nerve and amnion anastomosis groups was 8.63% and 9.68%, respectively. The experimental time was set at 7 200 seconds to collect 100 sets of data. The specimens were continuously given liquid spray to maintain humidity. At the end of the experiment, the stress relaxation data and curve were automatically output using the automatic control electronic universal testing machine (Shimadzu).
Creep experiment
Specimens in the creep experiment were treated identically to those in the stress relaxation experiment regarding clamping mode, temperature, data acquisition and preconditioning. Stress was given to the specimens at a speed of 0.5 GPa/min. When strain reached 8.63%, stress was 0.4 MPa and 0.31 MPa in the sciatic nerve and amniotic membrane groups, respectively. At the end of the experiment, the creep data and curve were automatically output using the automatic control electronic universal testing machine (Shimadzu).
Scanning electron microscopy for ultrastructural observation of human sciatic nerve and amniotic tissue cross-sections
Tissue specimens from each group were analyzed, before and after stress relaxation and creep experimentation, and were randomly selected (n = 1/group). All specimens were cut into 5 mm segments, pre-fixed in 4% glutaraldehyde, post-fixed with 1% osmium tetroxide, dehydrated with acetone gradient, critical point dried and gold sputter- coated in a vacuum. Nerve and amniotic tissue cross- sections were observed using a field emission scanning electron microscope (LEO, Schwerte, Germany) to visualize nerve cell, myelin sheath, axon and nerve basement membrane structures.
Statistical analysis
Using biomechanical calculation software (Department of Engineering Mechanics, Jilin University, China), the stress relaxation and creep data were normalized to establish the normalized stress relaxation function and creep function equations. Data were expressed as means ± SD and analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Data difference between groups was compared using one-way analysis of variance and the Sceffe method, paired t-test. A P value less than 0.05 was considered a significant difference.
Footnotes
Conflicts of interest: None declared.
Ethical approval: The experiment was approved by the Ethics Committee, China-Japan Union Hospital of Jilin University in China.
(Reviewed by Finnie E, Norman C, Chen ZG, Jiang XM)
(Edited by Wang J, Yang Y, Li CH, Song LP)
REFERENCES
- 1.Mohammad J, Shenaq J, Rabinovsky E, et al. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg. 2000;105(2):660–666. doi: 10.1097/00006534-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Mligiliche N, Endo K, Okamoto K, et al. Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J Biomed Mater Res. 2002;63(5):591–600. doi: 10.1002/jbm.10349. [DOI] [PubMed] [Google Scholar]
- 3.Lu JZ, Jiang JJ, Xu JG, et al. Effects of chitosancollagen betamethasone dipropionate film on nerve scarring and regeneration of peripheral nerves in rats following injuries. neural injury and functional reconstruction. Shenjing Sunshang yu Gongneng Chongjian. 2011;6(4):241–244. [Google Scholar]
- 4.O’Neill AC, Randolph MA, Bujold KE, et al. Photochemical sealing improves outcome following peripheral neurorrhaphy. J Surg Res. 2009;151(1):33–39. doi: 10.1016/j.jss.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Kadam SS, Sudhakar M, Nair PD, et al. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy. 2010;12(8):982–991. doi: 10.3109/14653249.2010.509546. [DOI] [PubMed] [Google Scholar]
- 6.Henry FP, Goyal NA, David WS, et al. Improving electrophysiologic and histologic outcomes by photochemically sealing amnion to the peripheral nerve repair site. Surgery. 2009;145(3):313–321. doi: 10.1016/j.surg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Cheng FC. Enhancement of regeneration with glia cell line-derived neurotrophic factor-transduced human amniotic fluid mesenchymal stem cells after sciatic nerve crush injury. J Neurosurg. 2010;112(4):868–879. doi: 10.3171/2009.8.JNS09850. [DOI] [PubMed] [Google Scholar]
- 8.Kadam SS, Sudhakar M, Nair PD, et al. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy. 2010;12(8):982–991. doi: 10.3109/14653249.2010.509546. [DOI] [PubMed] [Google Scholar]
- 9.Wolford LM, Rodrigues DB. Autogenous grafts/allografts/ conduits for bridging peripheral trigeminal nerve gaps. Atlas Oral Maxillofac Surg Clin North Am. 2011;19(1):91–107. doi: 10.1016/j.cxom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Meng H, Li M, You F, et al. Assessment of processed human amniotic membrane as a protective barrier in rat model of sciatic nerve injury. Neurosci Lett. 2011;27(496):48–53. doi: 10.1016/j.neulet.2011.03.090. [DOI] [PubMed] [Google Scholar]
- 11.Wolbank S, Peterbauer A, Fahrner M, et al. Dose-dependent immunomodulatory effect of human stem cells from amnioticmembrane: A comparison with human mesenchymal stem cells from adiposetissue. Tissue Eng. 2007;13(6):1173–1183. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kang HM, Kim H, et al. Ex vivo characteristics of human amniotic membrane-derived stem cells. Cloning Stem Cells. 2007;9(4):581–594. doi: 10.1089/clo.2007.0027. [DOI] [PubMed] [Google Scholar]
- 13.Schmid M, Fellermann K, Fritz P, et al. Attenuated induction of epithelial and leukocyte serine antiproteases elafin and secretory leukocvte protease inhibitor in Crohn's disease. J Leukoc Biol. 2007;81(4):907–915. doi: 10.1189/jlb.0906581. [DOI] [PubMed] [Google Scholar]
- 14.Mligiliche N, Endo K, Okamoto K, et al. Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J Biomed Mater Res. 2002;63(5):591–600. doi: 10.1002/jbm.10349. [DOI] [PubMed] [Google Scholar]
- 15.Mohammad J, Shenaq J, Rabinovsky E, et al. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg. 2000;105(2):660–666. doi: 10.1097/00006534-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Ma HS, Zhang ZJ, Li XH. Experimental study on viscoelasticity of the upper branch of fetal brachial plexus. Zhongguo Shengwu Yixue Gongcheng Xuebao. 2004;23(3):274–277. [Google Scholar]


