Abstract
Trigeminal neuralgia is a syndrome due to dysfunctional hyperactivity of the trigeminal nerve, and is characterized by a sudden, usually unilateral, recurrent lancinating pain arising from one or more divisions of the nerve. The most accepted pathogenetic mechanism for trigeminal neuralgia is compression of the nerve at its dorsal root entry zone or in its distal course. In this paper, we report four cases with trigeminal neuralgia due to an unknown mechanism after an intracranial intervention. The onset of trigeminal neuralgia after surgical interventions that are unrelated to the trigeminal nerve suggests that in patients with greater individual susceptibility, nerve contact with the vascular structure due to postoperative pressure and changes in cerebrospinal fluid flow may cause the onset of pain.
Keywords: neural regeneration, peripheral nerve injury, asymptomatic vascular compression of the cranial nerves, cranial nerves, dorsal root entry zone, dorsal root exit zone, etiology, hyperactive dysfunction syndrome, individual susceptibility, risk factors, trigeminal neuralgia, neuroregeneration
Research Highlights
-
(1)
Contact and/or compression of the trigeminal nerve does not always result in trigeminal neuralgia.
-
(2)
The pathophysiology of trigeminal neuralgia still needs to be elucidated. Hypertension, atherosclerotic vascular changes and aging are important risk factors for trigeminal neuralgia.
-
(3)
This paper reports four cases of trigeminal neuralgia of unknown etiology and concludes that subjects with greater individual susceptibility are more prone to develop trigeminal neuralgia in the presence of vascular contact and/or compression. This finding provides new insight into the pathophysiology of trigeminal neuralgia.
INTRODUCTION
Trigeminal neuralgia is a syndrome resulting from dysfunctional hyperactivity of the trigeminal nerve[1], and is defined as a sudden, usually unilateral, recurrent lancinating pain arising from one or more divisions of the nerve[2,3]. The incidence of trigeminal neuralgia is 4.5 per 100 000 people[4]. It is found more often in women than in men (ratio 1.74:1) and is most common from 50 to 69 years of age[5]. Hypertension, arteriosclerotic vascular changes, aging, individual sensitivity, familial history, and race are important risk factors for trigeminal neuralgia. Genetic transmission of trigeminal neuralgia has also been reported, and there is autosomal dominant inheritance in 1–2% of all cases[6,7]. Clinical observations have shown that stress and emotional factors can also precipitate various forms of neuralgia[8]. Pain associated with trigeminal neuralgia is most frequently due to arterial or venous compression of the trigeminal nerve near the root entry zone in the brainstem[3,4,9]. However, the etiology is still undetermined in a small proportion of patients[10] because contact and/or compression do not occur in all trigeminal neuralgia cases[10,11,12]. Other causes of trigeminal neuralgia include multiple sclerosis, masses in the posterior fossa or supratentorial region, central nervous system infections or local abscess[13], abnormalities of the craniovertebral junction such as Chiari malformations, bony disorders such as Paget's disease, osteogenesis imperfecta, and arteriovenous malformations which cause demyelination throughout the length of the nerve[2,4,9,14].
This paper reports four cases of trigeminal neuralgia occurring after intracranial intervention. Although the patients did not have trigeminal neuralgia in the preoperative period, trigeminal neuralgia arose due to an unknown mechanism after surgery. We hypothesize that risk factors determine individual susceptibility of developing trigeminal neuralgia.
CASE REPORTS
Case 1
A 61-year-old male was admitted to the neurosurgery department due to right trigeminal neuralgia in the region of the first division of the trigeminal nerve. The patient's history included an endovascular intervention for right occipital dural arteriovenous fistula 10 years prior in another institution (Figure 1A). After 6 months of this intervention, the patient had right facial pain. Alcohol blockade was applied to the first branch of the right trigeminal nerve. On follow-up cerebral angiography 10 years after surgery, enlargement of the right superior cerebellar artery, compression of the trigeminal nerve, and feeding of the right occipital dural arteriovenous fistula were observed (Figure 1B). Due to the patient's persistent complaint of facial pain, the daily dosage of carbamazepine was raised from 600 mg to 1 200 mg. The trigeminal neuralgia resolved 2 weeks later.
Figure 1.
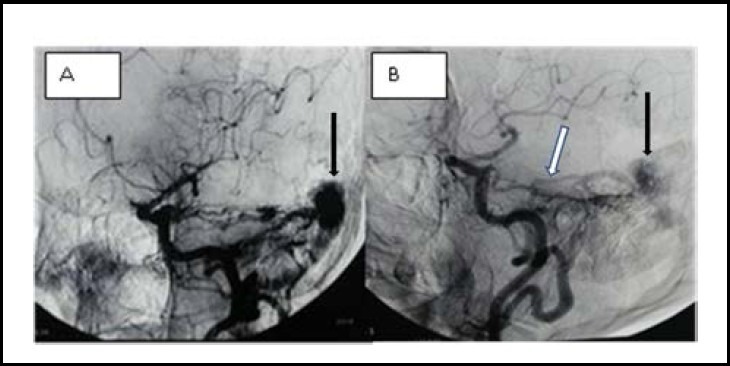
Angiography of a dural arteriovenous fistula (AVF) before (A) and 10 years after intervention for right occipital AVF (B).
White arrow shows the enlargement of the superior cerebellar artery which feeds the residual occipital dural AVF 10 years after surgery. While black arrow in figure A shows the AVF, and in figure B it shows the residual occipital dural AVF.
Case 2
A 25-year-old female was admitted to our department because of pain and numbness in both arms, as well as neck pain. Upon cervical MRI, a Chiari malformation was discovered (Figure 2A). The patient underwent uncomplicated suboccipital craniectomy, C1 laminectomy, and duraplasty for decompression (Figure 2B). During the early postoperative period, the patient had trigeminal neuralgia at the second division of the right trigeminal nerve. Carbamazepine therapy was applied with a step-by-step dose increase up to 1 200 mg per day, and the pain resolved 4 months after surgery.
Figure 2.
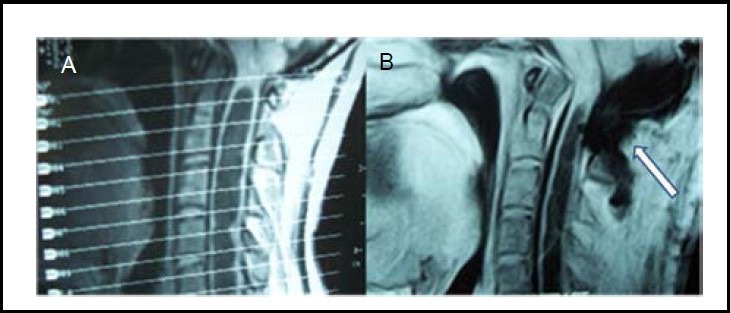
Preoperative (A) and postoperative (B) sagittal section of cervical MRI 1 month after suboccipital craniectomy.
White arrow shows suboccipital decompression.
Case 3
A 50-year-old female was admitted to the neurosurgery department with headache, nausea and vomiting. Cranial MRI revealed a left cerebellar large intraparenchymal tumor (Figure 3A). A left occipital craniotomy was performed and the tumor was completely excised (Figure 3B). On postoperative day 6, the patient developed left facial pain with trigeminal neuralgia characteristics at the first division of the trigeminal nerve. Carbamazepine therapy was applied at a dose of 800 mg per day, and the pain resolved after 3 months of treatment.
Figure 3.
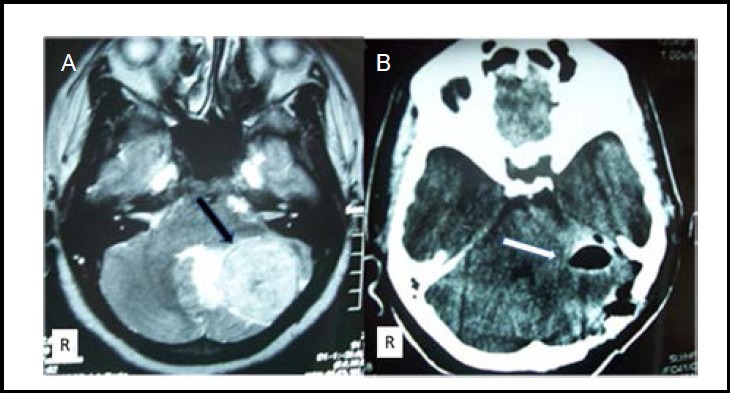
Preoperative cranial MRI (A) and postoperative cranial computed tomography image 6 days after left occipital craniotomy (B).
Black arrow shows cerebellar tumor. White arrow shows total resection of tumor. R: Right.
Case 4
A 31-year-old female was admitted to the neurosurgery department with headache, dizziness, pain and numbness in both arms. The patient's history included three surgeries for Chiari malformation. Cervical MRI showed basilar invagination (Figure 4A) and C1–2 anterior transarticular screw fixation was performed (Figure 4B). Although her complaints associated with the Chiari malformation resolved, she developed left facial pain with trigeminal neuralgia characteristics at the second division of the right trigeminal nerve on postoperative day 1. Carbamazepine therapy was applied with a step-by-step dose increase up to 600 mg per day, and the pain resolved 3 months after surgery. While the trigeminal neuralgia disappeared, occipital neuralgia began after cessation of carbamazepine therapy. The patient continued to use a low dosage of carbamazepine for occipital neuralgia.
Figure 4.
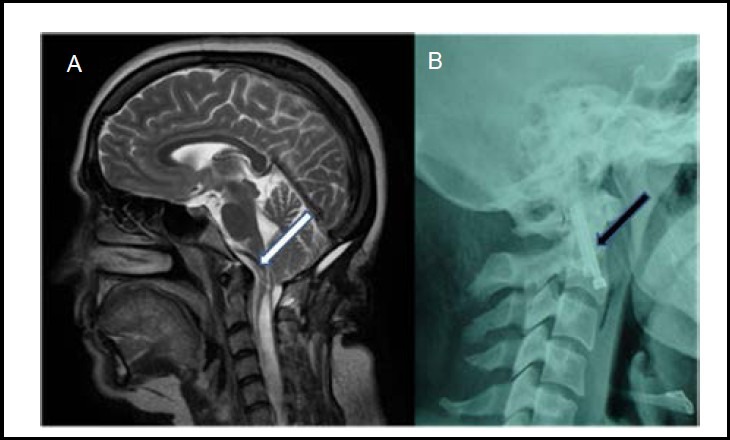
Preoperative cervical MRI (A) and cervical X-ray images after anterior transarticular screw fixation at C1–2 (B).
White arrow shows pressure from the odontoid tip on the brainstem. Black arrow shows the C1–2 transarticular screw fixation.
DISCUSSION
The pathophysiology of trigeminal neuralgia is not fully understood, although existing hypotheses point to the increased excitability of afferent trigeminal nerve afferents[15]. The most widely accepted theory is compression of the trigeminal root adjacent to the pons[4,5,16] at the dorsal root entry zone. The dorsal root entry zone is the junction between the peripheral and central myelin of Schwann cells and is approximately 1.0–2.5 mm in length[17]. This region is also referred to as the Obersteiner-Redlich zone[18]. The central branches of the unipolar ganglion cells enter the pons and arrive at the brainstem and spinal nuclei through this transition zone[15]. The most common etiology of trigeminal neuralgia (80–90%) is compression of one or more arteries or veins in the dorsal root entry zone of the trigeminal nerve[4,16]. Generally, the anterior inferior cerebellar artery, posterior inferior cerebellar artery and vertebral arteries are responsible for this compression[19]. These anatomical variations cause ectopic, spontaneous nerve impulses in some of the trigeminal nerve afferents[4,15,19]. Compression of this region causes nerve root demyelination[4]. This compression may also be caused by tumors, saccular aneurysms, osteomas and arteriovenous malformations in the posterior fossa[2,4,5,15]. Nurmikko and Eldbridge[15] reported that this nerve compression rarely occurs in the more peripheral regions of the dorsal root entry zone. In the demyelinating disease multiple sclerosis, demyelination of this region has been suggested to cause trigeminal neuralgia[4]. An important unresolved question is whether all contact and/or compression of the nerve produces trigeminal neuralgia, as anatomical and radiological studies show that neurovascular contact does not necessarily result in the condition[10,11,12,20,21,22]. Ueda et al[10] examined a total of 290 trigeminal nerves in a group of 145 patients using three-dimensional spoiled gradient recalled acquisition in steady state GRASS imaging. Four out of 145 patients had trigeminal neuralgia. The authors identified neurovascular contact in 83 out of 290 nerves, 43 on the right side and 40 on the left side, and noted that multivessel contact or deviation commonly seen in asymptomatic nerves is important. In addition, Meaney et al[11] demonstrated neurovascular compression in trigeminal neuralgia with MRI and stated that asymptomatic nerves can show vascular compression or multi-level contact. In a cadaveric study, Hamlyn[22] examined 35 fresh cadavers who were not known to suffer from neurological disease and found that 14% had neurovascular contact, and an additional 26% had vessels “near” the nerve.
Vascular pathologies, such as arteriovenous malformation and arteriovenous fistula, are well known causes of trigeminal neuralgia that result in compression and/or contact of the trigeminal nerve. These lesions are usually treated successfully[23,24]. However, trigeminal neuralgia after endovascular treatment is uncommon. Sumioka et al[23] treated a 66-year-old male who presented with typical right trigeminal neuralgia due to arteriovenous malformation by transposition of the superior cerebellar artery after posterior fossa exploration. Additionally, Ott and colleagues[24] treated a dural arteriovenous fistula by embolization, and the trigeminal neuralgia symptoms resolved completely. However, follow-up cerebral angiography of our first case showed enlargement of the right superior cerebellar artery, which feeds the dural arteriovenous fistula. This enlargement may explain the onset of facial pain 6 months after the endovascular surgery. Specifically, the artery may have been contacting the nerve with each pulsation. In addition to the enlargement of the artery, individual susceptibility may also have contributed to the onset of neuralgia.
Direct compression of the trigeminal nerve by intracranial masses is reported as another cause of trigeminal neuralgia[3,19,25]. Perrini et al[3] reported trigeminal neuralgia resolving after gross total resection of the paramedian tentorial meningioma in a 50-year-old woman. Cirak et al[25] surgically treated a 30-year-old female with trigeminal neuralgia who had an epidermoid tumor in the posterior fossa. Harrison and colleagues[19] surgically treated a patient who had hemifacial spasms due to a large distant ipsilateral posterior fossa meningioma. In our third case, the patient's complaints occurred on postoperative day 6 after removal of the left-side cerebellar tumor. During surgery, there was no contact with the trigeminal nerve. Therefore, the complication could not have resulted from trigeminal nerve injury. Direct compression of the trigeminal nerve is not the only cause of trigeminal neuralgia, and other hypotheses have been proposed[2,9,14,26,27,28] for trigeminal neuralgia onset. Cheng and Chang[9] surgically treated a 46-year-old female with trigeminal neuralgia caused by contralateral supratentorial meningioma, and Eftekhar et al[26] surgically treated a patient who presented with vestibular schwannoma with contralateral facial pain. The authors proposed that displacement and distortion of the brainstem and compression of the contralateral trigeminal nerve in Meckel's cave by large mass lesions may have resulted in the atypical presentation[9,26]. Postoperative cranial tomography was performed and brainstem displacement was not observed. The lack of direct compression suggests that other pathophysiological factors underlay the neuralgia. Postoperative pressure and changes in cerebrospinal fluid flow in the posterior fossa after surgery may cause the nerve to contact the vascular structure. Thus, individual susceptibility may contribute to the onset of trigeminal neuralgia.
Papanastassiou and colleagues[2] treated a 63-year-old male who had a Chiari type I malformation with syrinx and presented with trigeminal neuralgia. After uncomplicated suboccipital craniectomy, C1 laminectomy and duraplasty for decompression, the facial pain resolved. The authors suggested four mechanisms for the appearance of trigeminal neuralgia in Chiari malformations: (1) vascular compression of the nerve root entry zone or small posterior fossa; (2) stretching of the trigeminal nerve, resulting in demyelination; (3) microischemic changes; and (4) brainstem compression. The patient in our second case suffered from facial pain early in the postoperative period, which resolved with carbamazepine therapy after 4 months. Thus, the small posterior fossa, brainstem compression, demyelination and microischemic changes cannot underlie this complication. The occurrence and resolution of symptoms without a clear cause suggests that individual susceptibility may contribute to the condition. Anatomical and radiological studies have shown that not all neurovascular contact results in trigeminal neuralgia[10,11,12,20,21,22]. Therefore, we suggest that in patients with elevated individual susceptibility, contact of the nerve with the vascular structure due to postoperative pressure and cerebrospinal fluid flow changes may cause the onset of pain, which is temporary and disappears. Gnanalingham et al[14] reported a case of a 31-year-old male presenting with trigeminal neuralgia secondary to Chiari malformation and hydrocephalus. He was treated with a ventriculoperitoneal shunt, and the trigeminal neuralgia disappeared. The authors suggested the symptoms and causes of trigeminal neuralgia may have been due to neurovascular conflict, related to raised intracranial pressure from the hydrocephalus or small posterior fossa. Schwartz and colleagues[28] reported a case of a 36-year-old woman with trigeminal neuralgia who had a lumbar spine tumor and communicating hydrocephalus. Facial pain resolved after debulking of the tumor. The authors hypothesized that local mechanical changes in the hydrocephalus caused compression around Meckel's cave, resulting in trigeminal neuralgia in their patient. Additionally, Monzillo et al[27] reported a case of a 54-year-old female with paroxysmal hemicrania-tic secondary to Chiari malformation with syringomyelia. The patient was treated with carbamazepine, and the authors suggested compression or deformity of the trigeminal nucleus in the brainstem due to a small posterior fossa as one possible explanation. An alternative explanation is that the trigeminal pathways of pain, including the C2 and C3 levels, were affected by the syringomyelia. The patient in our fourth case suffered from facial pain of unknown etiology in the early postoperative period, which was resolved with carbamazepine therapy after 3 months. We hypothesize that in patients with greater individual susceptibility, contact of the nerve with the vascular structure, resulting from postoperative pressure and cerebrospinal fluid flow changes, may cause the onset of pain. Greater individual susceptibility probably contributed to earlier pain development, and symptoms were relieved when neurovascular contact and/or compression disappeared after the pressure and cerebrospinal fluid flow changes returned to normal.
If contact and/or compression of the trigeminal nerve necessarily results in neuralgia, asymptomatic patients with vascular compressions would not have been observed. A literature review of trigeminal neuralgia revealed anatomical and radiological relationships among symptoms, the vessels and the trigeminal nerve[10,11,12,20,21,22]. Klun and Prestor[12] studied neurovascular relationships in 130 trigeminal root entry zones of 65 cadavers with no history of facial or trigeminal pain disorders during their lifetime. A total of 42 examples of contact with the root entry zone and 10 examples of compression by arteries or veins were identified. The authors suggested that vascular compressions are the predominant, but not sole, cause of trigeminal neuralgia. In another anatomical study, Rames and Premkumar[20] studied 50 fresh cadavers asymptomatic for trigeminal neuralgia or other facial pain during their lifetime. They examined 100 trigeminal root entry zones using either a transtentorial (34 cadavers) or infratentorial approach (16 cadavers). A vascular relationship was observed in 39 root entry zones (39%), and they concluded that a neurovascular relationship at the trigeminal root entry zone is not uncommon in an asymptomatic population[20]. Fukuda and colleagues[21] noted that neurovascular contact was also observed in 15% of the asymptomatic nerves on MRI. Yang et al[1] reported that a genetic tendency could also determine whether neurovascular compression results in clinical symptoms of trigeminal neuralgia, because 25% of the study population had trigeminal nerve compression without symptoms of trigeminal neuralgia[29]. Although trigeminal neuralgia may be caused by neurovascular compression alone, we consider that differences in individual susceptibility may underlie the observed variation in the development of trigeminal neuralgia.
CONCLUSION
(1) The pathophysiology of trigeminal neuralgia is still unclear and requires further study. Consequently, definitive causes cannot be identified. The most commonly accepted mechanism of trigeminal neuralgia onset is compression of the nerve at its dorsal root entry zone or in its distal trajectory. (2) Subjects with greater individual susceptibility are more prone to develop trigeminal neuralgia. (3) Based on our study of a small number of patients, trigeminal neuralgia after intracranial operations in susceptible patients is transient. Carbamazepine is the drug of choice for treatment, and can be used for a period of time until the pain resolves. (4) Additional clinical data and histopathological studies are required to clarify the pathophysiology of trigeminal neuralgia.
Footnotes
Conflicts of interest: None declared.
(Reviewed by Patel B, Wysong S, Qu LT, Dong CX)
(Edited by Li CH, Song LP)
REFERENCES
- 1.Yang KH, Na JH, Kong DS, et al. Combined hyperactive dysfunction syndrome of the cranial nerves. J Korean Neurosurg Soc. 2009;46(4):351–354. doi: 10.3340/jkns.2009.46.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papanastassiou AM, Schwartz RB, Friedlander RM. Chiari I malformation as a cause of trigeminal neuralgia: case report. Neurosurgery. 2008;63(3):E614–615. doi: 10.1227/01.NEU.0000324726.93370.5C. [DOI] [PubMed] [Google Scholar]
- 3.Perrini P, Rasile F, Leggate J. Trigeminal neuralgia as initial symptom of paramedian tentorial meningioma. Neurol Sci. 2009;30(1):81–83. doi: 10.1007/s10072-009-0014-1. [DOI] [PubMed] [Google Scholar]
- 4.Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347–2360. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- 5.Delzell JE, Jr, Grelle AR. Trigeminal neuralgia. New treatment options for a well-known cause of facial pain. Arch Fam Med. 1999;8(3):264–268. doi: 10.1001/archfami.8.3.264. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Yu CY, Huang L, et al. Familial neuralgia of occipital and intermedius nerves in a Chinese family. J Headache Pain. 2011;12(4):497–500. doi: 10.1007/s10194-011-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riederer F, Sandor PS, Linnebank M, et al. Familial occipital and nervus intermedius neuralgia in a Swiss family. J Headache Pain. 2010;11(4):335–338. doi: 10.1007/s10194-010-0207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter JB, Patrinely JR, Jankovic J, et al. Familial hemifacial spasm. Arch Ophthalmol. 1990;108(2):249–250. doi: 10.1001/archopht.1990.01070040101040. [DOI] [PubMed] [Google Scholar]
- 9.Cheng WC, Chang CN. Trigeminal neuralgia caused by contralateral supratentorial meningioma. J Clin Neurosci. 2008;15(10):1162–1163. doi: 10.1016/j.jocn.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Ueda F, Suzuki M, Fujinaga Y, et al. In vivo anatomical analysis of arterial contact with trigeminal nerve: detection with three-dimensional spoiled grass imaging. Br J Radiol. 1999;72(861):838–845. doi: 10.1259/bjr.72.861.10645189. [DOI] [PubMed] [Google Scholar]
- 11.Meaney JF, Eldridge PR, Dunn LT, et al. Demonstration of neurovascular compression in trigeminal neuralgia with magnetic resonance imaging. Comparison with surgical findings in 52 consecutive operative cases. J Neurosurg. 1995;83(5):799–805. doi: 10.3171/jns.1995.83.5.0799. [DOI] [PubMed] [Google Scholar]
- 12.Klun B, Prestor B. Microvascular relations of the trigeminal nerve: an anatomical study. Neurosurgery. 1986;19(4):535–539. doi: 10.1227/00006123-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Bekar A, Kocaeli H, Yilmaz E, et al. Trigeminal neuralgia caused by a pontine abscess: case report. Neurosurgery. 2004;55(6):1434. [PubMed] [Google Scholar]
- 14.Gnanalingham K, Joshi SM, Lopez B, et al. Trigeminal neuralgia secondary to Chiari's malformation-treatment with ventriculoperitoneal shunt. Surg Neurol. 2005;63(6):586–589. doi: 10.1016/j.surneu.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Nurmikko TJ, Eldridge PR. Trigeminal neuralgia- pathophysiology, diagnosis and current treatment. Br J Anaesth. 2001;87(1):117–132. doi: 10.1093/bja/87.1.117. [DOI] [PubMed] [Google Scholar]
- 16.Janetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1):159–162. doi: 10.3171/jns.1967.26.1part2.0159. [DOI] [PubMed] [Google Scholar]
- 17.Prasad S, Galetta S. Trigeminal neuralgia: historical notes and current concepts. Neurologist. 2009;15(2):87–94. doi: 10.1097/NRL.0b013e3181775ac3. [DOI] [PubMed] [Google Scholar]
- 18.Alfieri A, Fleischhammer J, Strauss C, et al. The central myelin-peripheral myelin transitional zone of the nervus intermedius and its implications for microsurgery in the cerebellopontine angle. Clin Anat. 2012;25(7):882–888. doi: 10.1002/ca.22025. [DOI] [PubMed] [Google Scholar]
- 19.Harrison GS, Chovan P, Lee JH. Hemifacial spasm due to a large distant ipsilateral posterior fossa meningioma. Skull Base Surg. 2000;10(1):43–45. doi: 10.1055/s-2000-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rames VG, Premkumar G. An anatomical study of the neurovascular relationships at the trigeminal root entry zone. J Clin Neurosci. 2009;16(7):934–936. doi: 10.1016/j.jocn.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda H, Ishikawa M, Okumura R. Demonstration of neurovascular compression in trigeminal neuralgia and hemifacial spasm with magnetic resonance imaging: comparison with surgical findings in 60 consecutive cases. Surg Neurol. 2003;59(2):93–99. doi: 10.1016/s0090-3019(02)00993-x. [DOI] [PubMed] [Google Scholar]
- 22.Hamlyn PJ. Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. 2. Neurovascular compression of the trigeminal nerve in cadaveric controls and patients with trigeminal neuralgia: quantification and influence of method. Clin Anat. 1997;10(6):380–388. doi: 10.1002/(SICI)1098-2353(1997)10:6<380::AID-CA2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Sumioka S, Kondo A, Tanabe H, et al. Intrinsic arteriovenous malformation embedded in the trigeminal nerve of a patient with trigeminal neuralgia. Neurol Med Chir (Tokyo) 2011;51(9):639–641. doi: 10.2176/nmc.51.639. [DOI] [PubMed] [Google Scholar]
- 24.Ott D, Bien S, Krasznai L. Embolization of a tentorial dural arterio-venous fistula presenting as atypical trigeminal neuralgia. Headache. 1993;33(9):503–508. doi: 10.1111/j.1526-4610.1993.hed3309503.x. [DOI] [PubMed] [Google Scholar]
- 25.Cirak B, Kiymaz N, Arslanoglu A. Trigeminal neuralgia caused by intracranial epidermoid tumor: report of a case and review of the different therapeutic modalities. Pain Physician. 2004;7(1):129–132. [PubMed] [Google Scholar]
- 26.Eftekhar B, Gheini M, Ghodsi M, et al. Vestibular schwannoma with contralateral facial pain-case report. BMC Neurol. 2003;3:2. doi: 10.1186/1471-2377-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monzillo P, Nemoto P, Costa A, et al. Paroxysmal hemicrania-tic and Chiari I malformation: an unusual association. Cephalalgia. 2007;27(12):1408–1412. doi: 10.1111/j.1468-2982.2007.01362.x. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz NE, Rosenberg S, So YT. Action at a distance: a lumbar spine tumor presenting as trigeminal neuralgia. Clin Neurol Neurosurg. 2006;108(8):806–808. doi: 10.1016/j.clineuro.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Jia DZ, Li G. Bioresonance hypothesis: a new mechanism on the pathogenesis of trigeminal neuralgia. Med Hypotheses. 2010;74(3):505–507. doi: 10.1016/j.mehy.2009.09.056. [DOI] [PubMed] [Google Scholar]


