Abstract
Voxel-based morphometry is gaining considerable interest for studies examining Parkinson's disease dementia patients. In this study, 12 patients with clinically defined Parkinson's disease and dementia and 12 non-demented patients with Parkinson's disease were examined using a T1WI three-dimensional fast spoiled gradient echo sequence. Gray matter data were analyzed using a voxel-based morphometry method and independent sample t-test based on Statistical Parametric Mapping 5 software. Differences in gray matter volume were represented with statistical parametric mapping. Compared with Parkinson's disease patients without dementia, decreased gray matter volume in Parkinson's disease dementia patients was observed in the bilateral superior temporal gyrus, bilateral posterior cingulate and left cingulate gyrus, right parahippocampal gyrus and hippocampus, right precuneus and right cuneus, left inferior frontal gyrus and left insular lobe. No increased gray matter volume was apparent. These data indicate that gray matter atrophy in the limbic system and cerebral neocortex is related to the presence of dementia.
Keywords: neural regeneration, neurodegenerative diseases, neuroimaging, voxel-based morphometry, Parkinson's disease, dementia, gray matter abnormality, limbic system, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
The voxel-based morphometry method is an objective, comprehensive, accurate and quantitative approach for the assessment of whole brain structure, and is regarded as the preferred method to detect the changes in brain morphology.
-
(2)
There are varied conclusions in the measurement of brain atrophy using voxel-based morphometry in movement disorder diseases such as Parkinson's disease and other nervous system diseases.
-
(3)
We performed a prospective randomized controlled study to analyze the abnormal gray matter areas and distribution characteristics in patients with Parkinson's disease dementia using voxel-based morphometry. The occurrence of Parkinson's disease dementia was closely related to the limbic system and new cortex atrophy in the brain.
INTRODUCTION
Parkinson's disease is a multi-system disease, with characteristics of typical motor symptoms and various non-motor symptoms, abnormal behavior, personality changes and cognitive impairment, which may lead to the occurrence of dementia[1,2,3]. Mild cognitive impairment is a preliminary indicator of dementia, and cognitive impairment can be detected in the early stage of Parkinson's disease[4,5]. However, this may be occasionally neglected due to inapparent cognitive impairment and clinical manifestations[6]. It is estimated that 70–80% of Parkinson's disease patients eventually develop dementia[7], resulting in an annual rate of 10% for Parkinson's disease dementia[8]. The risk of dementia in Parkinson's disease patients is six times higher than in the healthy population[9]. Thus, Parkinson's disease dementia has serious impact on social function and quality of life in middle-aged and elderly people.
The etiology of Parkinson's disease dementia remains unclear, but may be attributed to age, education level, course of disease and concomitant symptoms such as depression, visual hallucinations, memory and language[2,7,10,11,12,13,14]. The pathology of Parkinson's disease dementia remains controversial, although it was previously reported to be associated with degeneration of subcortical structure, especially the substantia nigra pars compacta[15], basal nuclei, amygdala and thalamus[16], basal ganglia[17] and nucleus coeruleus deletions[18]. In addition, the cerebral cortex structure changes are regarded as a pathological cause, and the occurrence of dementia was related with Lewy bodies in the cortex[19,20].
In a 4-year longitudinal follow-up, Janvin et al[21] reported that the prevalence of dementia in Parkinson's disease patients with mild cognitive impairment was significantly higher than that in patients without cognitive impairment. The cognitive impairment in Parkinson's disease patients may aggravate with disease duration, and eventually develops into dementia. However, some patients do not develop dementia or show no significant dementia, despite evidence of cognitive impairment at the early stage[9,21,22]. The pathophysiology of cognitive functional impairment in Parkinson's disease is very complex, and includes dopaminergic and cholinergic neurons and other neurotransmitter systems, as well as diffuse nerve degeneration, which may involve neuropathological changes in Parkinson's disease and Alzheimer's disease[23,24].
Conventional imaging methods are not completely effective in the diagnosis of Parkinson's disease, and morphological changes may appear at the advanced stage, including a fuzzy and narrowed substantia nigra zona compacta, and a decrease in signal intensity and volume measurement[7]. Susceptibility-weighted imaging can quantitatively detect iron content in the substantia nigra zona compacta, thus contributing to the diagnosis and monitoring of disease progression[25]. Functional imaging diagnosis methods include positron emission tomography, single photon emission computed tomography, functional MRI and diffusion tensor imaging. Positron emission tomography and single photon emission CT are sensitive approaches to determine the metabolism and transport of dopaminergic neurons in the striatum, allowing early diagnosis, clinical classification and treatment of Parkinson's disease[16]. The functional MRI and diffusion tensor imaging can also detect changes in microstructure, metabolism, biochemistry and other neuropathological damage in the brain prior to the appearance of morphological changes, which helps to understand the pathological mechanism[23]. Functional imaging is necessary for the assessment of brain function and the early diagnosis of Parkinson's disease. However, the sensitivity and specificity need to be improved.
Voxel-based morphometry is a new method used to detect morphological changes in Parkinson's disease patients with and without dementia[15,26]. This technique can identify morphological changes in whole brain regions in vivo[27,28,29,30], and is a comprehensive and objective image technique for analysis of brain structure, with no unbiased statistics and no need of priori assumption.
The aim of our study was to quantitatively analyze the gray matter structure in Parkinson's disease patients with or without dementia using the voxel-based morphometry method, and to preliminarily explore the brain structural changes in a broader attempt to investigate the morphological changes and pathological mechanism of gray matter of the brain associated with Parkinson's disease and dementia.
RESULTS
Quantitative analysis of subjects
Twenty-four patients with Parkinson's disease were included in this study, including 12 demented cases (Parkinson's disease dementia group) and 12 non-demented cases (Parkinson's disease group). All patients were involved in the final analysis without any loss.
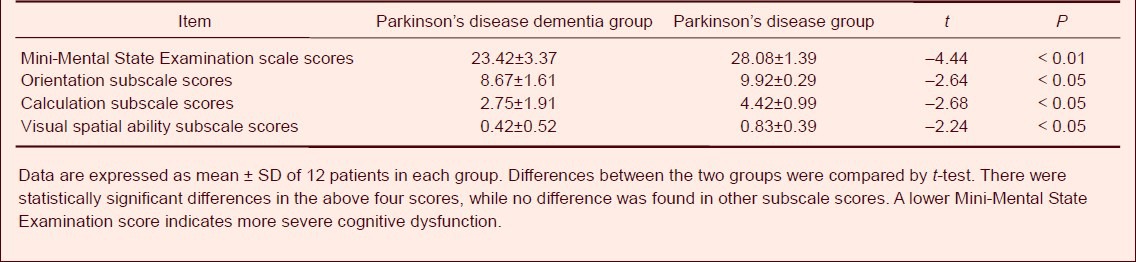
Baseline information of subjects
The 24 patients with Parkinson's disease were evaluated using the following examinations. The Parkinson's disease dementia group was stage 2–4 by Hoehn & Yahr staging scale, the Mini-Mental State Examination (MMSE) score was 15–25, the Montreal cognitive assessment (MoCA) score was 9–17, the Unified Parkinson's Disease Rating Scale (UPDRS-III) score was 21–71, the activity of daily living (ADL) score was 32–77, education level was 0–15 years and the course of disease was 2–13 years. The Parkinson disease group was stage 1–3 by Hoehn & Yahr staging scale, MMSE score was 26–30, MoCA score was 24–29, UPDRS-III score was 3–34, ADL score was 20–30, education level was 5–15 years and the course of disease was 1–11 years. The Parkinson's disease dementia patients showed more severe cognitive impairment and motor system damage, longer course of disease and lower education level than Parkinson's disease patients. There was no significant difference between the two groups with respect to age, gender and non-motor symptom score (P > 0.05; Table 1). In this study, the age was regarded as a covariate in statistical analysis.
Table 1.
Baseline information of Parkinson's disease patients with and without dementia
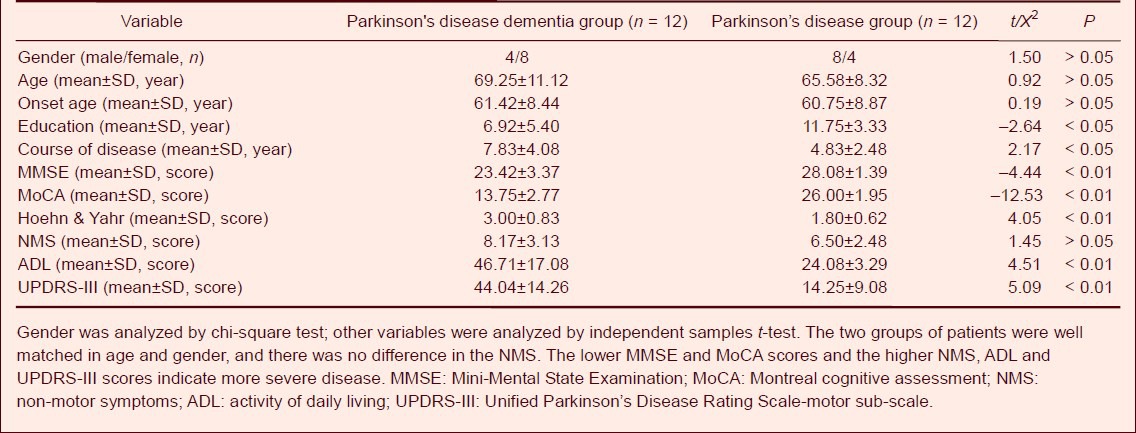
Comparison of cognitive functions in Parkinson's disease patients with and without dementia
The MMSE and MoCA scores in the Parkinson's disease dementia group were significantly decreased compared with Parkinson's disease (P < 0.01). In the MoCA subscale, the scores of visual spatial ability and executive function, naming, attention, language, abstraction, delaying and orientation were significantly lower in the Parkinson's disease dementia group than those in the Parkinson's disease group (P < 0.05 or P < 0.01; Table 2). In the MMSE subscale, the scores of visual spatial ability, calculation ability and orientation ability were also significantly decreased compared with the Parkinson's disease group (P < 0.05). There were no significant differences between the two groups in the other subscale scores (Table 3).
Table 2.
Comparison of Montreal cognitive assessment scale scores in Parkinson's disease patients with and without dementia
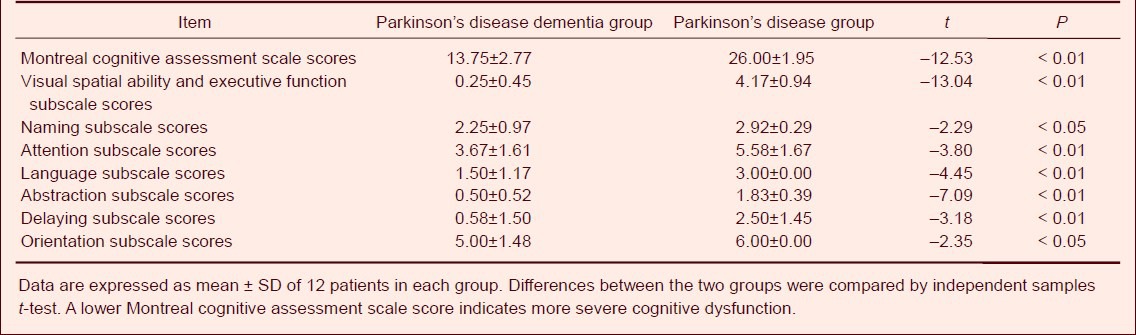
Table 3.
Comparison of Mini-Mental State Examination scale scores in Parkinson's disease patients with and without dementia
Abnormality of gray matter in Parkinson's disease patients with and without dementia
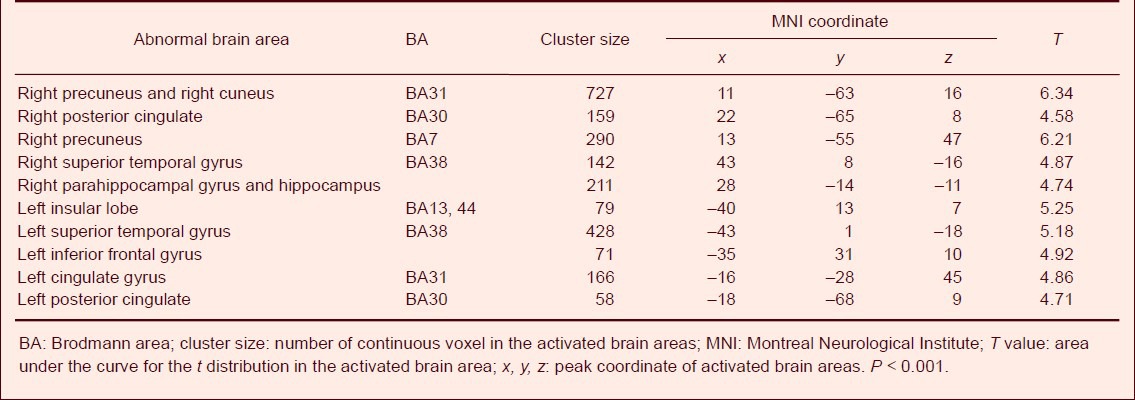
Using the voxel-based morphometry method, MRI three-dimensional fast spoiled gradient echo sequence scanning results showed decreased gray matter volume in Parkinson's disease dementia patients in the bilateral temporal lobe (bilateral superior temporal gyrus and left inferior frontal gyrus), limbic system (bilateral posterior cingulate, left cingulate gyrus, right parahippocampal gyrus and hippocampus), right parietal lobe and occipital lobe (right precuneus and right cuneus) and the left insular lobe compared with Parkinson's disease patients without dementia (Figure 1, Table 4). No apparently increase in gray matter volume was observed.
Figure 1.
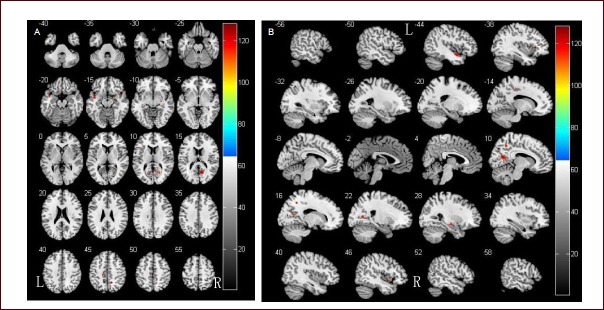
Comparison of gray matter volume in Parkinson's disease patients with and without dementia.
(A) Results of statistical analysis are represented as pseudo-color on axial template brain mapping in the Montreal Neurological Institute (MNI) standard coordinate.
(B) Results of statistical analysis are represented as pseudo-color on sagittal template brain mapping in the MNI standard coordinate.
Two-sample t-test showed that brain gray matter volume was significantly reduced in Parkinson's disease dementia patients.
R: Right; L: left.
Table 4.
Abnormal brain areas with decreased gray matter volume in Parkinson's disease dementia patients compared with Parkinson's disease patients without dementia (age-adjusted)
DISCUSSION
Parkinson's disease dementia is the cognitive dysfunction that occurs at least 1 year after clinical diagnosis of Parkinson's disease, and is characterized by insidious onset and slow progression. It is involved in more than two out of four key cognitive impairments (attention disorder, execution dysfunction, visual spatial dysfunction and free memory dysfunction), and seriously affects the patient's daily life.
The dementia accompanying Parkinson's disease can be defined by any one of the following appearances: apathy, depression or anxiety, hallucinations, delusions or daytime lethargy[31]. Parkinson's disease-related dementia is a subcortical dementia, which is the main performance of early Parkinson's disease, especially execution function. Advanced Parkinson's disease dementia patients exhibit both subcortical dementia and cortical dementia characteristics, mainly in the execution, visual-spatial ability, attention and memory[32,33,34,35,36]. In this study, we collected 12 patients who were clinically diagnosed with Parkinson's disease dementia, and who all presented with more than two cognitive impairments.
Morphological studies have shown that cortical atrophy is closely related to cognitive impairment in Parkinson's disease, and Parkinson's disease patients with cognitive impairment and (or) visual hallucinations are more susceptible to dementia[2,14,15,37]. Compared with normal elderly subjects, Parkinson's disease dementia patients exhibited a wider range of gray matter and subcortical gray matter volume reductions[38,39,40]. In these studies, decreased gray matter reductions were found in the frontal lobe[38,40,41] and temporal lobe, especially in the hippocampus and medial temporal lobe structures such as the amygdala[38,39], parahippocampal gyrus[39,40,41] and fusiform gyrus[39]. A reduction in subcortical gray matter occurred in the caudate nucleus, nucleocapsid, thalamus and nucleus accumbens septum[38,39,40,41], while reduced volumes were also observed in the anterior cingulate gyrus[38,40,41] and the parietal and occipital gray matter[38,39]. Burton et al[39] reported that the reduction in occipital gray matter volume was greater in patients with Parkinson's disease dementia than those without dementia. Summerfield et al[40] also found a greater reduction in gray matter volume in the left superior temporal gyrus and right parahippocampal gyrus in patients with Parkinson's disease dementia compared with Parkinson's disease patients. In support, our experimental findings revealed gray matter atrophy in the occipital and parietal lobe, superior temporal gyrus, limbic and paralimbic system (bilateral cingulate gyrus, right parahippocampal gyrus, hippocampus and left insula lobe). Beyer et al[38] and Nagano-Saito et al[41] found greater gray matter atrophy in patients with Parkinson's disease-related dementia, including the frontal lobe, temporal lobe, hippocampus, parahippocampal gyrus, superior temporal gyrus, anterior cingulate gyrus, parietal lobe, caudate nucleus and thalamus[38,41]. Although there is an inconsistency among these studies, our data support that Parkinson's disease dementia patients show greater cortical and subcortical gray matter loss than Parkinson's disease patients, especially in the frontal lobe, temporal lobe, limbic system, caudate nucleus and thalamus. Therefore, detection of these specific anomalies is helpful for diagnosing dementia in Parkinson's disease patients.
Burton et al[39] compared the atrophy pattern in Parkinson's disease dementia patients with those of Alzheimer's disease and dementia with Lewy bodies, and found a similar atrophy pattern between Parkinson's disease dementia and dementia with Lewy bodies, which involved a relatively complete medial temporal lobe. However, some studies have demonstrated a similar pattern in Alzheimer's disease patients[40,41]. This variation may be related to the clinical definition criteria of dementia. Burton et al[39] insisted on the diagnosis criteria for dementia with Lewy bodies, while some authors have adopted the Diagnostic and Statistical Manual of Mental Disorders-Fourth edition[40,41]. There are many contributing factors underlying the variations in studies of Parkinson's disease dementia, including the number of cases, clinical characteristics and disease severity.
A normal control group was not included in our study, as we compared cerebral gray matter in Parkinson's disease patients with and without dementia in a broader attempt to detect abnormal brain areas that are associated with dementia. The neurological scale showed that the Parkinson's disease dementia group had significantly decreased scores in the subscales of visuospatial and executive function, abstraction, language and attention, while significantly increased scores of UPDRS-III, ADL and Hoehn & Yahr staging scale, compared with Parkinson's disease patients. In addition, apparent gray matter atrophy at the temporal lobe, parietal lobe, occipital cortex, limbic system and paralimbic system was observed in patients with Parkinson's disease dementia, as previously described[2,14,15,37,42,43]. We found no differences in the subcortical structure between the Parkinson's disease dementia group and Parkinson's disease group, likely because some Parkinson's disease patients exhibited mild cognitive impairment and the sample size was low. Parietal and occipital cortex atrophy was reported to be associated with visual hallucinations in Parkinson's disease patients[42], and visual hallucination anomaly may increase the susceptibility to dementia[2,14,15]. Ramirez-Ruiz et al[37] also reported that visual hallucination anomaly is a strong predictive factor for dementia. Camicioli et al[43] found a correlation between precuneus atrophy and executive function in Parkinson's disease dementia. The limbic and paralimbic systems are considered important for cognition, memory, attention and learning[29,30,44]. In the present study, we found a significant volume loss in the bilateral cingulate gyrus, right hippocampus, parahippocampal gyrus and left insula lobe. Interestingly, the hippocampus[26,45] and cingulate gyrus[46,47] are the main targets of Lewy body attack. Hippocampal atrophy is a common feature of dementia, and hippocampal degeneration has been previously reported[48,49]. A reduction in hippocampal volume is a common feature of Lewy body dementia and Alzheimer's disease[50,51,52]. Furthermore, the cingulate cortex is particularly vulnerable to Lewy body attack in Parkinson's disease dementia patients[20,53], and cingulate gyrus volume reduction occurs in Parkinson's disease dementia patients with attention disorder[54]. Autopsy studies have also revealed the appearance of different quantities of Lewy bodies in all patients with Parkinson's disease, suggesting that there is a gradient change of neuropathological mechanism in the cingulate and medial temporal lobe structures in Parkinson's disease patients[55].
The pathogenesis of Parkinson's disease dementia is complex, but generally results from cortical-subcortical dopamine loop damage between the basal ganglia and frontal lobe, as well as dopaminergic depletion in the prefrontal lobe caused by a decrease in intrastriatal dopamine[55]. Parkinson's disease patients also present non-dopaminergic defects, leading to cognitive impairment and dementia. Cooper et al[56] suggested that the pathological mechanisms of cognitive impairment in Parkinson's disease involve the extrastriatal dopaminergic system or non-dopaminergic system, such as changes in 5-hydroxytryptamine metabolism, which may underlie depression in Parkinson's disease patients. Zgaljardic and colleagues[57] found that the physiopathological basis of cognitive impairment partially reflects dopaminergic neurotransmitter reduction and destruction of the serotonergic, noradrenergic and cholinergic systems in patients with Parkinson's disease. Mitochondrial abnormalities in the cerebral cortex and Parkinson's disease-associated synaptic protein dysmetabolism can also trigger cell damage, leading to cognitive impairment-related cortical atrophy[58]. These studies may explain the appearance of limbic system and cortical atrophy in Parkinson's disease dementia patients.
Neuropathological and neuroimaging studies have shown that the severity of gray matter loss in patients with Parkinson's disease dementia is associated with the onset time of dementia. For example, dementia symptoms at an early stage indicates a significant cortical and subcortical degeneration and atrophy[59,60]. Changes of cerebral morphology in the limbic system[37,45] and cortical area[26,45,46] were detected in Parkinson's disease dementia patients at autopsy, which was consistent with our voxel-based morphometry data. Thus, voxel-based morphometry is a useful approach for in vivo assessment of brain structure in Parkinson's disease dementia. Due to the limitations of case size, we failed to statistically analyze the onset time, duration and cognitive impairment in patients with Parkinson's disease dementia.
SUBJECTS AND METHODS
Design
A randomized, controlled, retrospective study.
Time and setting
Experiments were performed in the Affiliated Hospital of Nantong University, China in August 2012.
Subjects
Parkinson's disease patients were admitted from the Affiliated Hospital of Nantong University in China between January 2011 and August 2012. All patients were definitively diagnosed according to clinical diagnosis criteria of Parkinson's Disease Society Brain Bank[61] and Neurology Branch of Parkinson's Disease and Movement Disorders Group of Chinese Medical Association in 2006[62]. For patients aged less than 80 years or those with education for more than 10 years, Parkinson's disease dementia was clinically defined based on the criteria of the Neurology Branch of Parkinson's Disease and Movement Disorders Group of Chinese Medical Association in 2011[63]. For other subjects, the recommendations for the Diagnosis and Management of Alzheimer's Disease and Other Disorders Associated With Dementia (EFNS guideline by Waldemar et al[64] were used.
All subjects were detected using MMSE and MoCA (the lower scores indicated more severe cognitive impairment), non-motor symptoms questionnaire (the higher scores indicated more significant non-motor symptoms), ADL scale, UPDRS-III and revised Hoehn & Yahr staging scale (the higher scores and stages indicated more serious illness). The above clinical assessment was performed by professional experienced physicians in Department of Neurology of the Affiliated Hospital of Nantong University, China.
Inclusion criteria for Parkinson's disease dementia patients
Patients with Parkinson's disease dementia met all diagnostic criteria of Parkinson's disease dementia and presented cognitive impairment, with MMSE score less than 25 points, MoCA score less than 17 points and ADL score higher than 30 points simultaneously. Subjects with inconsistent scores were excluded.
Inclusion criteria for Parkinson's disease without dementia patients
Patients with Parkinson's disease met all the diagnostic criteria of Parkinson's disease and presented no cognitive impairment, with MMSE score higher than 26 points, MoCA score higher than 24 points and ADL score lower than 30 points simultaneously. Subjects with inconsistent scores were excluded.
Exclusion criteria
(1) Patients with advanced, severe or unstable diseases that affected brain function and cognitive function assessment. (2) Patients with a history of intracranial hemorrhage, infarction and other cerebral organic disease, as well as previous history of craniocerebral operation or acute cerebral vascular disease within the most recent 3 months. (3) Patients with seizures or mental illness. (4) Patients with secondary Parkinson's disease (Parkinson's syndrome) and Parkinson's plus syndrome.
A final 24 patients with Parkinson's disease (aged 51–79 years) were involved in the result analysis. The age at the onset was 45–75 years, the course of disease was 2–13 years and education level was 0–15 years. All patients were evaluated as 1–4 stages by Hoehn-Yahr stage. There were 12 males and 12 females, 12 dementia patients and 12 non-demented patients. Conventional symptomatic treatment was levodopa administration.
All subjects were informed of examination content and signed the informed consent prior to experimentation. This study met the Declaration of Helsinki ethical requirements.
Methods
MRI scanning
Patients were scanned using a GE Signa HDX 3.0 T superconducting MR scanner (GE Healthcare, Bethesda, MD, USA), with an 8-channel orthogonal coil. The patients were given drugs to control head movement before the scanning. All patients underwent three-dimensional fast spoiled gradient echo sequence scanning, with the sagittal image as a reference and the thalamus as the center, and the baseline was parallel to the junction line. Scan parameters: repetition time 7.3 ms, echo time 3.4 ms, reversing time 450 ms, twist angle 12°, excitation frequency 1, slice thickness 1.2 mm, non-interval scanning, field of view 24 cm × 24 cm, matrix 256 × 256, voxel 0.47 mm × 0.47 mm × 1.20 mm. A total of 124 layer axial images were collected in the whole brain. Routine MRI scanning was also performed to exclude intracranial hemorrhage, infarction or other cerebral organic disease and previous history of craniocerebral operation.
Data processing
The three-dimensional scanning data of all subjects were pre-processed using VBM5 toolbox (http://dbm.neuro. uni-jena.de/vbm/) of statistical parametric mapping 5 software (http//www.fil.ion.ucl.ac.uk/spm/software/spm5) in the Matlab 7.1 platform. The following processes were included: (1) Image conversion: Original images were transformed from DICOM format into SPM-discriminating NIFTI format. (2) Spatial normalization: Original transversion images were registered to the Montreal Neurological Institute (MNI) standard template through standard 12 affine transformation using VBM5 software, then the images after registration were corrected and sampled at 1 mm × 1 mm × 1 mm pixels. (3) Segmentation and modulation: Three-dimensional images after transversion and registration were segmented into gray matter, white matter and cerebrospinal fluid, and the volume of gray matter image was modulated. (4) Smooth: Gray matter images after volume modulation were spatially smoothed using an 8-mm full width at half maximum Gauss Kernel to improve the image signal-to-noise ratio (Figure 2).
Figure 2.
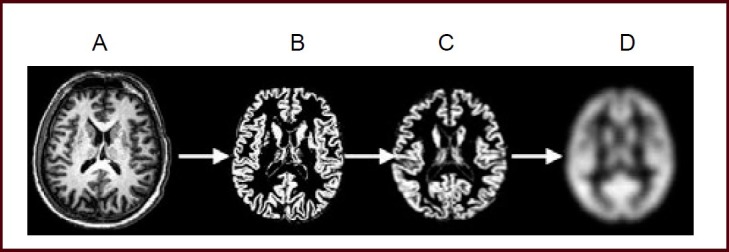
Preprocessing of MRI three-dimensional fast spoiled gradient echo sequence scanning.
The scanning process includes image conversion (A), spatial normalization (B), tissue segmentation and volume modulation (C) and image smoothing (D).
Statistical analysis
Data were statistically analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Differences in gender was compared using chi-square test between the two groups, while age, course of disease and other measurement data were compared using an independent samples t-test, and were expressed as mean ± SD. P < 0.05 was considered statistically significant. The gray matter structure between the two groups (age adjusted) was compared with an independent samples t-test in SPM software, with the age as a covariate for statistics. P < 0.001 (no correction), adjacent brain areas > 50 voxels gray matter, was regarded as statistically significant, and statistical parametric mapping was obtained. The xjView 8.2 software (http://www.alivelearn.net/xjview8/) was applied to record voxel brain area (represented with pseudo color), with significant differences, activation volume (cluster), activation intensity (statistically analyzed with t-test and expressed as T value; T value is proportional to the intensity), MNI coordinates and Brodmann partition determined.
Acknowledgments
We thank the Department of Radiology, Affiliated Hospital of Nantong University, China for providing scanning equipment.
Footnotes
Funding: This work was supported by the Medical Clinical Science and Technology Development Fund of Jiangsu University, No. JLY20120122; Innovative Climb Program of Natural Science Foundation of Jiangsu Province, No. BK2008010; Natural Science Foundation of Nantong University, China, No.11Z001; Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of interest: None declared.
Ethical approval: This study received the permission from the Ethics Committee of Nantong University in China.
(Reviewed by Dean J, Norman C, Zhang XM, Wang XP)
(Edited by Wang LM, Yang Y, Li CH, Song LP)
REFERENCES
- 1.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. 1837. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 3.Emre M. Dementia associated with Parkinson's disease. Lancet Neurol. 2003;2(4):229–237. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 4.Foltynie T, Brayne CE, Robbins TW, et al. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPalGN study. Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 5.Muslimovic D, Post B, Speelman JD, et al. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 6.Mamikonyan E, Moberg PJ, Siderowf A, et al. Mild cognitive impairment is common in Parkinson's disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15(3):226–231. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord. 2005;20(10):1255–1263. doi: 10.1002/mds.20527. [DOI] [PubMed] [Google Scholar]
- 8.de Lau LM, Schipper CM, Hofman A, et al. Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam Study. Arch Neurol. 2005;62(8):1265–1269. doi: 10.1001/archneur.62.8.1265. [DOI] [PubMed] [Google Scholar]
- 9.Aarsland D, Andersen K, Larsen JP, et al. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology. 2001;56(6):730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 10.Galvin JE. Cognitive change in Parkinson disease. Alzheimer Dis Assoc Disord. 2006;20(4):302–310. doi: 10.1097/01.wad.0000213858.27731.f8. [DOI] [PubMed] [Google Scholar]
- 11.Starkstein SE, Bolduc PL, Mayberg HS, et al. Cognitive impairments and depression in Parkinson's disease: a follow-up study. J Neurol Neurosurg Psychiatry. 1990;53(7):597–602. doi: 10.1136/jnnp.53.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson's disease: a community-based, 4 year longitudinal study. J Geriatr Psychiatry Neurol. 2005;18(3):149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- 13.Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67(9):1605–16611. doi: 10.1212/01.wnl.0000242630.52203.8f. [DOI] [PubMed] [Google Scholar]
- 14.Santangelo G, Trojano L, Vitale C, et al. A neuropsychological longitudinal study in Parkinson's patients with and without visual hallucinations. Mov Disord. 2007;22(16):2418–2425. doi: 10.1002/mds.21746. [DOI] [PubMed] [Google Scholar]
- 15.Song SK, Lee JE, Park HJ, et al. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord. 2011;26(2):289–296. doi: 10.1002/mds.23477. [DOI] [PubMed] [Google Scholar]
- 16.Nikolaus S, Antke C, Beu M, et al. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Rev Neurosci. 2010;21(2):119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- 17.Perry EK, Curtis M, Dick DJ, et al. Cholinergic correlates of cognitive impairment in Parkinson's disease: comparisons with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1985;48(5):413–421. doi: 10.1136/jnnp.48.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweig RM, Cardillo JE, Cohen M, et al. The locus ceruleus and dementia in Parkinson's disease. Neurology. 1993;43(5):986–991. doi: 10.1212/wnl.43.5.986. [DOI] [PubMed] [Google Scholar]
- 19.Apaydin H, Ahlskog E, Parisi JE, et al. Parkinson disease neuropathology. Later-developing dementia and loss of the levodopa response. Arch Neurol. 2002;59(1):102–112. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- 20.Hurtig HI, Trojanowski JQ, Galvin J, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54(10):1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 21.Janvin CC, Larsen JP, Aarsland D, et al. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21(9):1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 22.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 23.Carbon M, Marie RM. Functional imaging of cognition in Parkinson's disease. Curr Opin Neurol. 2003;16(4):475–480. doi: 10.1097/01.wco.0000084225.82329.3c. [DOI] [PubMed] [Google Scholar]
- 24.De Leonibus E, Manago F, Giordani F, et al. Metabotropic glutamate receptors 5 blockade reverses spatial memory deficits in a mouse model of Parkinson's disease. Neuropsychopharmacology. 2009;34(3):729–738. doi: 10.1038/npp.2008.129. [DOI] [PubMed] [Google Scholar]
- 25.Haller S, Badoud S, Nguyen D, et al. Differentiation between Parkinson disease and other forms of Parkinsonism using support vector machine analysis of susceptibility-weighted imaging (SWI): initial results. Eur Radiol. 2013;23(1):12–19. doi: 10.1007/s00330-012-2579-y. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Kim SS, Tae WS, et al. Brain volumetry in Parkinson's disease with and without dementia: where are the differences? Acta Radiol. doi: 10.1177/0284185113476029. in press. [DOI] [PubMed] [Google Scholar]
- 27.Frisoni GB, Testa C, Zorzan A, et al. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73(6):657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda H, Kitayama N, Ohnishi T, et al. Longitudinal evaluation of both morphologic and functional changes in the same individuals with Alzheimer's disease. J Nucl Med. 2002;43(3):304–311. [PubMed] [Google Scholar]
- 29.Busatto GF, Garrido GE, Almeida OP, et al. A voxel-based morphometry study of temporal lobe grey matter reductions in Alzheimer's disease. Neurobiol Aging. 2003;24(2):221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 30.Burton EJ, Karas G, Paling SM, et al. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage. 2002;17(2):618–630. [PubMed] [Google Scholar]
- 31.Antonelli F, Ray N, Strafella AP, et al. Imaging cognitive and behavioral symptoms in Parkinson's disease. Expert Rev Neurother. 2010;10(12):1827–1838. doi: 10.1586/ern.10.173. [DOI] [PubMed] [Google Scholar]
- 32.Janvin CC, Larsen JP, Salmon DP, et al. Cognitive profiles of individual patients with Parkinson's disease and dementia: comparison with dementia with lewy bodies and Alzheimer's disease. Mov Disord. 2006;21(3):337–342. doi: 10.1002/mds.20726. [DOI] [PubMed] [Google Scholar]
- 33.Poewe W, Gauthier S, Aarsland D, et al. Diagnosis and management of Parkinson's disease dementia. Int J Clin Pract. 2008;62(10):1581–1587. doi: 10.1111/j.1742-1241.2008.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollenhauer B, Förstl H, Deusehil G, et al. Lewy body and parkinsonian dementia: common, but often misdiagnosed conditions. Dtsch Arztebl Int. 2010;107(39):684–691. doi: 10.3238/arztebl.2010.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filoteo JV, Salmon DP, Schiehser DM, et al. Verbal learning and memory in patients with Dementia with Lewy Bodies or Parkinson's disease with Dementia. J Clin Exp Neuropsychol. 2009;31(7):823–834. doi: 10.1080/13803390802572401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson's disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57(2):304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez-Ruiz B, Junque C, Marti MJ, et al. Cognitive changes in Parkinson's disease patients with visual hallucinations. Dement Geriatr Cogn Disord. 2007;23(5):281–288. doi: 10.1159/000100850. [DOI] [PubMed] [Google Scholar]
- 38.Beyer MK, Janvin CC, Larsen JP, et al. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78(3):254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burton EJ, McKeith IG, Burn DJ, et al. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 40.Summerfield C, Junque C, Tolosa E, et al. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol. 2005;62(2):281–285. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- 41.Nagano-Saito A, Washimi Y, Arahata Y, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64(2):224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez-Ruiz B, Marti MJ, Tolosa E, et al. Cerebral atrophy in Parkinson's disease patients with visual hallucinations. Eur J Neurol. 2007;14(7):750–756. doi: 10.1111/j.1468-1331.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- 43.Camicioli R, Gee M, Bouchard TP, et al. Voxelbased morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in Parkinsonism. Parkinsonism Relat Disord. 2009;15(3):187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 45.Churchyard A, Lees AJ. The relationship between dementia and direct involvement of the hippocampus and amygdala in Parkinson's disease. Neurology. 1997;49(6):1570–1576. doi: 10.1212/wnl.49.6.1570. [DOI] [PubMed] [Google Scholar]
- 46.Mattila PM, Rinne JO, Helenius H, et al. Alpha-synuclein- immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol. 2000;100(3):285–290. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- 47.Kovari E, Gold G, Herrmann FR. Lewy body densities in the entorhinal and anterior cingulated cortex predict cognitive deficits in Parkinson's disease. Acta Neuropathol. 2003;106(1):83–88. doi: 10.1007/s00401-003-0705-2. [DOI] [PubMed] [Google Scholar]
- 48.Laakso MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer's disease, Parkinson's disease with and without dementia, and in vascular dementia: an MRI study. Neurology. 1996;46(3):678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 49.Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol. 2000;247(Suppl 2):II3–10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 50.Mattila PM, Röyttä M, Torikka H, et al. Cortical Lewy bodies and Alzheimer-type changes in patients with Parkinson's disease. Acta Neuropathol. 1998;95(6):576–582. doi: 10.1007/s004010050843. [DOI] [PubMed] [Google Scholar]
- 51.Mattila PM, Rinne JO, Helenius H, et al. Neuritic degeneration in the hippocampus and amygdala in Parkinson's disease in relation to Alzheimer pathology. Acta Neuropathol. 1999;98(2):157–164. doi: 10.1007/s004010051064. [DOI] [PubMed] [Google Scholar]
- 52.de Vos RA, Cansen EN, Stam FC, et al. Lewy body disease: clinicopathological correlations in 18 consecutive cases of Parkinson's disease with and without dementia. Clin Neurol Neurosurg. 1995;97(1):13–22. doi: 10.1016/0303-8467(94)00060-j. [DOI] [PubMed] [Google Scholar]
- 53.Hishikawa N, Hashizume Y, Yoshida M, et al. Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol. 2003;105(4):341–350. doi: 10.1007/s00401-002-0651-4. [DOI] [PubMed] [Google Scholar]
- 54.Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31(2):148–165. doi: 10.1006/brcg.1996.0039. [DOI] [PubMed] [Google Scholar]
- 55.Zgaljardic DJ, Borod JC, Foldi NS, et al. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to hontostriatal circuitry. Cogn Behav Neurol. 2003;16(4):193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Cooper JA, Sagar HJ, Jordan N, et al. Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain. 1991;114(Pt 5):2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- 57.Zgaljardic DJ, Foldi NS, Borod JC. Cognitive and behavioral dysfunction in Parkinson's disease: neurochemical and clinicopathological contributions. J Neural Transm. 2004;111(10-11):1287–1301. doi: 10.1007/s00702-004-0178-z. [DOI] [PubMed] [Google Scholar]
- 58.Ferrer I. Early involvement of the cerebral cortex in Parkinson's disease: convergence of multiple metabolic defects. Prog Neurobiol. 2009;88(2):89–103. doi: 10.1016/j.pneurobio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Beyer MK, Aarsland D. Grey matter atrophy in early versus late dementia in Parkinson's disease. Parkinsonism Relat Disord. 2008;14(8):620–625. doi: 10.1016/j.parkreldis.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 60.Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931–1934. doi: 10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- 61.Daniel SE, Lees AJ. Parkinson's Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl. 1993;39:165–172. [PubMed] [Google Scholar]
- 62.Neurology Branch of Parkinson Disease and Movement Disorders Group of Chinese Medical Association. The diagnosis of Parkinson's disease. Zhonghua Shenjingke Zazhi. 2006;39(6):408–409. [Google Scholar]
- 63.Neurology Branch of Parkinson Disease and Movement Disorders Group of Chinese Medical Association. Guideline for diagnosis and treatment of Parkinson's disease with dementia. Zhonghua Shenjingke Zazhi. 2011;44(9):635–637. [Google Scholar]
- 64.Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14(1):e1–26. doi: 10.1111/j.1468-1331.2006.01605.x. [DOI] [PubMed] [Google Scholar]


