Abstract
One feature of the amino acid sequence of P2X receptors identified from mammalian species, Xenopus laevis and zebrafish is the conservation of ten cysteines in the extracellular loop. Little information is available about the role of these conserved ectodomain cysteines in the function of P2X receptors. Here, we investigated the possibility that ten conserved cysteine residues in the extracellular loop of the rat P2X4 receptor may regulate zinc potentiation of the receptor using a series of individual cysteine to alanine point mutations and functional characterization of recombinant receptors expressed in Xenopus oocytes. For the C116A, C132A, C159A, C165A, C217A and C227A mutants, 10 μM zinc did not significantly affect the current activated by an EC40 concentration of ATP. By contrast, 5 μM zinc shifted the ATP concentration-response curve to the right in a parallel manner for both the C261A and C270A mutants and the magnitudes of those shifts were similar to that of the wildtype receptor. Interestingly, for the C126A and C149A mutants, 5 μM zinc potentiated ATP-activated current, but increased the maximal response to ATP by by 90% and 81% respectively, without significantly changing the EC50 value of ATP. Thus, these results suggest that cysteines and disulfide bonds between cysteines are differentially involved in the potentiation of the rat P2X4 receptor by zinc.
Keywords: P2X, P2X4 receptor, Cysteine, Disulfide bond, Mutation, Zinc, Efficacy
1. Introduction
P2X receptors are ATP-gated cation channels expressed widely in excitable and non-excitable cells, and a growing body of evidence indicates that P2X receptors are involved in diverse physiological processes, such as synaptic transmission, the sensing of taste and pain, as well as inflammation (Jarvis and Khakh, 2009; Surprenant and North, 2009; Kaczmarek-Hajek et al., 2012). To date, seven mammalian P2X receptor subunits, designated P2X1 to P2X7, have been cloned (Jarvis and Khakh, 2009; Surprenant and North, 2009; Kaczmarek-Hajek et al., 2012). All of these subunits are thought to consist of two transmembrane domains, a large extracellular loop, and intracellular amino- and carboxy-terminals (Jarvis and Khakh, 2009; Surprenant and North, 2009; Kaczmarek-Hajek et al., 2012). When expressed in Xenopus oocytes or cell lines, these subunits can form functional homomeric or heteromeric ATP-gated cation channels composed of three subunits (Jarvis and Khakh, 2009; Surprenant and North, 2009; Kaczmarek-Hajek et al., 2012). Recently, the first crystal structure of P2X receptors, zebrafish P2X4 receptor in a closed state, has been reported (Kawate et al., 2009) which provides a major advance in the understanding of P2X receptor channels.
One striking feature of the amino acid sequence of P2X receptors is the conservation of ten cysteines in the extracellular loop (Kawate et al., 2009). So far, some information about the role of these cysteines in P2X receptor function is available from several studies. For instance, cysteine residues in the extracellular loop form disulfide bonds in human P2X1 (Ennion and Evans, 2002), rat P2X2 (Clyne et al., 2002), rat P2X4 (Yi et al., 2009; Rokic et al., 2010) and rat P2X7 receptors (Jindrichova et al., 2012), and are involved in receptor trafficking to the cell surface in human P2X1 (Ennion and Evans, 2002) and rat P2X7 (Jindrichova et al., 2012) receptors, zinc and proton modulation in rat P2X2 (Clyne et al., 2002) receptors, and in regulation of the inhibitory effect of ethanol (Yi et al., 2009) as well as in agonist binding and channel gating in rat P2X4 receptors (Rokic et al., 2010). However, more information is needed to better understand the conserved extracellular systeines in P2X receptors influence the function of these receptor-channels. Of the known P2X receptor subunits, the P2X4 has been found to be one of the most abundant P2X subunits in brain, with particularly dense expression in the hippocampus and cerebral cortex (Collo et al., 1996; Kaczmarek-Hajek et al., 2012; Surprenant and North, 2009), and previous studies have revealed that the function of this subunit can be potentiated by low micromolar concentrations of zinc (Xiong et al., 1999; Coddou et al., 2007). Therefore, in the present study, we investigated whether conserved cysteine residues in the extracellular loop of rat P2X4 receptors are involved in the potentiation procuced by zinc.
2. Materials and methods
2.1 DNA site-directed mutagenesis
A P2X4 receptor cDNA cloned from rat superior cervical ganglion (SCG) was kindly provided by Dr. Gary Buell (Serono Pharmaceutical Research Institute, Geneva, Switzerland). Site-directed mutagenesis of C116, C126, C132, C149, C159, C165, C217, C227, C261 and C270 in the rat P2X4 cDNA was performed using the Quikchange kit (Stratagene, Inc., La Jolla, CA, USA) as described previously (Yi et al., 2009). Briefly, sixteen cycles of amplification by polymerase chain reaction catalyzed by Pfu DNA polymerase were performed with the following temperature protocol: strand separation 98°C, primer annealing 50°C and primer extension 68°C for 20 min, and the parental template was digested with Dpn I, the mutated P2X4 receptor construct transformed into competent host cells, and the transformed cells plated on ampicillin-containing agar plates. Individual clones were grown in Luria-Bertani medium; DNA was isolated and then sequenced to verify each mutation. Each mutant is referred to by the original amino acid (one letter code) followed by the residue number and the substituted amino acid (one letter code).
2.2 Expression of receptors in Xenopus oocytes and two-electrode voltage-clamp recording
The preparation of cRNA, expression of receptors in Xenopus oocytes and two-electrode voltage-clamp recording from Xenopus oocytes were performed as described previously (Xiong et al., 2005; Yi et al., 2009). Briefly, oocytes were placed in a recording chamber and impaled with two sharp microelectrodes filled with 3M KCl (tip resistances: 0.5 – 1.5 MΩ), and constantly superfused at the rate of ~2.5 ml/min with modified Ringer solution containing (in mM): 96 NaCl, 2 KCl, 1.8 BaCl2, 1 MgCl2, 5 HEPES (pH 7.4; Ba2+ replaced Ca2+ to prevent the activation of the endogenous calcium-activated chloride current in these cells). Agonist and other chemical solutions were prepared in the bathing solution. Solutions of ATP, added as the Na+ salt, were prepared daily. Oocytes were recorded 2–5 day after RNA injection at room temperature using a Geneclamp Amplifier (Axon Instruments Inc., Foster City, CA, USA), and voltage-clamped at −70 mV.
2.3 Drugs and chemicals
The drugs and chemicals used in these experiments were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO).
2.4 Data analysis
Data were statistically compared using analysis of variance (ANOVA) followed by the Dunnett’s test, as noted. Statistical analysis of concentration-response data was performed using the nonlinear curve-fitting program ALLFIT (DeLean et al., 1978), which uses an ANOVA procedure. Values reported for concentrations yielding 50% of the maximal effect (EC50) and the slope factor (n) are those obtained by fitting the data to the equation:
where X and Y are concentration and response, respectively, Emax is the maximal response. Current amplitudes reported are peak values. Average values are expressed as mean ± S.E.M. with n equal to the number of cells studied.
3. Results
3.1 Effect of cysteine mutations on zinc potentiation of rat P2X4 receptors
It has previously been reported that, using a series of individual cysteine to alanine point mutations, ten mutated receptors generated robust inward current in response to ATP in Xenopus oocytes expressing the P2X4 receptor cloned from rat SCG and the mutation produced less than a 6-fold change in the EC50 value of the ATP concentration-response curve (Yi et al., 2009). Similarly, in the present study extracellular ATP evoked inward current in each of the ten mutated receptors (Fig. 1,2). ATP, at concentrations up to 300 μM, did not activate detectable current in uninjected Xenopus oocytes (n = 7, data not shown).
Fig. 1.
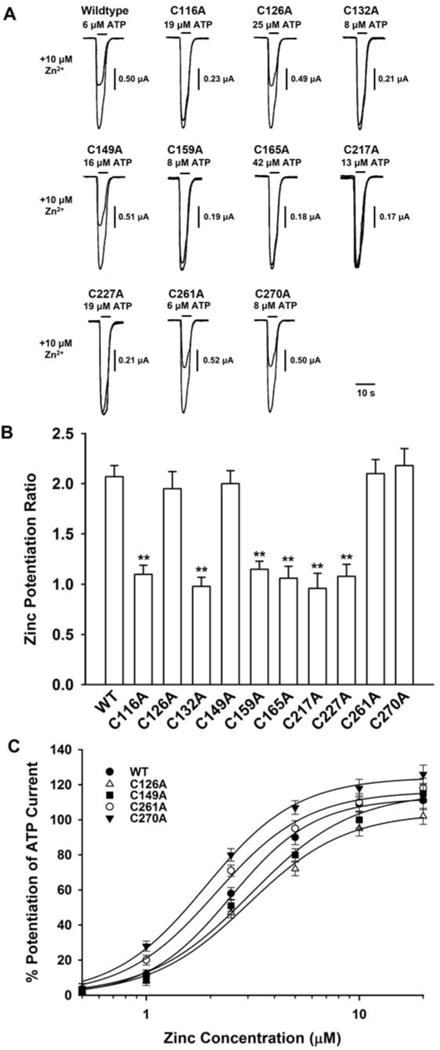
Effect of cysteine mutation on zinc potentiation of ATP-activated current in rat P2X4 receptors. (A) Records showing currents activated by an ATP concentration that was close to the EC40 value of the ATP concentration-response curve in WT (6μM), C116A (19μM), C126A (25μM), C132A (8μM), C149A (16μM), C159A (8μM), C165A (42μM), C217A (13μM), C227A (19μM), C261A (6μM) and C270A (8μM) rat P2X4 receptors in the absence and presence of 10 μM zinc. (B) Graph plotting mean zinc potentiaion ratio of 7–9 oocytes per construct. The zinc potentiation ratio is the ratio of the current amplitude in the presence of ATP and 10 μM zinc to the current amplitude of ATP alone. **, Values differed significantly from WT (ANOVA and Dunnett’s test; p < 0.01). (C) Graph plotting average percentage potentiation of ATP-activated current as a function of zinc concentration for the mutants indicated. Each data point is the average of 7 – 10 cells; error bars not visible are smaller than the size of the symbols. In (B) and (C), as ATP sensitivity was different in WT and mutated receptors, an ATP concentration that was close to the EC40 value of each ATP concentration-response curve was used for each mutant which is indicated in (A).
Fig. 2.
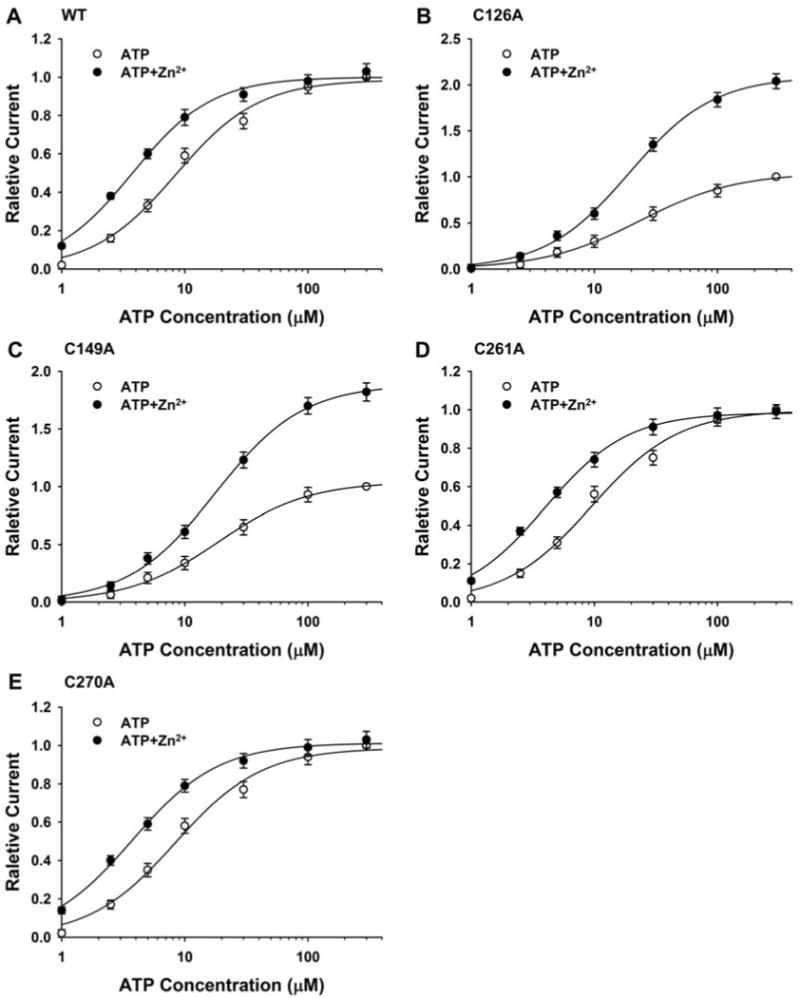
Effect of ATP concentration on zinc potentiation of ATP-activated current in the cysteine-mutated rat P2X4 receptors. (A–E) Graphs plotting the amplitude of ATP-activated current in the presence of 5 μM zinc relative to the amplitude of ATP-activated current in the absence of zinc, as a function of ATP concentration in oocytes expressing WT, C126A, C149A, C261A and C270A receptors. Each data point is the average current from 6 – 9 cells; error bars not visible are smaller than the size of the symbols.
Conserved extracellular cysteines in P2X receptors have been predicted to form disulfide bonds. The recently reported crystal structure of zebrafish P2X4 receptor provides direct evidence for the existence of these disulfide bonds which form between cysteines in the following order (P2X4 receptor numbering): C116–C165 (SS1), C126–C149 (SS2), C132–C159 (SS3), C217–C227 (SS4) and C261–C270 (SS5) (Kawate et al., 2009). To investigate whether cysteine residues in the extracellular loop might be involved in the potentiation of P2X4 receptor function by zinc, effects of zinc on the cysteine to alanine mutants were examined. Because ATP sensitivity was different in WT and mutated receptors (Yi et al., 2009) and zinc potentiation of ATP-activated current depends on ATP concentration (Xiong et al., 1999; Yi et al., 2009), an ATP concentration that was close to the EC40 value of the ATP concentration-response curve was used in these experiments for the WT and each mutated receptor; the EC40 values were 6, 19, 25, 8, 16, 8, 42, 13, 19, 6, and 8 μM for WT, C116A, C126A, C132A, C149A, C159A, C165A, C217A, C227A, C261A and C270A receptors, respectively (Yi et al., 2009). Similar to the WT receptor, 10 μM zinc markedly potentiated ATP-activated current in C126A and C149A (which generate SS2), C261A and C270A receptors (which generate SS5) (Fig. 1A,B). By contrast, 10 μM zinc did not produce any significant effects in C116A and C165A, C132A and C159A, C217A and C227A receptors (which generate SS1, SS3 and SS4) (Fig. 1A,B).
To study the potentiation of zinc in mutated rat P2X4 receptors, we constructed zinc concentration-response curves for potentiation of ATP-activated current for WT, C126A, C149A, C261A and C270A receptors (Fig. 1C). As shown in Fig. 1C and Table 1, zinc potentiated ATP-activated current in WT receptors with an EC50 value of 2.4 μM. All of the alanine-substitution mutant subunits were also potentiated by zinc, but zinc sensitivity varied among the mutant receptors. In the C126A mutant receptor, the EC50 value for zinc was 3.7 μM, while in the C270A receptor the EC50 value was 1.7 μM; both of these values differed significantly from the WT value (ANOVA; P < 0.05). In contrast, there were no significant differences observed among the slope factors of the concentration-response curves for the various mutants (ANOVA; P < 0.05). Over the concentration range 0.5 to 20 μM zinc, the application of zinc alone did not activate detectable current in Xenopus oocytes expressing WT and each of the six mutated rat P2X4 receptors (n = 5–6 for WT and each mutant, data not shown).
Table 1.
Summary of data on the effect of cysteine mutation on zinc potentiation of ATP-activated current in rat P2X4 receptors.
| Parameter | WT | C126A | C149A | C261A | C270A |
|---|---|---|---|---|---|
| Zinc EC50 (μM)1 | 2.4±0.1 | 2.8±0.3 | 2.9±0.4 | 1.9±0.2 | 1.7±0.1 |
| Zinc slope factor | 1.7±0.2 | 1.7±0.4 | 1.5±0.4 | 1.7±0.3 | 1.7±0.2 |
| Zinc Emax | 113.2±2.4% | 106.3±5.9% | 120.1±8.1% | 118.8±4.1% | 126.8±2.1% |
| ATP EC50 | 6.9±1.4 | 22.3±2.1a | 17.6±1.8a | 7.8±1.5 | 6.4±1.2 |
| ATP slope factor | 1.0±0.2 | 0.9±0.2 | 1.1±0.1 | 1.0±0.2 | 0.9±0.2 |
| ATP EC50 with Zinc2 | 2.6±0.7b | 19.9±1.3 | 17.1±1.3 | 2.8±0.2b | 2.7±0.6 |
| ATP slope factor with Zinc | 1.0±0.2 | 1.2±0.1 | 1.1±0.1 | 1.0±0.04 | 1.0±0.1 |
| ATP Emax ratio with Zinc3 | 1.0±0.01 | 1.9±0.04a | 1.81±0.05a | 1.0±0.02 | 1.0±0.02 |
An ATP concentration that was close to the EC40 value of each ATP concentration-response curve was used for the WT and each mutant receptor.
5 μM zinc was used.
The ratio of the current amplitude in the presence of 300 μM ATP and 5 μM zinc to the current amplitude of ATP alone.
P < 0.01 vs WT and
P < 0.01 vs ATP EC50 without zinc in the same receptor. Values are expressed as mean peak current ± S.E.M. (n = 7 – 10 oocytes).
3.2 Effect of cysteine mutations on the mechanism of zinc potentiation of rat P2X4 receptors
To investigate further the effect of cysteine mutation on zinc potentiation of rat P2X4 receptors, we examined whether the concentration of ATP affects zinc potentiation in C126A, C149A, C261A and C270A receptors. As shown in Fig. 2 and Table 1, 5 μM zinc increased the Emax value of the ATP concentration-response curve by 90% and 81% (ANOVA, P < 0.01), without significantly changing the slope (P > 0.05) or the EC50 value of ATP concentration-response curve (P > 0.05) in C126 and C149A receptors. By contrast, for the C261A and C270A mutants, like the WT receptor, 5 μM zinc shifted the ATP concentration-response curve to the left, significantly increasing the EC50 value for the ATP concentration-response curve (P < 0.01), without significantly changing the slope (P > 0.05) or the Emax value of the ATP concentration-response curve (P > 0.05).
4. Discussion
Among all known P2X receptors cloned from mammalian species, Xenopus laevis and zebrafish, one striking feature of the amino acid sequence is the conservation of ten cysteines in the extracellular loop (Jarvis and Khakh, 2009; Surprenant and North, 2009; Kaczmarek-Hajek et al., 2012). Several previous studies have been carried out to explore the possible role of conserved ectodomain cysteines in the function of P2X receptors. For instance, Clyne et al. (Clyne et al., 2002) have found that some cysteines in the rat P2X2 receptor form a series of disulfide bonds, and are involved in regulating the ATP sensitivity as well as zinc and proton modulation. Ennion and Evans (Ennion and Evans, 2002) carried out similar experiments for the human P2X1 receptor and found that similar pairs of disulfide bonds are formed between cysteines, and some of these disulfide bonds are involved in receptor trafficking to the cell surface. Similar results have been reported by Jindrichova et al. (Jindrichova et al., 2012) in the rat P2X7 receptor. In the rat P2X4 receptor, Yi et al. (Yi et al., 2009) have found that some cysteines form disulfide bonds, and are differentially involved in the sensitivity of the receptor to ethanol and in the alteration of the mechanism of ethanol inhibition from competitive to noncompetitive. In addition, Rokic et al. (Rokic et al., 2010) have found that cysteines and disulfide bonds between cysteines in the rat P2X4 receptor are involved in agonist binding and channel gating. All of these findings suggest that these conserved cysteine residues play an important role in P2X receptor function.
In the present study, we investigated the possible imvolvement of cysteine residues in the extracellular loop of rat P2X4 receptors in the potentiation produced by zinc using site-directed mutagenesis, and functional characterization of recombinant receptors expressed in Xenopus oocytes. Similar to the previous study (Yi et al., 2009), each of the ten mutated receptors generated inward current in response to ATP and the cysteine to alanine mutation produced less than a 6-fold change in the EC50 value of the ATP concentration-response curve. These data suggest that individual cysteine residues and the disulfide bonds they form are not essential for the production of functional rat P2X4 receptors cloned from rat SCG (Yi et al., 2009). However, the present study also indicates that the cysteine residues in the extracellular loop of the rat P2X4 receptor play different roles in the potentiation of this receptor by zinc. For the C116A, C132A, C159A, C165A, C217A and C227A mutants, unlike the WT receptor, 10 μM zinc did not significantly affect ATP-activated current, suggesting that these six cysteine residues are essential for zinc to produce its potentiation in rat P2X4 receptors. By contrast, for C126A, C149A, C261A and C270A mutants, like the WT receptor, zinc potentiated the current activated by ATP. Interestingly, it has been reported that in seven cysteine mutants (C13A, C124A, C130A, C147A, C158A, C164A and C214A) of the rat P2X2 receptor zinc produces very little potentiation and in two cysteine mutants (C258A and C267A), like the WT receptor, zinc potentiates ATP-activated current (Clyne et al., 2002). In addition, it has been reported that Cys132, but not Cys126 are crucial for zinc-induced potentiation of the receptor activity in the rat P2X4 receptor (Coddou et al., 2007). In the present study, we further investigated the mechanism by which zinc potentiates the function of C126A, C149A, C261A and C270A receptors. For the C261A and C270A mutants, the EC50 values for zinc potentiation of ATP-activated current did not differ from that for the WT receptor. As the ATP concentration used for C261A, C270A and WT receptors are all close to the EC40 value of the ATP concentration-response curve, this observation suggests that mutation of cysteine 261 to alanine (C261A) or cysteine 270 to alanine (C270A) did not significantly alter the zinc sensitivity of the rat P2X4 receptor. In addition, 5 μM zinc shifted the ATP concentration-response curve to the left in a parallel manner for both the C261A and the C270A mutants and the magnitudes of those shifts were similar to that of the WT receptor, suggesting that the mechanism by which zinc potentiates the C261A and C270A mutants is not altered by these mutations. Surprisingly, for the C126A and C149A mutants, like the WT receptor, zinc potentiated ATP-activated current, but increased the maximal response to ATP by 90% and 81% respectively without significantly changing the EC50 value of the concentration-response curve. Thus, the results suggest that cysteines 126 and 149 are involved in determining the mechanism by which zinc potentiates the P2X4 receptor function.
The function of ligand-gated ion channels can be up or down regulated by altering agonist sensitivity or efficacy and they could be measured by EC50 or Emax of agonist concentration-response curve. The molecular basis of such regulation is poorly understood. So far, there is limited information available from three publications about the molecular determination of altering agonist sensitivity or efficacy in the rat P2X4 receptor. Coddou et al. (Coddou et al., 2007) have found that mutation of ectodomain histidine 140 or histidine 286 changes the mechanism by which zinc potentiates ATP-activated current from increasing ATP sensitivity to increasing ATP efficacy. Xiong et al. (Xiong et al., 2005) have identified a residue – histidine 241 – located in the extracellular loop that when mutated alters the mechanism by which ethanol inhibits receptor function from competitive to noncompetitive. Recently, Yi et al. (Yi et al., 2009) have found that cysteines 132, 159, 217 and 227 are involved in alteration of the mechanism of ethanol inhibition from competitive to noncompetitive. In the present study we found that mutations of cysteine 126 to alanine or cysteine 149 to alanine change the mechanism by which zinc potentiates receptor function from increasing ATP sensitivity to increasing ATP efficacy.
In the present study, we found that among ten cysteine residues of the rat P2X4 receptor only cysteine 261 and 270 mutations did not significantly affect zinc potentiation, suggesting that disulfide bond SS5 is not crucial in determining the sensitivity and mechanism of zinc potentiation. By contrast, unlike the WT receptor, zinc potentiation was not observed in six mutants (C116A, C132A, C159A, C165A, C217A and C227A), suggesting that disulfide bonds SS1, SS3 and SS4 are essential for zinc to produce its potentiation in rat P2X4 receptors. Interestingly, in the rat P2X2 receptor, zinc potentiation was observed in two mutants (C258A and C267A), and lost in seven mutants (C13A, C124A, C130A, C147A, C158A, C164A and C214A) (Clyne et al., 2002). In the present study, we also found that for the C126A and C149A mutants, zinc increased the maximal response to ATP without significantly changing the EC50 value of the concentration-response curve, thus suggesting that disulfide bond SS2 is involved in determining the mechanism by which zinc potentiates the P2X4 receptor function. So far, the zinc-binding site responsible for its excitatory action in P2X receptors has not been identified. A previous study in the rat P2X2 receptor suggests that conserved extracellular cysteines are unlikely to be exposed in the zinc-binding site (Clyne et al., 2002). If this is the case, it seems possible that disrupting disulfide bonds SS1, SS3 and SS4 may sufficiently change the receptor conformation to result in altered sensitivity to zinc. Similarly, a receptor conformational change produced by disrupting disulfide bond SS2 may result in a different mechanism of zinc potentiation.
5. Conclusion
Of the known P2X receptor subunits, the P2X4 subunit has been found to be one of the most abundant in brain, and its function can be potentiated by extracellular zinc. In the present study we found that cysteine 261 and 270 mutations did not significantly affect zinc potentiation. By contrast, zinc potentiation was not observed in C116A, C132A, C159A, C165A, C217A and C227A. Interestingly, cysteine 126 and 149 mutations changed the mechanism by which zinc potentiates ATP-activated current. These results suggest that disulfide bond SS5 is not crucial in determining the sensitivity and mechanism of zinc potentiation, disulfide bonds SS1, SS3 and SS4 are essential for zinc to produce its potentiation and SS2 is involved in determining the mechanism by which zinc potentiates the P2X4 receptor function. Taken together, the results presented in the present study indicate that cysteines and disulfide bonds between cysteines are differentially involved in the potentiation of the rat P2X4 receptor by zinc, which may also differ from that in rat P2X2 receptors. Our data could help to better understand the role of ten conserved ectodomain cysteines in the function of P2X receptors.
Acknowledgments
We thank Dr. Gary Buell for providing the cDNA for the P2X4 subunit. This work was supported by the Wuhan Science and Technology Foundation (200970634270 and 201150699189-23) and National Natural Science Foundaton of China (30970927).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Clyne JD, Wang LF, Hume RI. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J Neurosci. 2002;22:3873–3880. doi: 10.1523/JNEUROSCI.22-10-03873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Acuna-Castillo C, Bull P, Huidobro-Toro JP. Dissecting the facilitator and inhibitor allosteric metal sites of the P2X4 receptor channel: critical roles of CYS132 for zinc potentiation and ASP138 for copper inhibition. J Biol Chem. 2007;282:36879–36886. doi: 10.1074/jbc.M706925200. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978;235:E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Ennion SJ, Evans RJ. Conserved cysteine residues in the extracellular loop of the human P2X(1) receptor form disulfide bonds and are involved in receptor trafficking to the cell surface. Mol Pharmacol. 2002;61:303–311. doi: 10.1124/mol.61.2.303. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Jindrichova M, Kuzyk P, Li S, Stojilkovic SS, Zemkova H. Conserved ectodomain cysteines are essential for rat P2X7 receptor trafficking. Purinergic Signal. 2012;8:317–325. doi: 10.1007/s11302-012-9291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek-Hajek K, Lorinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors–recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokic MB, Tvrdonova V, Vavra V, Jindrichova M, Obsil T, Stojilkovic SS, Zemkova H. Roles of conserved ectodomain cysteines of the rat P2X4 purinoreceptor in agonist binding and channel gating. Physiol Res. 2010;59:927–935. doi: 10.33549/physiolres.931979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery J, Chiang YS, Stewart RR, Weight FF, Li C. Differential modulation by divalent cations of P2X2 and P2X4 receptor function. J Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- Xiong K, Hu XQ, Stewart RR, Weight FF, Li C. The mechanism by which ethanol inhibits rat P2X4 receptors is altered by mutation of histidine 241. Br J Pharmacol. 2005;145:576–586. doi: 10.1038/sj.bjp.0706192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CL, Liu YW, Xiong KM, Stewart RR, Peoples RW, Tian X, Zhou L, Ai YX, Li ZW, Wang QW, Li CY. Conserved extracellular cysteines differentially regulate the inhibitory effect of ethanol in rat P2X4 receptors. Biochem Biophys Res Commun. 2009;381:102–106. doi: 10.1016/j.bbrc.2009.02.018. [DOI] [PubMed] [Google Scholar]


