Abstract
Hydroxymethylglutaryl-coenzyme A reductase inhibitors or statins are the most effective medications for reducing elevated concentrations of low-density lipoprotein cholesterol (LDL-C). Statins reduce cardiac events in patients with coronary artery disease and previously healthy persons. Current recommendations for LDL-C treatment goals indicate that more patients will be treated with higher doses of these medications. Statins have been extremely well-tolerated in controlled clinical trials but are increasingly recognized to produce skeletal muscle myalgia, cramps, and weakness. The reported frequency of such mild symptoms is not clear, and muscle performance has not been examined with these medications. Accordingly, the present investigation, the Effect of Statins on Skeletal Muscle Function and Performance (STOMP) study, will recruit approximately 440 healthy persons. Participants will be randomly assigned to treatment with atorvastatin 80 mg/d or placebo. Handgrip, elbow and knee isometric and isokinetic strength, knee extensor endurance, and maximal aerobic exercise performance will be determined at baseline. Participants will undergo repeat testing after 6 months of treatment or after meeting the study definition of statin myalgia. This study will determine the effect of statins on skeletal muscle strength, endurance, and aerobic exercise performance and may ultimately help clinicians better evaluate statin-related muscle and exercise complaints.
Hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors or statins are the most effective medications for reducing elevated concentrations of low-density lipoprotein cholesterol (LDL-C). Recommendations of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III)1,2 indicate that more patients will be treated with higher doses of statins. Statins have been extremely well-tolerated in controlled clinical trails but can produce a skeletal myopathy with symptoms ranging from mild complaints such as myalgia, cramps, and weakness to rhabdomyolysis with renal failure. The frequency of statin-induced myalgia is not well-defined3–5 but may compromise medication compliance and quality of life. Muscle weakness can also occur with statin therapy,5 but muscle performance has not been carefully examined in statin users. The Effect of Statins on Skeletal Muscle Function and Performance (STOMP) study will administer atorvastatin 80 mg/d or placebo to healthy persons for 6 months or until participants meet the study definition of myalgia to determine the incidence of statin-induced muscle complaints (myalgia and cramps) and to examine the effect of statins on skeletal muscle strength and endurance and aerobic exercise performance.
STUDY RATIONALE
Statins are among the most widely prescribed therapies in the United States.6 While the most serious risk associated with statin use—rhabdomyolysis—is rare,3 statins are more frequently associated with “mild muscle complaints” including myalgia, cramps, and weakness. The reported incidence of myalgia during therapy with the more powerful statins is highly variable, ranging from 1% in pharmaceutical company reports4 to 25%5 in studies involving patients. To date, it has been difficult to reconcile these discrepant reports with clinical trial data because statin-induced muscle complaints are typically not thoroughly examined in pharmaceutical industry–sponsored trials.7,8 Moreover, most trials report statin-induced muscle symptoms only when creatine kinase (CK) values exceed 10 times the upper limit of normal.9 This practice may underestimate statin-related muscle effects because myalgia can occur in the absence of significantly elevated CK.5,10
Muscle weakness is also a clinically acknowledged complication of statin use. Data from direct assessments of muscle weakness (ie, strength testing) and statins are limited and have produced inconsistent conclusions. For example, one study found reductions of 10% to 40% in hip abduction strength in 4 patients receiving statins referred for myopathy.5 Another documented statin-induced myopathic proximal weakness in patients with neurological disease. 11 Conversely, a larger cross-sectional study of statin users vs nonusers found slightly improved performance on a sit-to-stand chair test (a measure of proximal muscle strength) with statin use in older community-dwelling adults.12
There are also sparse data on the effects of statins on aerobic exercise performance. Atorvastatin improves oxygen uptake kinetics in post–myocardial infarction patients13 but decreases maximal oxygen uptake in myopathic patients.14 Interestingly, in the latter study, the resting respiratory exchange ratio (RER; an index of fat and carbohydrate metabolism) increased with statin therapy in both asymptomatic and symptomatic statin users, consistent with other reports suggesting that statins decrease or impair fat oxidation.15–17 An altered RER attributable to statins could alter exercise metabolism and diminish aerobic exercise performance.
How statins produce myalgia and muscle injury is unknown. One theory maintains that blocking cholesterol synthesis reduces the cholesterol content of skeletal muscle cell membranes, making the membranes unstable. However, muscle injury has not been correlated with cholesterol reduction,18 and blocking cholesterol synthesis with squalene synthase inhibitors does not produce myotoxicity, making cholesterol reduction alone an unlikely mechanism.19 Alternatively, statins reduce the production of isoprenoids, such as ubiquinone. Ubiquinone, or coenzyme Q10, participates in electron transport during oxidative phosphorylation in mammalian mitochondria. Serum ubiquinone levels decrease with statin treatment possibly because ubiquinone is transported in the LDL-C particle.20 We recently published evidence of a role for ubiquinone in exercise-induced muscle damage during statin use,21 although a small pilot study found no effect of concurrent coenzyme Q10 supplementation on statin-induced myalgia in patients with a previous history of myalgia.22 Other potential mechanisms for statin-induced myalgia include alterations in calcium handling such that calcium leaking from the mitochondria directly increases cytosolic calcium and impairs sarcoplasmic reticulum calcium cycling.23,24
Therefore, the STOMP study will address the current gaps in the literature with respect to statins, myalgia, and muscle performance in what will be, to the best of our knowledge, the first randomized double-blind clinical trail conducted specifically to address the direct effects of statins on exercise performance and skeletal muscle strength and endurance in humans.
METHODS
Study Overview
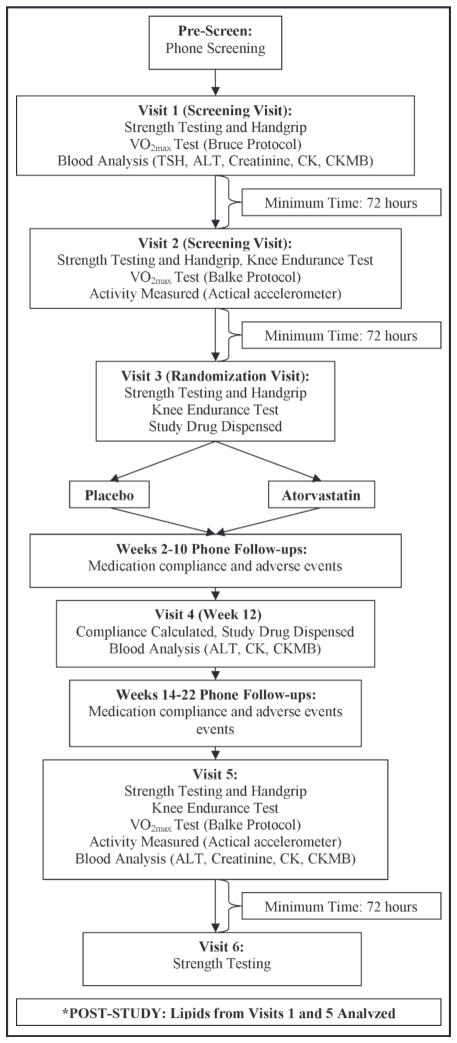
STOMP will recruit approximately 220 men and 220 women older than 20 years at 3 clinical sites (Hartford Hospital, University of Massachusetts, and University of Connecticut) over 5 years. Baseline lipid, liver, kidney, thyroid, and CK measurements will be obtained. Participants will complete a baseline muscle symptom questionnaire and undergo baseline exercise testing including a maximal exercise test with gas analysis; handgrip, elbow flexor, and knee extensor strength testing; and a knee extensor endurance exercise test. Participants will then be randomly assigned to identical placebo or atorvastatin 80 mg in a double-blind fashion. Participants will be called twice monthly to ascertain symptoms and undergo a safety assessment including CK values and liver function testing after 3 months of treatment. Participants will undergo repeat testing after 6 months of treatment or after development of muscle symptoms meeting the study definition of statin-induced myopathy. Baseline testing will be performed during 3 testing days. Six-month testing will be performed during 2 testing days. Testing days will be spaced 3 to 7 days apart. (Figure 1).
Figure 1.
Protocol for the Effect of Statins on Skeletal Muscle Function and Performance (STOMP) study. *Blood lipid analyses from visits 1 and 5 for total, low-density lipoprotein, and high-density lipoprotein cholesterol as well as triglycerides will be performed after the study is completed as described in the methods section to preserve blinding. TSH indicates thyroid-stimulating hormone; CK, creatine kinase; CKMB, creatine kinase MB; ALT, alanine aminotransferase.
Study Monitoring
The STOMP study is approved by the institutional review boards at Hartford Hospital, University of Massachusetts, and University of Connecticut in agreement with the guidelines set forth by the Declaration of Helsinki. A data safety and monitoring board (DSMB) comprising 2 physicians and a statistician will oversee the project with biannual meetings. The purpose of the DSMB is to conduct periodic assessments of data quality and timeliness, participant recruitment, accrual and retention, participant risk versus benefit, performance of trial sites, and other factors that can affect study outcomes. In addition, significant adverse event reports as well as safety data (CK and alanine aminotransferase [ALT] values) will be provided to the DSMB at each meeting. Members will discuss and analyze these data to determine whether the trial should be stopped. Stopping rules are as follows: (1) the presence of a significantly higher frequency of adverse events related to the drug and (2) the emergence of unexpected serious adverse experience(s) not specified in the study. Findings and recommendations of the DSMB will be reported regularly to the institutional review board and National Institutes of Health.
Study Drug Preparation
The study pharmacists will compound identical-looking atorvastatin and placebo capsules. Atorvastatin tablets will be ground up, covered with lactose secundum artem, and fit into opaque capsules. Placebo capsules will be identical and filled with lactose alone; the weight of the placebo and atorvastatin capsules will be similar (within 15% of each other as determined by weighing random samples of capsules during the compounding process). Drug will be dispensed to participants according to the study randomization code, which consists of a 1:1 randomization pattern with a block size of 10 participants/block.
Study Population
Age affects muscle strength and is a potential risk factor for statin myopathy,25 so equal numbers of male and female participants will be recruited into the 20 to 39, 40 to 59, and 60 and older age groups. Participants will be excluded if they have had cancer within 5 years, hepatic disease (ALT > 2 times normal), renal disease (creatinine > 2 mg/L), diagnosed cardiac disease, peripheral vascular disease, or diabetes mellitus. Those presently or previously treated with lipid-lowering medications will also be excluded to avoid biasing the sample toward those who tolerate statin treatment. Other exclusion factors include treatment with medications known to alter statin metabolism9; hypothyroidism or hyperthyroidism, because these conditions are known to be associated with statin intolerance; muscle weakness; and physical disabilities prohibiting the strength and exercise performance measurements. LDL-C levels will not be a criterion for inclusion or exclusion from this study because recent clinical trials suggest that statins will be used in high-risk individuals regardless of their pretreatment LDL-C values. All participants will be instructed in a standard lipid-lowering diet and asked to maintain this throughout the study. All alcohol, medication, and drug use will be queried and recorded, and study physicians will ensure that persons using medications known to affect muscle metabolism are not recruited (eg, growth hormone). Changes in medication and drug use will be recorded throughout the study.
Serologic Markers
Serum lipid (total cholesterol, LDL-C, high-density lipoprotein cholesterol, and triglycerides), ALT, creatinine, thyroid-stimulating hormone, and CK levels will be obtained at baseline and at 6 months. Plasma will be frozen and serum lipids will be measured at the end of the study to preserve blinding. Three-month levels of ALT and CK will be assessed to ensure participant safety on study drug. Participants with ALT levels > 3 times the upper normal limit and CK > 10 times the upper normal limit will be discontinued from the study.
Anthropometric Measurements
Body size is an important determinant of statin blood concentration and statin myopathy. Body weight will be measured at baseline and 6 months using a calibrated balance beam scale. Height will be determined using a wall-mounted tape measure. Waist circumference will be measured using a non-distensible tape measure.
Muscular Strength
Handgrip isometric strength of the dominant hand will be assessed using a handgrip dynamometer. Three maximal contractions for each isometric strength test will be assessed. Each contraction will last 3 seconds and 1 minute of rest will be allowed between contractions. An average of the 3 trials will be used as the criterion score. Elbow flexion/extension and knee extension/flexion isometric and isokinetic tests will be performed using a Biodex System 3 isokinetic dynamometer (Biodex Medical, Shirley, NY). Participants will warm up by performing 3 submaximal contractions before each test. For the elbow, participants will perform 3 isometric contractions with a duty cycle of 3:60 seconds at an elbow angle of 90 degrees. After 5 minutes’ rest, participants will perform 4 contractions at 1.05 rad/s (60 deg/s) followed by an additional 5 minutes’ rest and 4 contractions at 3.14 rad/s (180 deg/s). For the knee, participants will perform 3 isometric contractions with a knee angle of 110 deg. After 5 minutes’ rest, participants will perform 4 contractions at 1.05 rad/s (60 deg/s) followed by an additional 5 minutes’ rest and 4 contractions at 3.14 rad/s (180 deg/s). Data from the first study visit will be considered practice. Data from the second and third visits will be averaged to obtain baseline variables, and averaged data from the fifth and sixth visits will be used as the 6-month results.
Muscle Endurance Test
The knee endurance test consists of 30 consecutive maximal contractions at 3.14 rad/s. The fatigue index, a measure of muscular endurance, will be calculated as the percentage decrease in peak torque, as calculated by the following formula: .26
Aerobic Exercise Performance
Aerobic exercise performance and ventilatory threshold will be determined using a Parvomedics TrueOne 2400 metabolic cart (ParvoMedics Corp, Sandy, UT) and a breath-by-breath method. A physician- supervised Bruce Protocol treadmill test with electrocardiographic monitoring will be employed at the first visit to exclude those with documented cardiac ischemia. A modified Balke treadmill testing protocol will be used at the second and fifth visits and will be used to assess changes in aerobic exercise performance pre- and post-study drug administration.27
Habitual Physical Activity
Participants’ physical activity levels will be documented at baseline, 3 months, and 6 months using the Paffenbarger Physical Activity Questionnaire.28 Participants’ physical activity levels will also be objectively measured at baseline and 6 months using an Actiwatch (Actigraph, Pensicola, FL) accelerometer recorder for a 96-hour period pre- and post-treatment. These data will allow us to determine whether statin symptoms are more likely to develop in active participants and whether statin-treated participants decrease their level of activity even in the absence of symptomatic complaints.
Documentation of Myalgia/Muscle Symptoms
Participants will be contacted by phone twice monthly during the study to inquire about muscle complaints using the Short-Form McGill Pain Questionnaire (SF-MPQ)29 and Short-Form Brief Pain Inventory (BPI-SF).30 These surveys will be used to measure the location and intensity of participants’ muscle pain as well as the extent to which the muscle pain interferes with daily functioning. Both questionnaires will also be completed at all study visits. For the purpose of the study, participants will be considered to have statin-related complaints if all of the following occur:
They report new or increased myalgia, cramps, or muscle aching, unassociated with recent exercise.
These symptoms have persisted for at least 2 weeks.
The symptoms resolve within 2 weeks of stopping the study drug.
The symptoms reoccur within 4 weeks of restarting the medication.
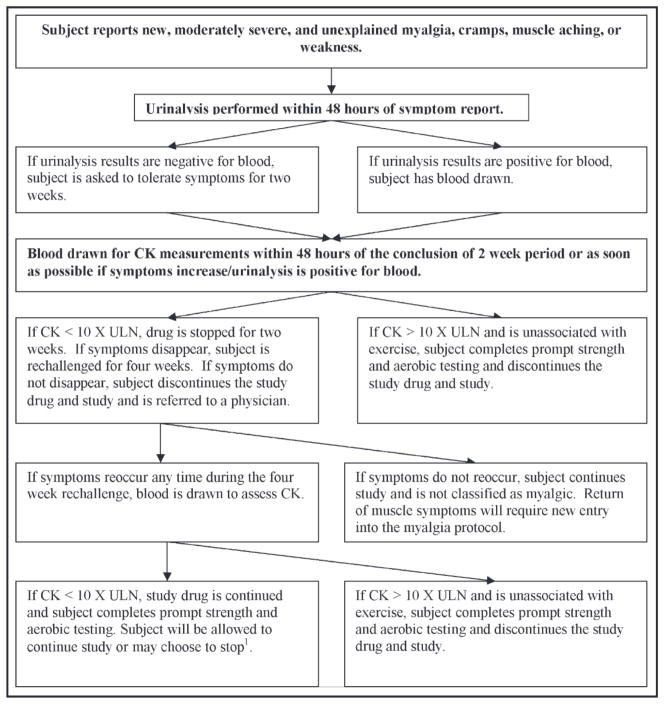
If a participant is classified as myalgic, he or she will undergo prompt physiologic testing of skeletal muscle strength and endurance and aerobic exercise performance (Figure 2).
Figure 2.
Myalgia decision tree. When a participant complains of new or increased myalgia, cramps, or muscle aching that persists for 2 weeks, he or she enters the structured protocol for definition of myalgia. CK indicates creatine kinase; UNL, upper normal limits. Strength and aerobic testing is defined as visits 5 and 6 from the study protocol (Figure 1). 1 Participants who choose to continue in the study will be assessed as planned at 6 months to investigate the effects of continued myalgia on muscle strength and aerobic performance.
Study Unblinding
After participants have completed the study, their pre- and post-study lipid results will be analyzed and their study drug and lipid levels before and after treatment revealed to them via a standardized letter. This will be performed by a Hartford Hospital research nurse who is not involved with any study procedures. The research nurse will keep lipid and unblinding results confidential from all other study personnel and investigators. The participant will be given specific instructions to direct any post-study questions regarding drug assignment and lipid levels to the research nurse only, so that the investigators and study personnel remain blinded until all data collection is completed. After the last participant has completed the study, investigators will be unblinded.
Standardization Among Sites
To control for any differences among sites, each site will use an identical protocol, source documents, and testing equipment purchased from the same manufacturers. Between-site and within-site quality assurance testing was performed before the start of the study. Furthermore, the principal investigator and study coordinator will monitor testing techniques at all 3 sites twice each year, and research personnel from each site will meet on a bimonthly basis. Data will be entered into a Web-based data entry system (Microsoft SQL Database) by designated study personnel at each site and checked for completeness, inconsistencies, and aberrant values by designated study personnel. In the instance of unexpected values in any database fields, source documents will be used to confirm validity.
Proposed Statistical Analyses
The study will not be analyzed in an “intention to treat” analysis because the goal is to determine the effect of treatment on exercise and muscle performance in participants taking atorvastatin. Consequently, active treatment participants who do not have a reduction in LDL-C by at least 25% will not be included in the analysis. Standard diagnostics will be used to determine whether assumptions of variance homogeneity, normality, and existence of outliers are met for all data. To determine the incidence of statin-induced mild muscle complaints, the proportion of participants with complaints will be compared between the statin and placebo groups using Pearson’s chi-square. Possible effect modifiers (eg, baseline LDL-C, sex, age, imbalances in the randomization) will be explored using logistic regression with interaction terms. To determine the effects of statins on skeletal muscle function and aerobic performance, we will investigate whether statin participants differ from placebo participants in outcome measures derived from physiologic testing (eg, muscle strength, maximal oxygen uptake, ventilatory threshold). For each outcome measure, a repeated measures analysis of variance will be used to model pre- and post-study comparisons, with between-participant effects of study drug modeled alone and in interaction with potential modifiers such as age and sex. The influence of continuous covariates (eg, baseline LDL-C, baseline muscle strength, baseline CK levels) will be analyzed with analysis of covariance. To address the potential difficulty of missing response data when participants discontinue the study because of muscle complaints, we will use two approaches: averaging the physiologic measurements obtained when participants withdraw and using multiple imputation techniques to account for missing data.
DISCUSSION
STOMP is, to our knowledge, the first clinical trial designed specifically to determine the incidence of mild muscle complaints associated with statin use as well as the effects of statins on skeletal muscle endurance and strength and aerobic exercise performance. STOMP is funded by the National Heart, Lung and Blood Institute, and data collection began in February 2008. To date, 264 participants are enrolled in the study.
Future Studies
During the initial visit, blood will be obtained into EDTA-containing tubes for white cell separation to obtain DNA for genetic testing. Samples will be stored for future genetic analyses, which will investigate the presence of variants in selected candidate genes in participants in whom myalgia does and does not develop and/or muscle performance changes develop during statin therapy. Candidate genes will be identified from our prior muscle expression profiling results during statin therapy21 and published literature regarding other genes related to muscle size and strength and statin metabolism and myopathy. This genetic analysis will be conducted at a later date in a separate study. In addition, archived serum and plasma samples from each participant at baseline, mid-study, and post-study are being stored for future investigations involving factors that may modulate the relationship between atorvastatin and muscle outcomes. Three years after the primary data analysis is completed, we will make the data from this study available to other investigators who wish to conduct additional analyses.
Study Limitations
The rates of mild muscle complaints, such as myalgia, in pharmaceutical industry–sponsored trials have averaged only 1% to 3%. Consequently, it is possible that our selected sample size will not include sufficient myalgic patients to determine a true incidence. However, most clinicians estimate the rate of myalgia on statins as considerably higher than 3%, and one recognized statin myalgia expert maintains that the rate is 25% in clinically treated patients.5 Indeed, current literature suggests that 7% to 20% of patients taking statins complain of musculoskeletal pain at various statin doses.31–36 We have accordingly powered our current study based on estimates that 10% of statin participants and 2% of nonstatin participants will have muscle complaints. Nevertheless, failing to document myalgia and other mild statin myopathic effects in this sample size using the strict criteria we will employ will in itself be an important finding.
We selected atorvastatin because it is the most widely used statin. However, the 80-mg atorvastatin dose chosen for the study is not used frequently, possibly reducing the generalizability of the results to clinical practice. The rationale for selecting 80 mg was because recent research indicates a reduction in cardiac events with high-dose statin therapy,37 and the use of high doses of powerful statins will likely increase in accordance with NCEP ATP III recommendations. Therefore, the current study, while not investigating the effects of all types and doses of statins on muscle function and myalgia, will have the potential to shape future recommendations regarding high-dose statin therapy and may also stimulate subsequent investigations regarding dose-response effects of statins on skeletal muscle.
Participants in STOMP will be treated with a statin for only 6 months. Clinically, many patients who complain of weakness possibly associated with statin therapy experience such problems after years of treatment. In contrast, many patients with statin rhabdomyolysis experience it within the first year of statin therapy.38 We selected 6 months as the treatment duration to facilitate participant retention and to provide sufficient time for data collection and analysis within the usual duration of National Institutes of Health support, but this duration will not be sufficient to detect delayed-onset muscle symptoms or effects.
The STOMP study population may not be fully representative of the typical individual who initiates statin therapy, as participants in STOMP are free of overt cardiovascular or metabolic disease and more likely to have low cholesterol because they must be statin-naïve to enter the study. However, given the large sample size of the study population, we will be able to investigate the interactions between various baseline characteristics on our outcome variables to determine whether individual participant characteristics influence the effect of statins on skeletal muscle or predispose an individual to myopathy. Moreover, we hypothesize that changes in muscle function and aerobic performance from statins will be greater in a population with more comorbidities, including those known to increase susceptibility to statin myopathy. Thus, results from the current study may actually underestimate the true effects of statins on myalgia, muscle strength and endurance, and aerobic performance.
SUMMARY
The use of statins is widespread. Given the recent NCEP ATP III guidelines as well as multiple studies demonstrating that statins reduce the incidence of cardiac events, the prescription of statins for cardiovascular health will likely continue to increase. However, the lack of data concerning the direct effects of statins on skeletal muscle function represents a significant gap in clinical knowledge that will be addressed by the STOMP study. STOMP aims to quantify the common complaints of muscle weakness and cramping often reported by patients receiving statin therapy by developing a strict definition of statin-induced myopathy. STOMP will also investigate the effects of statins on skeletal muscle strength and endurance as well as aerobic performance in a large, statin-naïve study sample. These results should help clinicians better understand the effects of statins on skeletal muscle function and may ultimately yield clinical tests useful in evaluating statin-related muscle and exercise complaints.
Acknowledgments
The authors wish to acknowledge the following individuals for their scientific contributions: Gualberto Ruano, MD, PhD, Theodore Holford, PhD, JoAnne Foody, MD, Pamela Hartigan, PhD, and Ira Ockene, MD. The STOMP study is funded by NHLBI/NIH grant RO1 HL081893A2 (P. Thompson). Dr Thompson is also a consultant for AstraZenica International, Merck & Company, Inc., Schering-Plough Corporation, Takeda Pharmaceutical Company Limited, Roche, and Genomas and is a member of the speaker’s bureau for Merck & Company, Inc., Pfizer, Inc., Abbott Labs, AstraZenica International, and Schering-Plough Corporation.
References
- 1.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3):720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346(7):539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- 4.Physician’s Desk Reference, Inc. Physicians’ Desk Reference. Montvale, NJ: Medical Economics; 2002. [Google Scholar]
- 5.Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137(7):581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Wynn RL. The top 50 prescription medications dispensed in pharmacies in 2007. Gen Dent. 2008;56(7):604–607. [PubMed] [Google Scholar]
- 7.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 9.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289(13):1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 10.Soininen K, Niemi M, Kilkki E, et al. Muscle symptoms associated with statins: a series of twenty patients. Basic Clin Pharmacol Toxicol. 2006;98(1):51–54. doi: 10.1111/j.1742-7843.2006.pto_193.x. [DOI] [PubMed] [Google Scholar]
- 11.Dobkin BH. Underappreciated statin-induced myopathic weakness causes disability. Neurorehabil Neural Repair. 2005;19(3):259–263. doi: 10.1177/1545968305277167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agostini JV, Tinetti ME, Han L, et al. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. 2007;55(3):420–425. doi: 10.1111/j.1532-5415.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M, Tumminello G, Reina G, et al. Atorvastatin therapy improves exercise oxygen uptake kinetics in post-myocardial infarction patients. Eur J Clin Invest. 2007;37(6):454–462. doi: 10.1111/j.1365-2362.2007.01805.x. [DOI] [PubMed] [Google Scholar]
- 14.Phillips PS, Phillips CT, Sullivan MJ, et al. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis. 2004;177(1):183–188. doi: 10.1016/j.atherosclerosis.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Paolisso G, Barbagallo M, Petrella G, et al. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients. Atherosclerosis. 2000;150(1):121–127. doi: 10.1016/s0021-9150(99)00352-4. [DOI] [PubMed] [Google Scholar]
- 16.Fisher NM, Meksawan K, Limprasertkul A, et al. Statin therapy depresses total body fat oxidation in the absence of genetic limitations to fat oxidation. J Inherit Metab Dis. 2007;30(3):388–399. doi: 10.1007/s10545-007-0449-6. [DOI] [PubMed] [Google Scholar]
- 17.Head A, Jakeman PM, Kendall MJ, et al. The impact of a short course of three lipid lowering drugs on fat oxidation during exercise in healthy volunteers. Postgrad Med J. 1993;69(809):197–203. doi: 10.1136/pgmj.69.809.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pederson TR, Kjekshus J, Berg K, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 19.Flint OP, Masters BA, Gregg RE, Durham SK. Inhibition of cholesterol synthesis by squalene synthase inhibitors does not induce myotoxicity in vitro. Toxicol Appl Pharmacol. 1997;145(1):91–98. doi: 10.1006/taap.1997.8131. [DOI] [PubMed] [Google Scholar]
- 20.Ghirlanda G, Oradei A, Manto A, et al. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J Clin Pharmacol. 1993;33(3):226–229. doi: 10.1002/j.1552-4604.1993.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 21.Urso ML, Clarkson PM, Hittel D, et al. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. Arterioscler Thromb Vasc Biol. 2005;25(12):2560–2566. doi: 10.1161/01.ATV.0000190608.28704.71. [DOI] [PubMed] [Google Scholar]
- 22.Young JM, Florkowski CM, Molyneux SL, et al. Effect of coenzyme Q(10) supplementation on simvastatin-induced myalgia. Am J Cardiol. 2007;100(9):1400–1403. doi: 10.1016/j.amjcard.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Liantonio A, Giannuzzi V, Cippone V, et al. Fluvastatin and atorvastatin affect calcium homeostasis of rat skeletal muscle fibers in vivo and in vitro by impairing the sarcoplasmic reticulum/mitochondria Ca2+-release system. J Pharmacol Exp Ther. 2007;321(2):626–634. doi: 10.1124/jpet.106.118331. [DOI] [PubMed] [Google Scholar]
- 24.Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol. 2008;8(3):333–338. doi: 10.1016/j.coph.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40(3):567–572. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 26.Katsiaras A, Newman AB, Kriska A, et al. Skeletal muscle fatigue, strength, and quality in the elderly: the Health ABC Study. J Appl Physiol. 2005;99(1):210–216. doi: 10.1152/japplphysiol.01276.2004. [DOI] [PubMed] [Google Scholar]
- 27.Whaley ME, Brubaker PH, Otto RM, editors. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 7. Philadelphia, PA: Lippincott Williams and Wilkins; 2006. pp. 99–102. [Google Scholar]
- 28.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 29.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 30.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Bradford RH, Shear CL, Chremos AN, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med. 1991;151(1):43–49. doi: 10.1001/archinte.151.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Gaist D, Rodriguez LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology. 2001;12(5):565–569. doi: 10.1097/00001648-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen TR, Berg K, Cook TJ, et al. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1996;156(18):2085–2092. [PubMed] [Google Scholar]
- 34.Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116(6):408–416. doi: 10.1016/j.amjmed.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol. 2002;40(2):163–171. doi: 10.1097/00005344-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Ucar M, Mjorndal T, Dahlqvist R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf. 2000;22(6):441–457. doi: 10.2165/00002018-200022060-00003. [DOI] [PubMed] [Google Scholar]
- 37.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 38.Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy–a genomewide study. N Engl J Med. 2008;359(8):789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]