Abstract
Programmed death-ligand 1 (PD-L1) blockade is accepted as a novel strategy for the reactivation of exhausted T cells that express programmed death-1 (PD-1). However, the mechanism of PD-L1-mediated inhibitory signalling after PD-L1 cross-linking by anti-PD-L1 monoclonal antibody (mAb) or PD-1–immunogloblin fusion protein (PD-1-Ig) is still unknown, although it may induce cell death of PD-L1+ cells required for regular immune reactions. In this study, PD-1-Ig or anti-PD-L1 mAb treatment was tested in cell lines that expressed PD-L1 and bovine lymphocytes to investigate whether the treatment induces immune reactivation or PD-L1-mediated cell death. PD-L1 cross-linking by PD-1-Ig or anti-PD-L1 mAb primarily increased the number of dead cells in PD-L1high cells, but not in PD-L1low cells; these cells were prepared from Cos-7 cells in which bovine PD-L1 expression was induced by transfection. The PD-L1-mediated cell death also occurred in Cos-7 and HeLa cells transfected with vectors only encoding the extracellular region of PD-L1. In bovine lymphocytes, the anti-PD-L1 mAb treatment up-regulated interferon-γ (IFN-γ) production, whereas PD-1-Ig treatment decreased this cytokine production and cell proliferation. The IFN-γ production in B-cell-depleted peripheral blood mononuclear cells was not reduced by PD-1-Ig treatment and the percentages of dead cells in PD-L1+ B cells were increased by PD-1-Ig treatment, indicating that PD-1-Ig-induced immunosuppression in bovine lymphocytes could be caused by PD-L1-mediated B-cell death. This study provides novel information for the understanding of signalling through PD-L1.
Keywords: bovine immunology, cell death, immunoglobulin fusion protein, programmed death-ligand 1
Introduction
Programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) are known as an immunoinhibitory receptor and its ligand that can induce exhausted T cells, and the PD-1/PD-L1 pathway is involved in immune dysfunction associated with several tumours and chronic infections.1,2 Treatment with anti-PD-1 or -PD-L1 monoclonal antibody (mAb) blocks this inhibitory pathway, significantly enhances the T-cell response, and improves clinical conditions.3–6 On the basis of these reports, clinical trials have been conducted in patients with cancer who were given anti-PD-1 and PD-L1 mAb.7–9
Immunoglobulin fusion protein is one of the protein-engineering techniques used to increase or decrease serum half-life.10 It is also suitable for the detection of the binding partner of ‘orphan receptors’ whose ligands are unknown11 and stimulation or blockage of the binding receptor. The PD-1/PD-L1 pathway has also been investigated using a soluble PD-1–immunogobulin fusion protein (PD-1-Ig). PD-1-Ig treatment blocked the pathway and overcame immunosuppression induced by either PD-L1 or PD-L2, another ligand of PD-1.12–14 However, the opposite phenomenon has also been reported; dendritic cells exposed to PD-1-Ig were altered to a suppressive phenotype, resulting in the inhibition of antigen-specific T-cell proliferation.15 Moreover, PD-1-Ig treatment promotes programmed cell death of activated CD4+ T and B cells.16,17 These reports suggest that the binding partners of PD-1-Ig, PD-L1 or PD-L2 have unknown functions.
It was once believed that PD-L1 was simply a ligand for PD-1 because the intracellular regions of mouse, human and bovine PD-L1 were only approximately 30 amino acids long, and there was no known functional domain.18 However, PD-L1-induced signalling is now believed to be present; this signalling may have unknown functions, however, with the accumulation of conflicting reports on PD-L1 as the activation or inhibitory receptor. For example, PD-L1-deficient T cells express lower Bcl-xl, which is an anti-apoptosis gene, than wild-type cells and are more sensitive to killing by cytotoxic T cells in vivo, indicating that PD-L1 is essential for the survival of activated T cells.19 It has also been reported that PD-L1 promotes stable immunological synapse formation.20 These two reports suggest that PD-L1 works as a receptor for immune activation. In contrast, PD-L1 cross-linking induced apoptosis of Epstein–Barr virus-transformed B cells, indicating that PD-L1 can act as an inhibitory receptor, which induces apoptotic signalling.17
PD-L1 ligation by CD80, which is also a binding partner with CD28 and cytotoxic T-lymphocyte antigen 4 (CTLA-4), complicates our understanding of PD-L1 function as a receptor. PD-L1 has been observed to interact with CD80 on T cells isolated from Cd28−/−, Ctla4−/−, or Pdcd1−/− mice and inhibits proliferation and cytokine production in PD-L1+ T cells.21,22 Meanwhile, several studies have demonstrated that PD-L1 is able to stimulate T-cell function.14,23,24 For example, T cells are activated by PD-L1 expressed on natural killer cells through independent mechanisms from PD-1.25
In our previous report, augmented PD-L1 expression was confirmed in bovine leukaemia virus (BLV) -infected cattle,18 and PD-L1 blockade was accepted as an appropriate strategy to improve immune responses against tumours and chronic infections.2,18 In this study, PD-L1-expressing cell lines and lymphocytes isolated from BLV-infected cattle were treated with bovine PD-1-Ig or anti-bovine PD-L1 mAb to investigate the effect of PD-L1 blockade and PD-L1 cross-linking on immune reactions and cell death.
Materials and methods
Expression of recombinant soluble bovine PD-L1-Ig fusion protein
The cDNA encoding the extracellular domain fragment of bovine PD-L1 without a leader sequence was amplified by PCR and inserted into the cloning site of a modified pCAGGS (provided by Dr J. Miyazaki, Osaka University26) that contained a mouse CD150 leader sequence at the N terminus and the Fc fragment of rabbit IgG at the C terminus.11 PD-L1-Ig was expressed in Chinese hamster ovary (CHO)-DG44 cells (Dr Y. Suzuki, Hokkaido University, unpublished data), stably transfected with Lipofectamine LTX reagent (Life Technologies, Carlsbad, CA), and purified from the media with Ab-Capcher ExTra (ProteNova, Tokushima, Japan), according to the manufacturer's protocol. The purification of PD-L1-Ig was confirmed by SDS–PAGE, and the quantity of PD-L1-Ig was determined by Rabbit IgG ELISA Quantification Set (Bethyl Laboratories, Montgomery, TX).
Stable expression of bovine PD-L1 on CHO-DG44 cells
Part of the bovine PD-L1 gene encoding the whole extracellular domain was cloned into pEGFP-N2 vector (Clontech, Mountain View, CA; Fig. 1). The plasmid that contained enhanced green fluorescent protein (EGFP) at the C terminus was transfected into CHO-DG44 cells using Lipofectamine LTX; the cells were selected by the medium containing G418 (800 μg/ml; Enzo Life Sciences, Farmingdale, NY) for 10 days and cloned by limiting dilution. The stable cell lines were screened for fluorescence using a FACSVerse® flow cytometer (BD Biosciences, San Jose, CA), and the three cell lines that showed the brightest fluorescence were used for screening of anti-bovine PD-L1 mAbs. PD-L1 expression on the cell membrane was determined by the LSM 700 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
Figure 1.
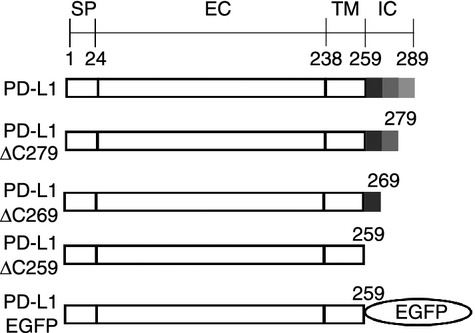
Schematic representation of programmed death ligand 1 (PD-L1), PD-L1-ΔC279, PD-L1-ΔC269, PD-L1-ΔC259, and PD-L1-EGFP. PD-L1, PD-L1-ΔC279, PD-L1-ΔC269, and PD-L1-ΔC259 were inserted in pCIneo and PD-L1-EGFP was inserted in pEGFP-N2. Numbers indicate the amino acid number of bovine PD-L1. Grey region indicates the intracellular domain of PD-L1. SP, signal peptide; EC, extracellular domain; TM, transmembrane domain; IC, intracellular domain.
Generation of anti-bovine PD-L1 mAb
A rat was immunized with 170 μg of PD-L1-Ig emulsified with complete Freund's adjuvant. After 24 hr, lymphocytes isolated from the iliac lymph node were fused with myeloma cells. Supernatants from the hybridomas were screened by flow cytometry using the three cell lines that stably expressed PD-L1 with EGFP and Cos-7 cells that were transiently transfected with bovine PD-L1 encoding pCIneo (Promega, Madison, WI). Hybridomas producing antibodies that recognized PD-L1 but not EGFP were cloned by limiting dilution. Rat immunization and hybridoma cultivation were performed at Cell Engineering Corporation (Osaka, Japan). In this study, two types of the mAb, 4G12 (rat IgG2a) and 5A2 (rat IgG1), were used.
Expression of recombinant soluble bovine PD-1-Ig
A gene encoding the extracellular domain of bovine PD-1 (amino acid numbers 1–171) coupled with the Fc region of bovine IgG1 (Fig. 2) was commercially synthesized according to preferential codon usage of mammalian cells in Medical and Biological Laboratories (Nagoya, Japan) and inserted into pDN11 (Dr Y. Suzuki, Hokkaido University, unpublished data). To reduce the antibody-dependent cell-mediated cytotoxicity response to PD-1-Ig treatment, the mutation was introduced into the binding sites for Fcγ receptors as described elsewhere (Fig. 2).27,28
Figure 2.
Amino acid sequences of the extracellular region of bovine programmed death 1 (PD-1), bovine IgG1-Fc region, and bovine PD-1-Ig. GenBank accession numbers are described in each title. Double lines indicate mutation sites for the reduction of the antibody-dependent cell-mediated cytotoxicity response.
CHO-DG44 cells were transfected with pDN11 that coded PD-1-Ig and were selected in CD OptiCHO AGT medium (Life Technologies) supplemented with 800 μg/ml G418. After 3 weeks, the cells were screened for the ability to produce PD-1-Ig by dot blotting and ELISA with anti-bovine IgG Fc (Rockland, Gilbertsville, PA). PD-1-Ig expression was also confirmed by SDS–PAGE and Western blotting using horseradish peroxidase-conjugated anti-bovine IgG Fc (Rockland), as previously described.29 Ten cell lines producing high amounts of PD-1-Ig were cloned by limiting dilution and screened again. Gene amplification was subsequently performed using medium, containing 60 nm methotrexate (Enzo Life Sciences), and screened again. PD-1-Ig was produced by the shake cultivation of the top three cell lines that produced the highest amount of PD-1-Ig in the medium without G418 and methotrexate and purified with Ab-Capcher ExTra, according to the manufacturer's protocol. To confirm the interaction between PD-1-Ig and PD-L1, PD-L1-expressing Cos-7 cells or CHO-DG44 cells were stained with PD-1-Ig and FITC-conjugated anti-bovine IgG Fc (Rockland) or PD-1-Ig, biotin-conjugated anti-bovine IgG Fc (Rockland), and allophycocyanin-conjugated streptavidin (BioLegend, Cambridge, UK).
Lymphocytes preparation and B-cell depletion
Peripheral blood mononuclear cells (PBMCs) were purified from the heparinized blood of BLV-infected cattle by density gradient centrifugation using Percoll (GE Healthcare, Chalfont St Giles, UK). This study was conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of Hokkaido University, Japan (approval number: 11-0059). Informed consents were obtained from clinical veterinarians and farmers before sample collection. BLV infection was diagnosed by amplifying BLV provirus by nested-PCR from genomic DNA of blood lymphocytes, as previously described.18
For B-cell depletion, PBMCs were stained with anti-IgM (BIG73A; VMRD, Pullman, WA) and anti-mouse IgG1 MicroBeads (Miltenyi Biotec, Bergish Gladbach, Germany) and depleted with the autoMACS Pro separator (Miltenyi Biotec) according to the manufacturer's protocol. We confirmed that > 95% of IgM+ B cells were depleted from the PBMCs by flow cytometry.
Plasmids for PD-L1 expression on the cell membrane
Schematic representation of PD-L1 proteins is given in Fig. 1. DNA fragments encoding whole PD-L1 and the C-terminal deletions of PD-L1 were amplified by forward primer (5'-CTA GCT AGC ACC ATG AGG ATA TAT AGT GTC TTA AC-3'), containing a restriction site for NheI, and following reverse primers containing a restriction site for XhoI; 5'-CAA TCT CGA GTT ACG TCT CCT CAA ACT GTG TAG-3' for whole PD-L1; 5'-CAA TCT CGA GTT ACT TTG AAT TCA TAT CTC GGG-3' for PD-L1-ΔC279; 5'-CAA TCT CGA GTT ATT CTA CAT CCA TCA TTC TCA C-3' for PD-L1-ΔC269; 5'-CAA TCT CGA GTT ACA GAC AGA AGA TGA CTG C-3' for PD-L1-ΔC259. These were cloned into pCIneo. The construction of PD-L1-EGFP is described above. The pcDNA3 (Life Technologies) encoding leukotriene B4 receptor (BLT1) tagged with Flag at the N-terminal was provided by Dr T. Yokomizo (Juntendo University, Japan30) and used as a negative control.
Cell apoptosis assays
Cos-7 and Hela cells were grown in RPMI-1640 medium (Sigma-Aldrich, St Louis, MO) supplemented with 10% fetal bovine serum (Cell Culture Technologies, Zurich, Switzerland) and a mixture of 2 mmol l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies). The cells were transfected with the above plasmids by Lipofectamine 2000 reagent (Life Technologies); PD-1-Ig (50 μg/ml) or anti-PD-L1 mAbs (4G12 and 5A2; 20 μg/ml) with goat anti-rat IgG Fc (12 μg/ml; Thermo Scientific, Waltham, MA) was added into the medium after 2 days of transfection. Bovine IgG (Sigma-Aldrich) or rat IgG (Sigma-Aldrich) was used as a negative control for PD-1-Ig or anti-PD-L1 mAbs. After 1 day, the cells were harvested, and PD-L1 or BLT1 expression was confirmed by EGFP fluorescence for PD-L1-EGFP by staining with anti-DYKDDDDK antibody31 (2H8; Trans Genic, Kumamoto, Japan) and phycoerythrin (PE)-conjugated anti-mouse IgG for BLT1-Flag and staining with anti-PD-L1 mAb (4G12) and anti-rat IgG PE (Beckman Coulter, Brea, CA) for other plasmids. After surface staining, the cells were stained with Annexin-V-allophycocyanin (BD Biosciences) and 7-aminoactinomycin D (7-AAD) (BD Biosciences), as per the manufacturer's instructions.
The PBMCs were cultivated with bovine IgG or PD-1-Ig (50 μg/ml) for 2 days. Cells were stained with following antibodies: anti-CD4 (CACT138A; VMRD) pre-labelled with Zenon PE (Life Technologies), anti-CD8 (CACT80C; VMRD) pre-labelled with Zenon Alexa Fluor 647 (Life Technologies), or anti-IgM (BIG73A) pre-labelled with Lightning-Link PE-Cy7 (Innova Biosciences, Cambridge, UK). To investigate the effect of PD-1-Ig on cell death in PD-L1+ cells, the cells were stained with anti-PD-L1 (4G12) with allophycocyanin-conjugated anti-rat IgM + IgG (Beckman Coulter) and anti-IgM with PE-conjugated anti-mouse IgG. After washing, the cells were stained with Annexin-V-FITC (BD Biosciences) and 7-AAD and analysed by flow cytometry.
Interferon-γ and interleukin-10 ELISA
The PBMCs or B-cell-depleted PBMCs were cultivated with bovine IgG or PD-1-Ig (50 μg/ml) in the presence or absence of PMA (Sigma-Aldrich) and ionomycin (20 ng/ml and 1 μg/ml; Sigma-Aldrich). After 2 days, the supernatants were collected and frozen for ELISA. Interferon-γ (IFN-γ) production was measured by ELISA for bovine IFN-γ (Mabtech, Nacka Strand, Sweden) according to the manufacturer's protocol. Sandwich ELISA of interleukin-10 (IL-10) was performed as previously described.29 One arbitrary unit was defined as the amount of IL-10 contained in 15·625 μl of the bovine plasma stimulated with concanavalin A and lipopolysaccharide (10 μg/ml each; Sigma-Aldrich). Reported data were expressed as mean of duplicate ELISA.
Proliferation assay
The PBMCs were cultivated with bovine IgG or PD-1-Ig (50 μg/ml) in the presence of PMA and ionomycin for 3 days as described above. [3H]Thymidine (0·5 μCi/well; ICN Biochemicals, Irvine, CA) was added to the culture, and the cells were cultured for an additional 6 hr and harvested onto glass filters using a 96-well cell harvester (Perkin Elmer, Waltham, MA). The incorporated radioactivity was measured using a liquid scintillation counter (Aloka, Tokyo, Japan). All samples were tested in triplicate, and the data were presented as the mean stimulation index.
Statistical analysis
Two-way analysis of variance and Wilcoxon matched-pairs test were performed using GraphPad Prism version 5.0. P-values of < 0·05 were considered to be statistically significant.
Results
Preparation of recombinant bovine PD-1-Ig and anti-bovine PD-L1 mAbs
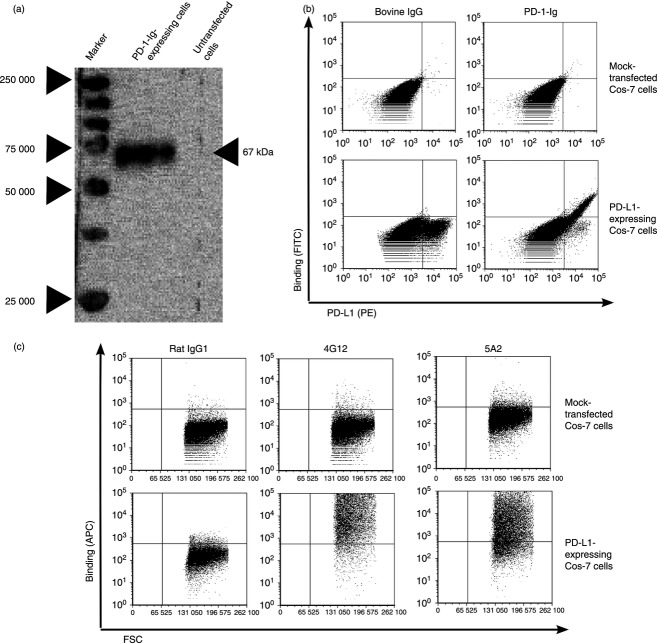
PD-1-Ig expression was confirmed by Western blotting with anti-bovine IgG Fc. PD-1-Ig was produced in the supernatant from PD-1-Ig-expressing CHO-DG44 cells and was detected at approximately 67 000 molecular weight (Fig. 3a). To confirm the binding of PD-1-Ig and anti-PD-L1 mAbs with PD-L1 on the cell membrane, Cos-7 and CHO-DG44 cells that expressed bovine PD-L1 were stained with PD-1-Ig and anti-PD-L1 mAbs. Both PD-1-Ig and two types of anti-PD-L1 mAbs (4G12 and 5A2) recognized the PD-L1-expressing cells (Fig. 3b,c and see Supporting information, Fig. S1).
Figure 3.
Establishment of programmed death 1–immunoglobulin (PD-1-Ig) and anti-bovine programmed death ligand 1 (PD-L1) monoclonal antibodies (mAbs). (a) Western blotting of PD-1-Ig. Anti-bovine IgG Fc antibody recognized PD-1-Ig at approximately 67 000 molecular weight from the supernatant of PD-1-Ig-expressing CHO-DG44 cells. (b, c) PD-1-Ig (b) and two types of anti-PD-L1 mAbs (c) recognized the PD-L1-expressing Cos-7 cells, not mock-transfected Cos-7 cells. Bovine IgG (for PD-1-Ig; b), rat IgG1 (for 5A2; c), and rat IgG2a (for 4G12, data not shown; c) were used as negative control.
PD-1-Ig treatment increased cell death in PD-L1-expressing cell lines
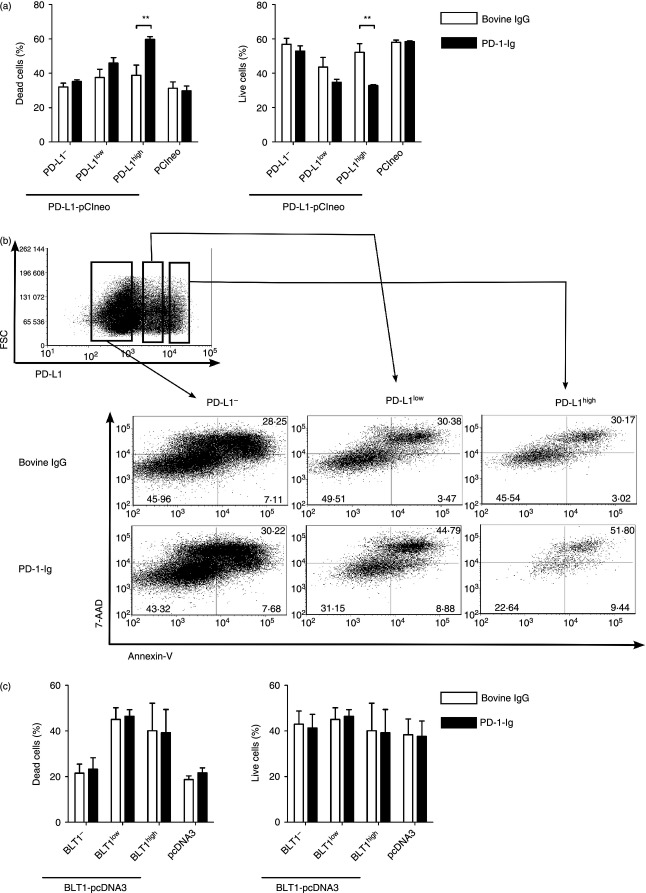
To investigate the influence of PD-L1 cross-linking by PD-1-Ig on the cell viability of PD-L1-expressing cells, Cos-7 cells were transiently transfected with the vectors encoding bovine PD-L1 and incubated with 50 μg/ml of PD-1-Ig. We confirmed that PD-1-Ig did not bind to both untransfected and mock-transfected Cos-7 and Hela cells at this concentration in advance (data not shown). Annexin-V and 7-AAD staining revealed that dead cells (Annexin-V+ 7-AAD+) increased and live cells (Annexin-V− 7-AAD−) decreased only in PD-L1high cells; however, this did not occur in PD-L1− cells when the cells were incubated with PD-1-Ig compared with bovine IgG (Fig. 4a,b). There was no induction of cell death by the PD-1-Ig treatment in BLT1high, BLT1low and BLT1– cells (Fig. 4c), indicating that PD-1-Ig treatment specifically induces the apoptosis of PD-L1-expressing cells through signalling that is involved in cell viability.
Figure 4.
Cell death in programmed death ligand 1 (PD-L1) -expressing cells treated with programmed death 1–immunoglobulin (PD-1-Ig). (a, c) Percentages of dead cells (Annexin-V+ 7-AAD+) and live cells (Annexin-V− 7-AAD−) among Cos-7 cells transfected with pCIneo encoding PD-L1 (a) or pcDNA3 encoding BLT1 (c) and treated with bovine IgG or PD-1-Ig (50 μg/ml). pCIneo or pcDNA3 was used as transfection control. PD-L1−/low/high cells were distinguished by staining with anti-PD-L1 monoclonal antibody. (b) Gating strategy and representative dot plots of PD-L1-expressing Cos-7 cells stained with Annexin-V and 7-AAD. Values in the quadrant indicate the percentage of the cells. Mean values ± SEM from three independent experiments are shown. Statistical comparisons between bovine IgG and PD-1-Ig were made using the two-way analysis of variance. Differences were considered statistically significant at P < 0·05 (**P < 0·01).
PD-1-Ig-induced cell death was not dependent on the intracellular region of PD-L1
There is no known signalling motif in the intracellular region of PD-L1.2 To confirm whether the intracellular region of PD-L1 was involved in PD-L1-mediated cell death, Cos-7 cells transfected with the vectors encoding the C-terminal deletion mutants of PD-L1 were treated with PD-1-Ig. As expected, PD-1-Ig treatment increased the proportions of dead cells in PD-L1high cells when PD-L1-ΔC279, PD-L1-ΔC269 and PD-L1-ΔC259 were transfected (Fig. 5a–c). Moreover, the percentages of dead cells in the PD-L1-EGFPhigh cells were augmented (Fig. 5d), indicating that PD-L1-mediated cell death occurred through a mechanism that was independent of the intracellular region of PD-L1. Cell mortality in the EGFP-expressing cells was not affected by PD-1-Ig treatment. The percentage of live PD-L1high cells decreased after PD-1-Ig treatment among the cells transfected with all plasmids other than the control plasmids (data not shown). When HeLa cells were transfected with PD-L1-ΔC259 and treated with PD-1-Ig, the proportions of dead cells in the PD-L1-expressing cells increased in the same manner as the Cos-7 cells (Fig. 5e). Meanwhile, PD-L1 cross-linking by one type of anti-PD-L1 mAb, 4G12, but not 5A2, also induced the augmentation of the proportion of dead cells in the PD-L1-EGFP-expressing cells (Fig. 5f), suggesting that the binding with PD-1 was not essential for PD-L1-mediated cell death.
Figure 5.
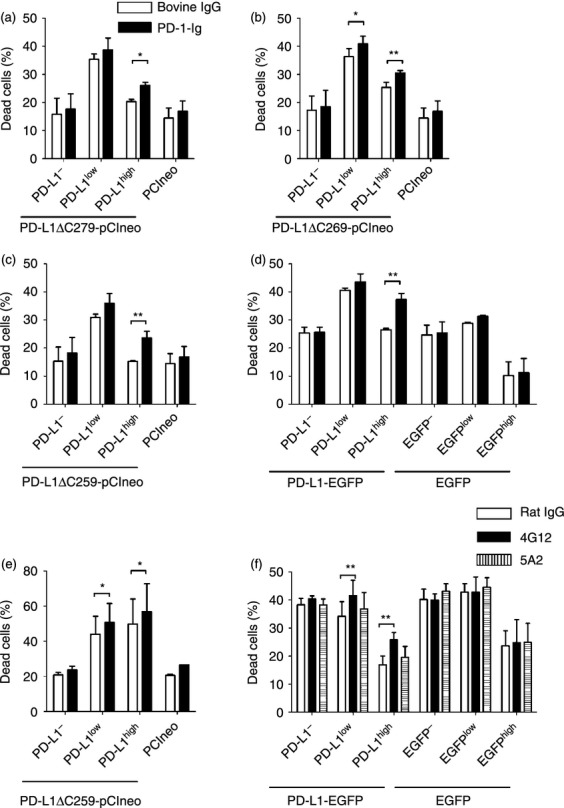
Cell death in cells transfected with plasmids encoding C-terminal deletion mutants of programmed death ligand 1 (PD-L1) and treated with programmed death 1–immunoglobulin (PD-1-Ig). (a–d) Percentages of dead cells in Cos-7 cells transfected with pCIneo encoding PD-L1-ΔC279 (a), PD-L1-ΔC269 (b), PD-L1-ΔC259 (c), and PD-L1-EGFP (d) after treatment of bovine IgG or PD-1-Ig (50 μg/ml). pCIneo or pEGFP-N2 was used as transfection control. PD-L1−/low/high cells were distinguished by staining with anti-PD-L1 monoclonal antibody (mAb) or EGFP fluorescence. (e) Same as (c), except that HeLa cells were transfected. (f) Same as (d), except that Cos-7 cells were incubated with two types of anti-PD-L1 mAbs (20 μg/ml; 4G12 and 5A2) and anti-rat IgG Fc (12 μg/ml). Mean values ± SEM from three independent experiments are shown. Statistical comparisons between bovine IgG and PD-1-Ig were made using the two-way analysis of variance. Differences were considered statistically significant at P < 0·05 (*P < 0·05; **P < 0·01).
PD-1-Ig treatment reduced cytokine production and cell proliferation in bovine lymphocytes
To investigate the effect of PD-L1 blockade on the immune function of bovine lymphocytes, the response of cell proliferation and cytokine production in PBMCs cultivated with PD-1-Ig or anti-PD-L1 mAbs were measured. Interferon-γ production was enhanced by PD-L1 blockade using two types of anti-PD-L1 mAbs, supporting the results of several previous reports2 (Fig. 6a). However, the cell proliferation response to PMA/ionomycin was decreased by PD-1-Ig treatment (Fig. 6b). PD-L1 blockade by PD-1-Ig also reduced IFN-γ and IL-10 production in PBMCs treated with PD-1-Ig in the presence or absence of PMA/ionomycin compared with those treated with bovine IgG (Fig. 6c,d).
Figure 6.
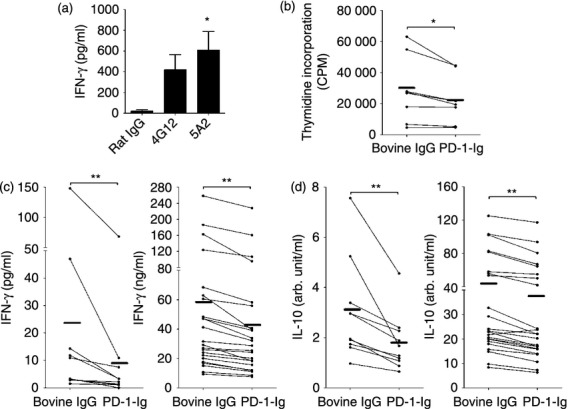
Effect of programmed death ligand 1 (PD-L1) blockade by anti-PD-L1 monoclonal antibodies (mAbs) and programmed death 1–immunoglobulin (PD-1-Ig) treatment on immune reaction in bovine lymphocytes. (a) Peripheral blood mononuclear cells (PBMCs) were cultured with rat IgG control or two types of anti-PD-L1 mAbs (4G12 and 5A2). Interferon-γ (IFN-γ) production was measured by ELISA (n = 8). (b–d) PBMCs were cultured with bovine IgG control or PD-1-Ig (50 μg/ml) in the presence or absence of PMA/ionomycin. Proliferation responses in the presence of PMA/ionomycin were measured by thymidine incorporation (b; n = 8). IFN-γ (c) and interleukin-10 (IL-10) (d) production were measured by ELISA (PMA/ionomycin in right; n = 23, no stimulant in left; n = 10). Each line indicates the mean values in each group. Statistical comparison between rat IgG, 4G12, and 5A2 was made using the one-way analysis of variance with Tukey's post-test. Those between rat IgM and 6G7 or bovine IgG and PD-1-Ig were made using the Wilcoxon matched-pairs test. Differences were considered statistically significant at P < 0·05 (*P < 0·05; **P < 0·01).
PD-1-Ig treatment increased B-cell death in bovine lymphocytes
To investigate the mechanism of PD-1-Ig-induced immunosuppression, annexin-V and 7-AAD staining was performed in PBMCs. PD-1-Ig treatment led to a decreased frequency of live cells (data not shown) and an increased frequency of dead cells in the IgM+ B cells, but not in the CD4+ and CD8+ T cells among the bovine lymphocytes (Fig. 7a). The reduction of IFN-γ production by PD-1-Ig treatment was diminished by B-cell depletion from PBMCs (Fig. 7b), whereas there was no effect on IL-10 production (Fig. 7c); this indicated that B-cell death was one of the mechanisms of PD-1-Ig-mediated immunosuppression. Because PD-L1 expression was primarily observed in B cells among bovine lymphocytes as previously described,18 it was hypothesized that PD-1-Ig binds to PD-L1+ B cells and also induces PD-L1 cross-linking and PD-L1-mediated cell death in B cells along with the observations in Cos-7 cells in Figs 4 and 5, resulting in immunosuppression. As expected, the percentages of dead PD-L1+ B cells were augmented by PD-1-Ig treatment (Fig. 7d) and that of the live cells decreased (data not shown). However, the percentages of dead PD-L1− B cells were also up-regulated (Fig. 7d).
Figure 7.
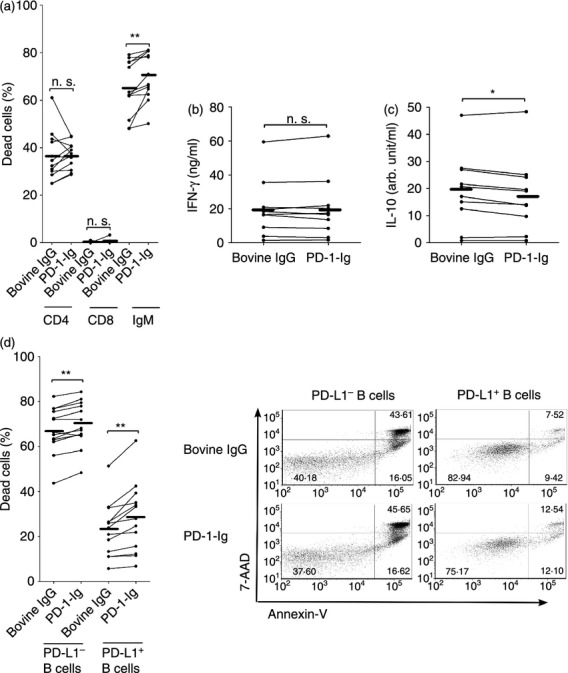
Effect of programmed death 1–immunoglobulin (PD-1-Ig) treatment on B-cell survival in bovine lymphocytes. (a) Percentages of dead cells in CD4+ T cells, CD8+ T cells, and IgM+ B cells among peripheral blood mononuclear cells (PBMCs) treated with bovine IgG or PD-1-Ig (n = 12). (b, c) interferon-γ (IFN-γ) (b) and interleukin-10 (IL-10) (c) productions in IgM+ B-cell-depleted PBMCs were measured by ELISA (n = 10). (d) Proportions of dead cells in PD-L1+ or PD-L1− B cells among PBMCs isolated from cattle were analysed (n = 13). Each line indicates the mean values in each group. Statistical comparisons between bovine IgG and PD-1-Ig were made using the Wilcoxon matched-pairs test. Differences were considered statistically significant at P < 0·05 (*P < 0·05; **P < 0·01).
Discussion
The blockade of the PD-1/PD-L1 pathway by anti-PD-L1 mAb is widely recognized as an appropriate strategy to enhance the immune reaction; it is also more effective than anti-PD-1 mAb because some types of anti-PD-L1 mAbs can inhibit the interaction of not only PD-1 and PD-L1 but also PD-L1 and CD80.2 Otherwise, some classes of anti-PD-L1 mAbs interfere with PD-L1 binding to CD80 but do not interfere with PD-L1 binding to PD-1. The treatment with this anti-PD-L1 mAb accelerated the symptoms of autoimmune-mediated diabetes in a mouse model,32 indicating that the PD-L1/CD80 and PD-1/PD-L1 pathways are involved in immunoinhibition. Moreover, Ghiotto et al.33 reported that another type of anti-PD-L1 mAb, which is not able to block the interaction of PD-1 with PD-L1, increased the binding of PD-1 with PD-L1. From these reports, it is important to carefully select clones that produce the appropriate anti-PD-L1 mAb for immune reactivation. In this study, two types of anti-PD-L1 mAbs that enhance IFN-γ production in bovine lymphocytes were examined. Of the two, 5A2 may be the more suitable antibody for immune activation because of the PD-L1 cross-linking by 5A2, and anti-rat IgG antibody did not induce PD-L1-mediated cell death in PD-L1-expressing Cos-7 cells. Although it remains to be determined whether they block the PD-1/PD-L1 or PD-L1/CD80 pathways, these antibodies may be useful tools for investigating PD-L1 function in the bovine immune system.
In this study, forced expression of PD-L1 in Cos-7 cells revealed that PD-L1high cells were more sensitive to PD-L1-mediated cell death than PD-L1low cells. These data indicated that a high expression level of PD-L1 is essential for the induction of PD-L1-mediated cell death. If PD-L1-mediated cell death occurs often in physiological conditions in vivo, anti-PD-L1 mAb treatment could offer two benefits: the blockade of the PD-1/PD-L1 pathway and the eradication of PD-L1high tumour cells or virus-infected cells. Actually, the reduction of PD-1+ CD8+ T cells by the treatment of PD-L2-Ig fusion protein in vivo tipped the balance in favour of non-exhausted PD-1low CD8+ T cells.34 Although there is a danger that PD-L1-mediated cell death may be induced in the PD-L1+ antigen-presenting cells required for T-cell activation, the clinical applications of the eradication of PD-L1+ lymphoma cells by PD-L1-mediated cell death may deserve consideration.
Meanwhile, bovine lymphocytes incubated with PD-1-Ig down-regulated the immune reaction in this study. It was also confirmed that the treatment with polyclonal anti-human PD-L1 antibody inhibited IFN-γ production in bovine lymphocytes (data not shown). These observations were believed to be attributed to PD-L1-mediated cell death in bovine lymphocytes, due to augmented PD-L1 expression in B cells isolated from BLV-infected cattle18 and that there was no down-regulation of IFN-γ in B-cell-depleted PBMCs treated with PD-1-Ig. However, the dead cells among the PD-L1− B cells were also increased in response to PD-1-Ig treatment. Although the increase in the number of apoptotic PD-L1+ B cells may sequentially affect apoptosis in PD-L1− B cells, the mechanism of PD-1-Ig-induced cell death in bovine lymphocytes still needs to be investigated.
This study and the previous report suggested the presence of PD-L1-induced inhibitory signalling into PD-L1+ cells.17 However, PD-L1 on cancer cells has been shown to act as an anti-apoptotic receptor, clarified with PD-1-Ig treatment.35 The reason why a consensus on the existence of PD-L1-induced signalling has not been reached is that the known signalling motifs or molecules are not present in the intracellular region of PD-L1. In this study, it was observed that the intracellular region of PD-L1 was not involved in PD-L1-mediated cell death in the Cos-7 and Hela cells. These data suggest that the extracellular region of PD-L1, and not the complex of PD-1 with PD-L1, may bind some molecules or receptors that induce inhibitory or anti-apoptosis signalling. Further investigation using the immunoprecipitation technique would be required to identify the unknown molecules that interact with PD-L1.
There are few reports that show PD-L1-mediated cell death in PD-L1-expressing cells ‘in vivo’ because several interactions of B7 family members, such as PD-1 and PD-L1/PD-L2, CTLA-4 and CD80/CD86, CD28 and CD80/CD86, and PD-L1 and CD80 pathways are involved in both the inhibition and activation of immune responses. Moreover, PD-L1 expression is up-regulated in an inflammatory environment that is enriched with IFN-γ and other cytokines. Those cytokines enhance resistance against apoptosis in the cells, and thereby, PD-L1-mediated cell death in vivo may rarely be observed. This study revealed that PD-L1-mediated cell death by PD-1-Ig can be induced without the intracellular region of PD-L1 and occurs only in PD-L1high cells. These data are useful for the clarification of the in vivo mechanism of PD-L1-mediated cell death.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and by a special grant for the Promotion of Basic Research Activities for Innovative Biosciences from the Bio-oriented Technology Research Advancement Institution (BRAIN). We are grateful to Dr Hideyuki Takahashi and Dr Yoshiyuki Goto, National Agriculture and Food Research Organization, BRAIN, for valuable advice and discussion. We wish to thank Dr Takehiko Yokomizo and Dr Toshiaki Okuno, Juntendo University, for plasmids and advice. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Glossary
- 7-AAD
7-aminoactinomycin D
- BLT1
leukotriene B4 receptor
- BLV
bovine leukaemia virus
- CHO
Chinese hamster ovary
- CTLA-4
cytotoxic T lymphocyte antigen 4
- EGFP
enhanced green fluorescent protein
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- mAb
monoclonal antibody
- PBMCs
peripheral blood mononuclear cells
- PE
phycoerythrin
- PD-1
programmed death-1
- PD-1-Ig
programmed death-1-immunoglobulin fusion protein
- PD-L1
programmed death-ligand 1
- PD-L1-Ig
programmed death-ligand 1-immunoglobulin fusion protein
Disclosures
The authors declare that they have no competing interests.
Authorship declaration
RI performed all of the studies contained in this manuscript, analysed data, and drafted the manuscript. SK analysed data and helped to draft the manuscript. TO participated in sample collection. KY, CN, and YS participated in the experiments, involving expression and purification of PD-1-Ig and PD-L1-Ig, and reviewed the manuscript. SM helped with the experimental design, data interpretation, and drafting the manuscript. KO supervised the study and reviewed the manuscript. All authors read and approved the final manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Recognition of programmed death-ligand 1 (PD-L1)-expressing CHO-DG44 cells by programmed death 1–immunoglobulin (PD-1-Ig) and anti-bovine PD-L1 monoclonal antibodies.
References
- 1.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–45. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hori J, Wang M, Miyashita M, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–35. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 4.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 5.Salama AD, Chitnis T, Imitola J, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–8. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;1:1–6. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jazayeri JA, Carroll GJ. Fc-based cytokines: prospects for engineering superior therapeutics. BioDrugs. 2008;22:11–26. doi: 10.2165/00063030-200822010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–6. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 12.Geng H, Zhang G-M, Xiao H, et al. HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD-1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int J Cancer. 2006;118:2657–64. doi: 10.1002/ijc.21795. [DOI] [PubMed] [Google Scholar]
- 13.He Y-F, Zhang G-M, Wang X-H, Zhang H, Yuan Y, Li D, Feng Z-H. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol. 2004;173:4919–28. doi: 10.4049/jimmunol.173.8.4919. [DOI] [PubMed] [Google Scholar]
- 14.Wan B, Nie H, Liu A, Feng G. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844–50. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 15.Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FBJ, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006;36:2472–82. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Matteson EL, et al. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2003;111:363–70. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Park G, Lee H, Song H, Choi I-H, Lee JW, Hur YD. Cross-linking of B7-H1 on EBV-transformed B cells induces apoptosis through reactive oxygen species production, JNK signaling activation, and fasL expression. J Immunol. 2008;181:6158–69. doi: 10.4049/jimmunol.181.9.6158. [DOI] [PubMed] [Google Scholar]
- 18.Ikebuchi R, Konnai S, Shirai T, Sunden Y, Murata S, Onuma M, Ohashi K. Increase of cells expressing PD-L1 in bovine leukemia virus infection and enhancement of anti-viral immune responses in vitro via PD-L1 blockade. Vet Res. 2011;42:103. doi: 10.1186/1297-9716-42-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulko V, Harris KJ, Liu X, Gibbons RM, Harrington SM, Krco CJ, Kwon ED, Dong H. B7-H1 expressed by activated CD8 T cells is essential for their survival. J Immunol. 2011;187:5606–14. doi: 10.4049/jimmunol.1003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinselmeyer BH, Heydari S, Sacristán C, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210:757–74. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butte MJ, Pena-Cruz V, Kim M-J, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol. 2008;45:3567–72. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 24.Subudhi S, Zhou P, Yerian L, et al. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest. 2004;113:694–700. doi: 10.1172/JCI19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saudemont A, Jouy N, Hetuin D, Quesnel B. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7-H1 that stimulates T cells. Blood. 2005;105:2428–35. doi: 10.1182/blood-2004-09-3458. [DOI] [PubMed] [Google Scholar]
- 26.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 27.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fc gamma receptor I binding and monocyte triggering activities. Eur J Immunol. 1999;29:2613–24. doi: 10.1002/(SICI)1521-4141(199908)29:08<2613::AID-IMMU2613>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Shields RL, Namenuk AK, Hong K, et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem. 2001;276:6591–604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 29.Ikebuchi R, Konnai S, Okagawa T, Yokoyama K, Nakajima C, Suzuki Y, Murata S, Ohashi K. Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cells in vitro. Vet Res. 2013;44:59. doi: 10.1186/1297-9716-44-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hase M, Yokomizo T, Shimizu T, Nakamura M. Characterization of an orphan G protein-coupled receptor, GPR20, that constitutively activates Gi proteins. J Biol Chem. 2008;283:12747–55. doi: 10.1074/jbc.M709487200. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki F, Okuno T, Saeki K, Min L, Onohara N, Kato H, Shimizu T, Yokomizo T. A high-affinity monoclonal antibody against the FLAG tag useful for G-protein-coupled receptor study. Anal Biochem. 2012;425:157–65. doi: 10.1016/j.ab.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Paterson AM, Brown KE, Keir ME, et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187:1097–105. doi: 10.4049/jimmunol.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghiotto M, Gauthier L, Serriari N, Pastor S, Truneh A, Nunes JA, Olive D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int Immunol. 2010;22:651–60. doi: 10.1093/intimm/dxq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mkrtichyan M, Najjar YG, Raulfs EC, Liu L, Langerman S, Guittard G, Ozbun L, Khleif SN. B7-DC-Ig enhances vaccine effect by a novel mechanism dependent on PD-1 expression level on T cell subsets. J Immunol. 2012;189:2338–47. doi: 10.4049/jimmunol.1103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–43. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Recognition of programmed death-ligand 1 (PD-L1)-expressing CHO-DG44 cells by programmed death 1–immunoglobulin (PD-1-Ig) and anti-bovine PD-L1 monoclonal antibodies.