Abstract
It is difficult for most of drug delivery system to really display a temporal and spatial release of entrapped drug once the systems are iv administrated. We hypothesized that the photothermal effect, mediated by a near-infrared (NIR) laser and hollow gold nanospheres (HAuNS), can modulate paclitaxel (PTX) release from polymer micelles, and further result in the enhanced antitumor activity of the micelles. We loaded PTX and HAuNS, which display strong plasmon absorption in the NIR region, into glycolipid-like polymer micelles with an excellent cell internalization capability. The surface of the micelles was conjugated successfully with a peptide, which has the specific-binding with EphB4, a member of the Eph family of receptor tyrosine kinases overexpressed on cell membrane of numerous tumors, to increase the delivery of PTX into tumor cells. Rapid and repetitive drug release from our polymer (HP-TCS) micelles could be readily achieved upon NIR laser irradiation. Our data demonstrated the specific-delivery of HPTCS micelles into positive-EphB4 tumors using a duel-tumor model after iv administration during the whole experiment process (1-48h). Interestingly, significantly higher uptake of the micelles by SKOV3 tumors (positive-EphB4) than A549 tumors (negtive-EphB4) was observed, with increased ratio on experiment time. However, the specific cell uptake was observed only during the short incubation time (1-4h) in vitro. Our data also indicated the treatment of tumor cells with the micelles followed by NIR laser irradiation showed significantly greater toxicity activity than the treatment with the micelles alone, free PTX and the micelles (without PTX loading) plus NIR laser irradiation. The enhanced toxicity activity to tumor cells should be attributed to the enhanced drug cellular uptake mediated by the glycolipid-like micelles, chemical toxicity of the released drug from the micelles due to the trigger of NIR laser, and the photothermal ablation under NIR laser irradiation.
Keywords: Hollow gold nanospheres, Polymer micelles, Tumor-homing peptide, Triggered release, Photothermal effect
1. Introduction
Controlled drug delivery systems have advanced over the last 60 years [1]. Numerous delivery systems have been developed, which in nanotechnology-based systems for targeting delivery to tumor have received great attention recently, including drug–polymer conjugates, drug-protein conjugates, liposomes, polymer micelles, dendrimers, drug nanocrystals and inorganic nanoparticles [2, 3]. Of there, polymer micelle is a very promising drug delivery system due to many obvious advantages, such as low cytotoxicity, nanoscale size, high bioavailability and drug-encapsulated ability. It is well know that only the released drug molecular can play the therapeutic activity after the drug is loaded into a carrier system. However, like most of drug delivery systems, polymer micelle also faces the defect, which is difficulty to decide when or where the encapsulated drugs should be released once the systems are iv administrated.
We developed glycolipid-like polymer micelles in the previous study, which was synthesized by a coupling reaction between the amino groups of chitosan oligosaccharide (CSO) and carboxyl group of stearic acid (SA) [4]. The obtained polymer (CSO-SA) micelles showed the excellent internalization into tumor cells, attributed to their components and special spatial structure (so-called “minor cores”, hydrophobic microdomains, near the surface of the micelles’ shell) [5-7]. Therefore, the micelles were employed to deliver the antitumor drug, paclitaxel (PTX), into tumor site. The tumor-cell killing efficiency of PTX encapsulated in micelles was improved sharply due to the increased intracellular delivery of the drug. However, it was found that only 13% of PTX was released from the micelles after 9h incubation under in vitro physiological environment. Even thinking of the decreasing pH value in the tumor site, the accumulative PTX release was still below 40% after 9h in the simulative tumor environment [5].
Hollow gold nanospheres (HAuNS) are a class of gold nanoparticles that have plasmon absorption in the near-infrared (NIR) region and that display a strong photothermal conducting property. HAuNS’ unique combination of small size (30–50 nm in diameter) and strong and tunable (520–950 nm) absorption band suggests that HAuNS are a promising photothermal conducting agent for a variety of biomedical applications, including imaging [8, 9] and cancer therapy [10]. Our previous studies have explored the potential utility of HAuNS as a delivery vehicle to shuttle biomolecules or to trigger drug release under NIR light irradiation [11-13].
In this study, we developed a drug delivery system, which in glycolipid-like polymer micelles were used to encapsulate PTX and HAuNS. HAuNS was modified hydrophobically to increase its encapsulation efficiency into the micelles. We hypothesized that the photothermal effect mediated by a NIR laser and HAuNS could modulate PTX release from the micelles. By the local irradiation in tumor site after iv administration of the micelles, we may optionally decide when and where the anticancer agent is released from the system. We also hypothesized the antitumor activity of the system can be significantly enhanced under the NIR irradiation. In order to enhance the interaction between the micelles and tumor cells, we conjugated a peptide TNYLFSPNGPIARAW (designated as TNYL) on the surface of the micelles, which displayed high binding affinity to EphB4, with an equilibrium dissociation constant (Kd) values of 1.98 to 23 nmol/L [14, 15]. The Eph receptors constitute the largest known family of receptor tyrosine kinases and have been reported to control various pathological processes, including tumor progression and angiogenesis [16-18]. Overexpression of EphB4 has also been observed in numerous tumor types [16, 17, 19-21]. Therefore, EphB4 is a particularly promising target for tumor-specific delivery of our system.
2. Materials and methods
2.1. Materials
Sodium citrate (>99%), cobalt chloride hexahydrate (99.99%), sodium borohydride (99%), and chloroauric acid trihydrate (American Chemical Society reagent grade) were purchased from Thermo Fisher Scientific (Waltham, MA) and were used as received. Octadecyl-3-Mercaptopionate (OMP) was from Chemical Indoustry Co.(Japan). Chitosan oligosaccharide (CSO) with about 18.0 kDa weight average molecular weight was obtained by enzymatic degradation of 95% deacetylated chitosan (Mw = 45.0 kDa), which was supplied by Yuhuan Marine Biochemistry Co., Ltd. (Zhejiang, China); stearic acid (SA) was from Shanghai Chemical Reagent Co. Ltd. (Shanghai, China); PTX was gifted from Zhejiang Hisun Pharmaceutical Co, Ltd, (Taizhou Zhejiang, China). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N,N-Disuccinimidyl carbonate (DSC), and 3-(4,5-dimethylthiazol-2-yl)-2,5 -diphenyltetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO). NH2-PEG2000-NH2 was purchased from Sigma-Aldrich Inc, (St Louis, MO). TNYL (sequence: TNYLFSPNGPIARAW) was provided from Baiaotai biotechnology Inc. (Guangzhou, China). All other solvents were of analytical or chromatographic grade.
5.2 Cell culture
SKOV3 (human ovarian carcinoma), and A549 (human lung adenocarcinoma) cell lines were obtained from Institute of Biochemistry and Cell Biology (Shanghai, China). The cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Life Technologies, Inc., Carlsbad, CA) at 37°C in a humidified atmosphere containing 5% CO2.
5.3. Preparation and hydrophobic modification of HAuNS
HAuNS were synthesized according to a previously reported method [11]. Briefly, cobalt nanoparticles were first synthesized by deoxygenating deionized water containing 4.5 mL of 1 mol/L sodium borohydride, 2.8 mL of 0.1 mol/L sodium citrate, and 1.0 mL of 0.4 mol/L cobalt chloride. After chloroauric acid was added into the solution containing cobalt nanoparticles, the cobalt immediately reduced the gold ions onto the surface of the nanoparticles and was simultaneously oxidized to cobalt oxide. Any remaining cobalt was further oxidized by air, resulting in the final product, HAuNS. The size of the HAuNS was determined using dynamic light scattering on a Zetasizer (3000 HS; Malvern Instruments, UK). The UV-visible spectra were recorded on a Beckman Coulter DU-800 UV-visible spectrometer.
HAuNS was further modified hydrophobically by OMP. Briefly, 0.2 mL HAuNS [200 optical density (OD), 10.0 mg/mL] was centrifuged at 8000 rpm for 10 min. The pellet of HAuNS was mixed with OMP (0.1 mmol) in 1 mL DMF solution. After overnight stirring, OMP modified HAuNS (OMP-HAuNS) was purified by the centrifugation (8000 rpm for 10 min), followed by washing twice with DMF, and then was identified by FT-IR analysis.
5.4. Synthesis of CSO-SA and TNYL conjugated CSO-SA
CSO-SA was synthesized via reaction of the carboxyl groups of SA with the amine groups of CSO in the presence of EDC [4]. Briefly, CSO (2.0 g) was dissolved in 50 mL of distilled water. SA (1.75 g) and EDC (10 mol/mol of SA) were dissolved in 40 mL of ethanol. CSO solution was heated to 80 °C under vigorous stirring accompanied by dropwise addition of SA solution. The reaction lasted 5 h. The byproducts were removed via ultrafiltration by Millipore Labscale TFF system [molecular weight cutoff (MWCO) 10 000, Billerica, MA]. The obtained product was lyophilized (Labconco, FreeZone 2.5 Plus, Kansas City, MO), further washed with ethanol, and separated by the centrifugation (3K30, Sigma Labrorzentrifugen GmbH, Germany).
CSO-SA was further conjugated with TNYL peptide. The reaction was performed at room temperature. Briefly, TNYL (10mg) was mixed with (Boc)2O [TNYL : (Boc)2O = 1:5.2, mol/mol] in dried DMF, followed by stirring for 12 h. Then, NH2-PEG2000-NH2 (11.9 mg) and EDC (11.4 mg) were added into above solution. Succinimidyl t-Boc-TNYL-PEG2000-NH2 was obtained by 9 h reaction after the addition of DSC (NH2-PEG2000-NH2 : DSC = 1:1, mol/mol), and further reacted with CSO-SA (CSO-SA : succinimidyl t-Boc-TNYL-PEG2000-NH2 = 1:1, mol/mol) for 24 h. Finally, the protection of (Boc)2O to TNYL peptide was removed by adding hydrochloric acid into the solution. The pH value of the solution was adjusted back to 7.0 using sodium hydroxide. For purification, the solution was dialyzed using a membrane (MWCO 7 kDa, Spectrum Laboratories) against distilled water for 24 h. TNYL conjugated COS-SA (TNTL-CSO-SA) was obtained by the lyophilization, and identified by 1H-NMR spectroscopy.
5.5. Preparation of HAuNS and PTX loaded TNYL-CSO-SA micelles
HAuNS and PTX loaded TNYL-CSO-SA (HP-TCS) micelles were prepared by dialysis method [5]. Briefly, OMP-HAuNS (0.5 mL of 100 OD), PTX (2 mg) and TNYL-CSO-SA (20 mg) were dispersed in the mixture solution (water : DMSO=1:9, v/v), followed by the stirring for 3 h. Then, the above solution was dialyzed using a membrane (MWCO 7 kDa, Spectrum Laboratories) against distilled water for 24 h, and was filtered through a 0.22 mm pore-sized membrane to remove free PTX and OMP-HAuNS. The powder of HP-TCS was obtained by lyophilization. The size distribution and zeta potential of HP-TCS micelles were measured by a Zetasizer. The concentration of PTX in the micelle solution was determined by high-performance liquid chromatography (HPLC).
5.6. NIR-light-triggered release of PTX from HP-TCS micelles
The sample was transferred into a dialysis membrane bag (MWCO 7 kDa), and then submerged into the medium containing 1 M sodium salicylate with pH 7.4 under the stirring (100 rpm) at 37 °C. For NIR laser irra diation, the sample was taken out from the dialysis bag at the designed time point, and put into a transparent glass tube. The samples were irradiated with the 808-nm NIR light at an output power of 3 or 6W over a period of 5min (Diomed 15 plus, Cambridge, UK), as our previous reported [12]. Then, the sample was put back into the dialysis bag to continue the release study. The released PTX in the medium was quantified by HPLC.
NIR-light-triggered release from HP-TCS micelles was further confirmed by an in vivo imaging system (Cambridge Research & Instrumentation, Inc., Woburn, MA). Firstly, DIR, a near-infrared lipophilic fluorescent tracer, was used as the model drug to be encapsulated into HP-TCS micelles as the above method in section 2.4. Then, the agarose gel lump was prepared using the reported methods with a little modification [22]. The release study was carried out as the showed in fig. S1. Briefly, we made a circular hole (1 cm diameter and 0.5 cm depth) in the agarose gel lump. The sample was put into the hole, and irradiated by NIR laser (output power of 3 W for 10 min). Then, the gel was observed by the in vivo imaging system. NIR-light-triggered release of DIR from HP-TCS micelles was identified by the diffused degree of DIR molecule in agarose gel.
5.7. Cellular uptake of HP-TCS micelles
EphB4 expression in cells was analyzed according to our previously reported [15]. Briefly, for western blot analysis, cells were treated by CelLytic M cell lysis buffer (Sigma-Aldrich) containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Equal amounts of protein from cells were run on a 4-12% NuPAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis gel (Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane. The EphB4 expression was probed with mouse anti-EphB4 antibody and Alexa Fluor 680-conjugated goat anti-mouse IgG (Invitrogen). Protein bands were visualized with a LI-COR Odyssey system (Lincoln, NE). For binding study, Cells were incubated with primary antibody (mouse anti-EphB4 antibody) for 1 h at 4 °C. The cells wer e then washed with PBS three times and incubated with secondary antibody (Alexa Fluor 680-conjugated goat anti-mouse IgG). The cells were then repeatedly rinsed with PBS and observed using a fluorescence microscope.
For investigating cellular uptake, HP-TCS micelles were further labeled with fluorescein isothiocyanate (FITC) via the reactive amino group of chitosan and the isothiocyanate group of FITC. Cells were transferred and cultured onto 20-mm glass cover slips in a 24-well plate and allowed to grow for 2 days. The medium was then replaced with 1 mL of fresh culture medium containing HP-TCS micelles (0.5 mg/mL). For the blocking experiments, cells were incubated firstly with free TNYL (1.0 mmol/L) for 0.5 hours and then with HP-TCS micelles. After different incubation period, cell nuclei were stained with 40, 6-diamidino-2-phenylindole (DAPI). The cell monolayer on the coverslip was removed, repeatedly rinsed with PBS, and then mounted for microscopic examination. The cellular fluorescence was examined under a fluorescence microscope (Leica DM4000 B; Leica, Solms, Germany) and further observed by a confocal microscopy (Olympus, Japan). For the quantitative analysis of cell uptake, cells were treated with trypsin after 2h incubation with HP-TCS micelles, and then re-suspended in PBS. The intensity of cellular fluorescence was determined by a flow-cytometer (FC500MCL, Beckman Coulter).
5.8. Photothermal ablation (PTA) in vitro
For assessing PTA effect in vitro, A549 and SKOV3 cells seeded onto a 96-well plate were treated with HP-TCS micelles (0.5 mg/mL; 40 g PTX/mL; 2 h) plus NIR laser, free HAuNS (6.0 OD/mL; 2 h) plus NIR laser, HP-TCS micelles (0.5 mg/mL; 40 g PTX/mL; 2 h) plus free TNYL (1.0 mmol/L) and NIR laser (blocking study), and NIR laser alone. Laser treatment was achieved with NIR laser light centered at 808 nm at an output power of 1.5 W for 3 min (15PLUS laser, Diomed, Cambridge, UK). Then, cell medium was refreshed, and 24h later cells were washed and stained with calcein AM (Invitrogen, Carlsbad, CA) for visualization of live cells and with EthD-1 (Invitrogen) for visualization of dead cells. Cells were observed using a fluorescence microscope.
5.9. In vitro cytotoxicity studies
Cytotoxicity was measured using MTT assay according to the manufacturer’s suggested procedures (Sigma-Aldrich). SKOV3 and A549 cells were exposed to free PTX, HP-TCS micelles, or H-TCS micelles (HAuNS loaded TNYL-CSO-SA micelles without PTX) for 48h. The cells further were irradiated by NIR laser once (output power of 3 W) at the beginning of incubation with HP-TCS or H-TCS micelles. The data are expressed as percentage of survival cells and are reported as the means of triplicate measurements.
5.10. In vivo imaging
All animal studies were carried out under Institutional Animal Care and Use Committee-approved protocols. To investigate the in vivo distribution, HP-TCS micelles further encapsulated DIR. It was found there was almost no DIR release from the micelles during the experiment, which indicated that DIR could be employed to trace the micelles. We intravenously injected female nude mice bearing subcutaneous A549 and SKOV3 tumors with HP-TCS micelles containing DIR. The mice were observed by the in vivo imaging system at the predetermined time after the injection. At the end of the experiment, the mice were killed. Various tissues, including tumors, were collected, weighted and observed by the in vivo imaging system. The fluorescent intensity, responding the amount of the micelles, was also read by the imaging system. The accumulation of HP-TCS micelles in various tissues was calculated as %ID/g (the percentage of the injected dose per gram of tissue).
2. Results
2.1. HP-TCS micelles
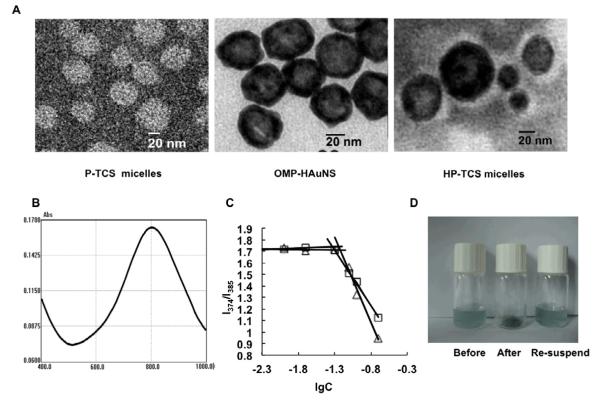
The surface of HAuNS is coated by citrate group according the synthesis process, which is high hydrophilic. In order to encapsulate HAuNS into the hydrophobic core of the micelles, OMP was conjugated onto the surface of HAuNS using the reaction between -SH in OMP with Au to obtain the hydrophobic HAuNS, which was identified by IR spectrum (fig. S2): The weak characteristic peak of mercapto group at the range of 2500cm−1~2900cm−1 was disappeared completely, comparing with OMP alone, while other characteristic peaks for OMP still existed. The results suggested that the group of -SH in OMP changed to -S- due to the reaction between -SH with Au. The average diameter of OMP-HAuNS was 42.5 nm, as determined by the dynamic light scattering (DLS) method, which was close to the result from TEM imaging (Fig. 1A). The absorption spectra showed that the plasma resonance peaks for OMP-HAuNS were in the NIR region (~800 nm) (Fig. 1B). Thus, OMP modification did not affect the spectrum characteristic of HAuNS.
Figure 1.
(A) TEM images of PTX loaded TNYL-CSO-SA (P-TCS) micelles, OMP modified HAuNS (OMP-HAuNS) and HAuNS & PTX loaded TNYL-CSO-SA (HP-TCS) micelles. The sample was stained by phosphotungstic acid (2% W/V) for 30 seconds before the observation. The white spheres indicated the polymer micelles. For HP-TCS micelles, HAuNS was encapsulated in the polymer micelles. (B) Absorption spectra of OMP-HAuNS. (C) Variation of intensity ratio (I1/I3) vs concentration of CSO-SA (square) and TNYL-CSO-SA (triangle). (D) The pictures for the solution containing HP-TCS micelles (before lyophilization), the powder of HP-TCS micelles (after lyophilization) and the solution containing HP-TCS micelles (the re-suspended).
The synthesis of TNYL-CSO-SA was confirmed from 1H NMR spectrum (fig. S3). TNYL-CSO-SA has a very sharp single peak at about 1.02~1.12 ppm, attributed to the methylene hydrogen proton peak of the leucine in TNYL. The double peaks at about 2.83-2.92 ppm for TNYL-CSO-SA were appeared, attributed to the methyl hydrogen proton peak of the tryptophan in TNYL. The peak at about 1.05-1.26 ppm was attributed to the methylene and methyl hydrogen of SA in TNYL-CSO-SA.
The aggregation behavior of TNYL-CSO-SA and CSO-SA in aqueous media was measured by fluorometry using pyrene as a fluorescent probe, as our previously reported [5]. The I1/I3 ratio, ie, the first to third highest energy bands in the pyrene emission spectra (I1, em = 374 nm; I3, em = 385 nm) was used to determine the critical micelle concentration (CMC) value of the polymer. CMC values of TNYL-CSO-SA and CSO-SA in distilled water were about 38 and 60 μg/mL respectively (Fig. 1C), which indicated that CSO-SA after TNYL conjugation still inherited the excellent dispersity and ability to self-assemble in aqueous environment.
Table 1 summarized the PTX encapsulating efficiency (EE) and physicochemical properties of HAuNS and PTX loaded TNYL-CSO-SA (HP-TCS) micelles, HAuNS loaded TNYL-CSO-SA (H-TCS) micelles, and PTX loaded TNYL-CSO-SA (P-TCS) micelles. The average diameter of P-TCS micelles was 49.6 nm, determined by DLS, which was accordant with that by TEM (Fig. 1A). Due to the encapsulation of HAuNS, the average diameter of HP-TCS micelles increased significantly to be 77.5 nm, determined by DLS and confirmed by TEM (Fig. 1A). PTX encapsulating efficiency of both HP-TCS and P-TCS micelles was about 80%, and almost all of feeding HAuNS after the hydrophobic modification was encapsulated into the micelles. It was found that the powder of lyophilized HP-TCS micelles could easily disperse in the aqueous medium to form the micelles again, with no significant size change as the comparison with that of the micelles before the lyophilization (Fig. 1D), which suggested that HP-TCS micelles would have higher physical-chemical stability cause they could be kept as solid state, instead of dispersing in the aqueous medium, before the injection for the administration.
Table 1.
Size, zeta potential, encapsulated efficiency (EE) and drug content (DC) (n=3)
| Marerial | Size | Zeta potential | EE | DC |
|---|---|---|---|---|
| HP-TCS micelles | 77.5±4.2 | 31.3±1.6 | 81.17 | 7.31 |
| H-TCS micelles | 71.5±3.3 | 33.4±2.1 | - | - |
| P-TCS micelles | 49.6±2.9 | 31.8±1.0 | 79.12 | 7.12 |
2.2. Triggered-release of PTX from HP-TCS micelles
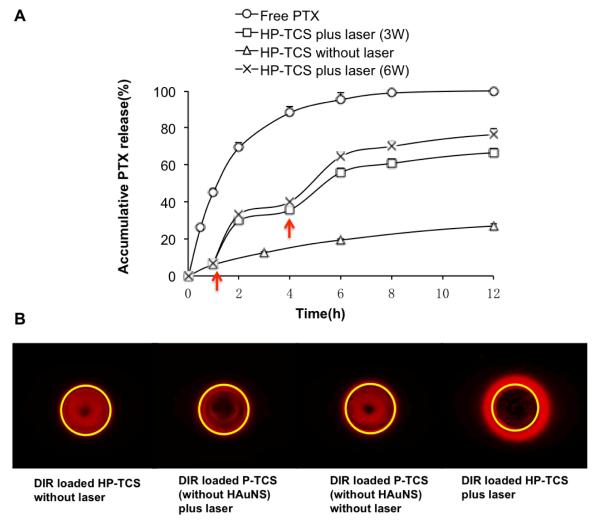
Figure 2A shows the characteristics of PTX release from HP-TCS micelles. As a control, almost all of free PTX was released within 8h. The solutions containing the micelles (2 mg/mL) were irradiated repeatedly using a NIR laser over a period of 5 min, followed by 3-h intervals with the laser turned off. After a NIR irradiation, a rapid increase in PTX release from HP-TCS micelles was observed during the following 1 or 2 h period, when the released PTX diffused through the dialysis membrane into the external medium. However, PTX release from the micelles was slow significantly under no NIR irradiation, with only about 25% PTX accumulative release after 12 h, defined as the percentage of released PTX to total entrapped PTX. For NIR irradiations at output power of 3W, the increased release of PTX was about 22.8% and 20.2% for first and second NIR irradiation respectively, which finally induced the total release of 66.6% PTX after 12 h. More PTX will be released from the micelles due to the increase of output power (up to 6 W), with total release of 76.3% PTX after 12h.
Figure 2.
(A) NIR-light-triggered release of PTX from HP-TCS micelles. Red arrows indicate the beginning time points of NIR laser irradiation. The sample was irradiated by NIR laser at the output power of 3W (square) or 6W (cross) over a period of 5 min for each time. The release of free PTX (circle) and HP-TCS without laser irradiation (triangle) were as controls. (B) The triggered-release of DIR from HP-TCS micelles mediated by NIR laser irradiation (3W for 10 min), which was observed by in vivo imaging system. Yellow circle indicated the location of the hole in the agarose gel, where the sample was put. Red fluorescent signal out of the yellow circle represented the released DIR molecule from the micelles.
Because only free DIR molecules, but not entrapped in the micelles, can diffuse in the agarose gel, we can observe the triggered-release of DIR by the imaging system (Fig. 2B). The yellow circle indicated the location of the hole, where the sample was put. For HP-TCS micelles loading DIR, much DIR molecule diffused out the hole under a NIR laser irradiation. However, as control, almost no DIR molecule diffused into the gel under the laser irradiation for P-TCS micelles (no HAuNS), like that for HP-TCS micelles or for P-TCS under no laser irradiation. These results obviously indicated HAuNS in the micelles produced the photothermal effect under NIR laser irradiation, which consequently triggered the release of DIR molecule from the micelles.
2.3. Cell uptake of HP-TCS micelles
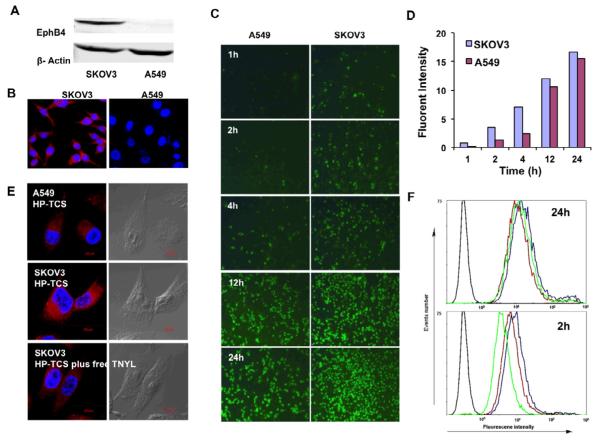
Both Western blotting analysis and immunostaining confirmed high expression levels of EphB4 receptor in SKOV3 cells, but only low expression level in A549 cells. Dark protein bands marking EphB4 (Fig. 3A) and strong cellular fluorescent signal (Fig. 3B) due to the binding with anti-EphB4 antibody was observed in SKOV3 cells, but not in A549 cells. Under the same conditions, significantly more HP-TCS micelles were internalized in the cells with high EphB4 receptor expression (SKOV3) than in the cells with low EphB4 receptor expression (A549) during a short incubation time (i.e. <4h) (Fig. 3C), which was confirmed quantitatively by a software “Image J” (Fig. 3D) [23]. The addition of free TNYL peptide into the culture medium induced a significant decrease in the cellular uptake of the micelles (Fig. 3E), which was further confirmed by the analysis of flow-cytometer. The average fluorescent intensity after 2h incubation, meaning the amount of cellular internalization of HP-TCS micelles, was 14.7, which was significantly higher than that in A549 cells (4.59), and decreased to be 9.26 with the addition of free TNYL peptide (Fig. 3F). These data demonstrated that HP-TCS micelles were taken up by EphB4-positive cells via receptor-mediated endocytosis. With the increasing incubation time (>4h) (Fig. 3C, D and F), the difference of cellular internalization of HP-TCS micelles between SKOV3 and A549 cells became smaller and smaller.
Figure 3.
(A) Western blot analysis of EphB4 expression in SKOV3 and A549 cell lines. (B) Immunostaining using anti-EphB4 antibody. High expression of EphB4 receptor was shown in SKOV3 cells (red), but not in A549 cells. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). (C) Cell uptake of HP-TCS micelles (FITC labeled) in A549 and SKOV3 cells with different incubation time. (D) The quantitative analysis based on the imaging in (C) by a software “Image J”. (E) Cell uptake in A549 and SKOV3 cells after 2h incubation with HP-TCS micelles and HP-TCS micelles plus free TNYL peptide (blocking). (F) Quantitative cell uptake, analyzed by a flow-cytometer, in A549 (green lines) and SKOV3 (blue lines) cells after 2h and 24h incubation with HP-TCS micelles and HP-TCS micelles plus free TNYL peptide (blocking, red lines).
2.4. Photothermal ablation in vitro
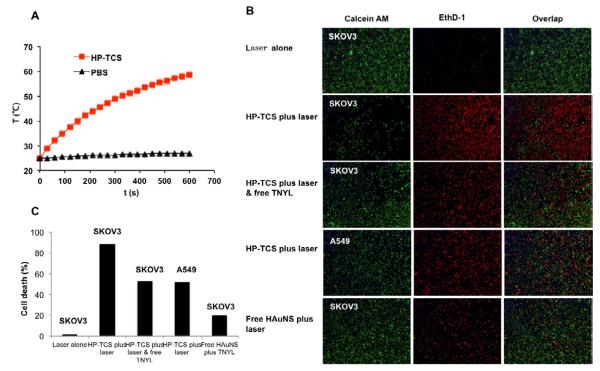
Continuous exposure of aqueous solution containing HP-TCS micelles (0.5 mg/mL) to NIR light (output power of 1.5 W) resulted in the rapid elevation of its temperature. The temperature of the solution was elevated by 34 °C after 10 min irradiation, which demonstrated that HP-TCS micelles induced the photothermal conversion under NIR laser irradiation due to encapsulating HAuNS. In comparison, no significant temperature change was observed when phosphate-buffered saline (PBS) was exposed to the laser light (Fig. 4A).
Figure 4.
(A) The temperature changes in aqueous solutions containing HP-TCS micelles (0.5 mg/mL) after exposure to NIR light at an output power of 1.5W. PBS=phosphate buffered saline. (B) Photothermal ablation in SKOV3 and A549 cells with HP-TCS micelle, HAuNS alone or HP-TCS micelles plus free TNYL (blocking). NIR laser was delivered at an output power of 1.5 W for 3 min. Cells were stained with calcein AM (green) and EthD-1 (red) for visualization of live and dead cells, respectively. (C) The quantitative analysis based on the imaging in (B) by the software “Image J”.
After NIR laser irradiation at an output power of 1.5 W for 3 min, significantly more SKOV3 cells was killed (stained red by EthD-1) due to the effect of photothermal ablation from HP-TCS micelles, compared with A549 cells, which was attributed to more HP-TCS micelles were internalized into SKVO3 cells than A549 cells after 2h incubation. Due to the addition of free TNYL peptide, the internalization of HP-TCS micelles into SKOV3 cells was decreased, resulting in the increased survival cells (stained green by calcein AM) under the same NIR irradiation condition (Fig. 4B). As a control, NIR laser irradiation alone under above condition induced hardly the death of the testing cells. These results indicated that HP-TCS micelles showed a higher capability of photothermal ablation mediated by NIR laser to the cells with high EphB4 expression, due to the increased cellular uptake of the micelles via receptor-mediated endocytosis. It was found that HAuNS, encapsulated in the micelles, showed stronger photothermal ablation effect for both cells (the cell death percentage of 88.6% and 52.3% for SKOV3 and A549, respectively) than free HAuNS (the cell death percentage of 19.6% for SKOV3) under the same number of the gold nanospheres (about 6.0 OD) and the same irradiation condition (Fig. 4B and C). The possible explain is that more HAuNS in the micelles is taken up into the cells (whether EphB4-positive or -negative) via the mediation of the micelles, which showed excellent internalization into tumor cells as our previously reported [5-7].
2.5. In vitro cytotoxicity assay
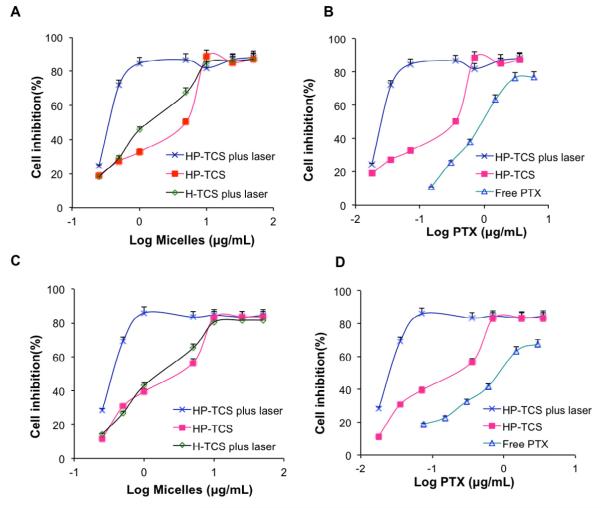
Figure 5 shows the cytotoxic effects of HP-TCS micelles, with or without NIR laser irradiation, in SKOV3 and A549 cells. The significantly higher cell-killing efficiency of HP-TCS micelles combined with NIR laser irradiation than that of the micelles alone was presented. For example, over 70% SKOV3 cells were killed after 48h incubation with HP-TCS micelles (0.5 mg/mL) combining with the irradiation. Contrarily, over 70% cells were survived after the incubation with the same concentration of the micelles, without NIR irradiation (Fig. 5A). The significantly enhanced cytotoxicity of HP-TCS micelles combined with NIR laser irradiation was also much higher than that of photothermal ablation alone (H-TCS micelles plus NIR) or PTX cytotoxicity alone (HP-TCS micelles without NIR laser) (Fig. 5A), which should be attribute to the combination and synergistic interaction of photothermal ablation of HAuNS and cytotoxic activity of released PTX, like our previously reported [12]. It was found that even HP-TCS micelles without NIR laser irradiation still showed significantly higher cytotoxicity than free PTX (Fig. 5B). It was mainly attributed to the excellent internalization of the micelles, resulting in the increased PTX concentration in the cells. Our previous work had demonstrated, by determining the amount of PTX in tumor cells, that the glycolipid-like polymer micelles significantly enhanced the cellular uptake of their payload compared with free drug alone [5]. Similar findings were observed with A549 cells (Fig. 5C and D).
Figure 5.
SKOV3 (A and B) and A549 (C and D) cell viability as a function of equivalent micelle or PTX concentration. Cells were treated with HP-TCS micelles plus NIR laser (cross), HP-TCS micelles (solid square), H-TCS micelles plus NIR laser (diamond) and free PTX (triangle).
It looks that there is no significant cytotoxic difference of HP-TCS micelles, with or without NIR irradiation, between EphB4-positive SKOV3 cells and EphB4-negative A549 cells. For example, IC50 values were 0.37 and 0.39 μg/mL for SKOV3 and A549 cells treated with HP-TCS micelles plus NIR laser. It was mainly because that the incubation for long time (i.e. 48h) induced no significant difference about cellular uptake of the micelles between both cells.
2.6. In vivo imaging
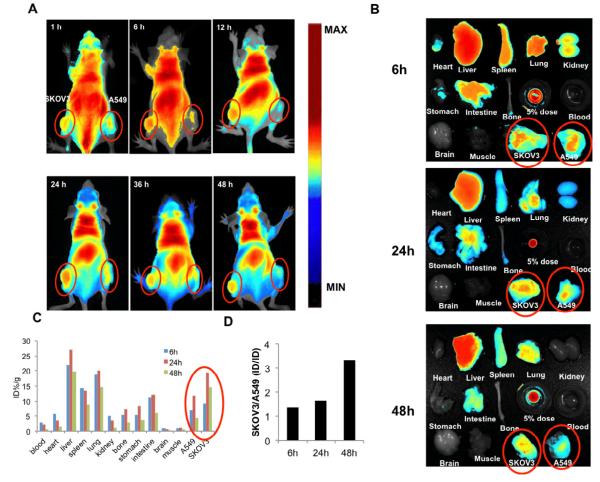
In order to investigate simultaneously the accumulation of HP-TCS micelles in tumors with positive- and negative-EphB4 expression, the tumor model was built by subcutaneous injection of SKOV3 and A549 cells in left and right side of the mice, respectively. It was very clear that significantly more HP-TCS micelles accumulated into SKOV3 tumors with positive-EphB4 expression than A549 tumors with negtive-EphB4 expression during the whole experiment process (Fig. 6A). The fluorescent intensity of various tissues after 6, 24 and 48h injection was observed (Fig. 6B), and was quantitated. There was the highest accumulation of HP-TCS micelles at 24h after injection in both SKOV3 (19.4 %, ID/g) and A549 (11.9 %, ID/g) tumors (Fig. 6C). Interestingly, unlike the observed from cell uptake of HP-TCS micelles in vitro, the difference about the accumulation of HP-TCS micelles between SKOV3 and A549 tumors increased with longer experiment time. For example, the ratio of accumulative amount of HP-TCS micelles in SKOV3 tumor to that in A549 tumors was 1.36, 1.63 and 3.33 at 6, 24 and 48h after injection, respectively (Fig. 6D).
Figure 6.
(A) The in vivo imaging of the mice, bearing SKOV3 and A549 tumors in left and right side respectively, at different time after iv injection of HP-TCS micelles encapsulating DIR. (B) The fluorescent imaging of various tissues at 48h after the iv injection of the micelles. (C) The accumulation of HP-TCS micelles in various tissues was calculated as %ID/g (the percentage of the injected dose per gram of tissue). The fluorescent intensity, responding the amount of the micelles, was read by the imaging system. (D) The accumulative ratio of HP-TCS micelles in SKOV3 tumor to A549 tumor at different time.
3. Discussion
Several methods for triggered release of agents from polymeric micelles have been described so far, including pH-sensitive, thermosensitive, ultrasound sensitive and light-sensitive micelles. Most methods were based on the special property of the polymer, such as the protonation [24, 25], low critical solution temperature or cloud point [26-29], and bond-break under low pH [30] or light irradiation [31]. As we known, a few studies have explored the triggered release of polymeric micelles based on a photothermal effect mediated by NIR light.
Our delivery system combined two advantages, derived from two materials. One is the glycolipid-like polymer, which was widely studied for the delivery of chemical and biologic agents in our previous works [4-7, 32-35]. However, few studies were focus on the real control of drug release from the micelles. The polymer was synthesized by the conjugation between chitosan oligosaccharide and stearic acid, presenting a so-called special spatial structure of multiple “minor-cores” in the aqueous medium, which induces its excellent internalization into cells and the increase of cellular uptake of the payload. The explanation is the “minor cores” can insert into the celluar membrane, exerting hydrophobic interactions with membrane components, resulting in membrane perturbation and fusion, thus facilitating endocytosis of the micelles. The other is HAuNS, which display a plasmon absorption in the NIR region and a strong photothermal conducting property. We employed HAuNS as photothermal ablation agent for cancer therapy, as a delivery vehicle to shuttle biomolecules or to trigger drug release under NIR light irradiation [11-13, 15]. In this study, as a result, the triggered-release of PTX from the established system responding to the turn-on of a NIR laser was observed (Fig. 2), which suggested that it was possible for the entrapped drug to really present a temporal and spatial control by a local NIR laser irradiation. And the significantly enhanced cytotoxic activity of the system to tumor cells was obtained (Fig. 5), which should be attributed to the enhanced drug cellular uptake mediated by the glycolipid-like micelles, triggered release of the drug from the system mediated by NIR laser and consequent chemical toxicity of the released drug, and the photothermal ablation under a NIR laser irradiation.
For the glycolipid-like micelles as a drug delivery system, besides controlling drug release, we need to improve tumor-specific delivery of the micelles. Therefore, a tumor-homing ligand, TNYL peptide, was conjugated on the surface of the micelles to increase the tumor-selective accumulation. Though the specific uptake of our delivery system to EphB4-positive cells happens obviously during the short incubation time, and became unconspicuous with the extension of incubation time in vitro (Fig. 3C, D and F), it is still worth looking forward to the increased tumor-specific delivery of the micelles after iv administration in vivo. It is because the interaction in vivo between the micelles in circulatory system and cells, not like that in vitro, will happen only in a very short time. The tumor cells with high EphB4 expression still have more chance to take up the micelles than the cells with low EphB4 expression. Consisting with the expected, the specific delivery of the micelles into EphB4-positive tumors was demonstrated by in vivo imaging system after iv administration, which clearly showed higher uptake of our micelles by SKOV3 tumors than A549 tumors, with increased ratio on experiment time (Fig. 6). Besides in vitro, the decreasing the system toxicity and enhanced antitumor activity of the micelles should be further studied in vivo, which will be our next work.
4. Conclusion
This work demonstrates the feasibility of modulated drug release using NIR light as the external stimulus. Rapid and repetitive drug release from our polymer micelles could be readily achieved upon NIR laser irradiation. Our data also indicated the treatment of tumor cells with HP-TCS micelles followed by NIR laser irradiation showed significantly greater toxicity activity than the treatment with HP-TCS micelles alone, free PTX and H-TCS micelles plus NIR laser irradiation. The enhanced cytotoxicity of HP-TCS micelles-plus-NIR laser against tumor cells can be attributed to the increased cellular uptake of PTX mediated by the glycolipid-like micelles, the cytotoxic effect of PTX released from the micelles and the photothermal effect mediated by HAuNS under NIR laser irradiation. Our data demonstrated that the specific-delivery of HP-TCS micelles into positive-EphB4 tumors was achieved after iv administration during the whole experiment process (1-48h). Significantly higher uptake of the micelles by SKOV3 tumors than A549 tumors was observed, with increased ratio on experiment time. However, the specific cell uptake was observed only during the short incubation time (1-4h) in vitro. It is anticipated that significantly enhanced antitumor activity of HP-TCS micelles plus NIR laser would be gained after HP-TCS micelles were iv administrated.
Supplementary Material
Acknowledgments
This work was supported by the National Nature Science Foundation of China (81001411), the National Basic Research Program of China (973 Program) under Contract 2009CB930300, National Nature Science Foundation of China (81072583), and a part by National Institutes of Health grants RC2 GM092599, Center of Cancer Nanotechnology Excellence grant (U54 CA151668)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release. 2008;132:153–63. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- [2].Park K. Nanotechnology: What it can do for drug delivery. J Control Release. 2007;120:1–3. doi: 10.1016/j.jconrel.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ojea-Jimenez I, Comenge J, Garcia-Fernandez L, Megson ZA, Casals E, Puntes VF. Engineered Inorganic Nanoparticles for Drug Delivery Applications. Curr Drug Metab. 2012 doi: 10.2174/13892002113149990008. [DOI] [PubMed] [Google Scholar]
- [4].Hu FQ, Zhao MD, Yuan H, You J, Du YZ, Zeng S. A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studies. Int J Pharm. 2006;315:158–66. doi: 10.1016/j.ijpharm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- [5].You J, Hu FQ, Du YZ, Yuan H. Polymeric micelles with glycolipid-like structure and multiple hydrophobic domains for mediating molecular target delivery of paclitaxel. Biomacromolecules. 2007;8:2450–6. doi: 10.1021/bm070365c. [DOI] [PubMed] [Google Scholar]
- [6].You J, Hu FQ, Du YZ, Yuan H, Ye BF. High cytotoxicity and resistant-cell reversal of novel paclitaxel loaded micelles by enhancing the molecular-target delivery of the drug. Nanotechnology. 2007;18:495101. doi: 10.1088/0957-4484/18/49/495101. [DOI] [PubMed] [Google Scholar]
- [7].You J, Hu FQ, Du YZ, Yuan H. Improved cytotoxicity of doxorubicin by enhancing its nuclear delivery mediated via nanosized micelles. Nanotechnology. 2008;19:255103. doi: 10.1088/0957-4484/19/25/255103. [DOI] [PubMed] [Google Scholar]
- [8].Schwartzberg AM, Oshiro TY, Zhang JZ, Huser T, Talley CE. Improving nanoprobes using surface-enhanced Raman scattering from 30-nm hollow gold particles. Anal Chem. 2006;78:4732–6. doi: 10.1021/ac060220g. [DOI] [PubMed] [Google Scholar]
- [9].Lu W, Huang Q, Ku G, Wen X, Zhou M, Guzatov D, Brecht P, Su R, Oraevsky A, Wang LV, et al. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials. 2010;31:2617–26. doi: 10.1016/j.biomaterials.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin Cancer Res. 2009;15:876–86. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 2010;4:1033–41. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].You J, Shao R, Wei X, Gupta S, Li C. Near-infrared light triggers release of Paclitaxel from biodegradable microspheres: photothermal effect and enhanced antitumor activity. Small. 2010;6:1022–31. doi: 10.1002/smll.201000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].You J, Zhang R, Zhang G, Zhong M, Liu Y, Van Pelt CS, Liang D, Wei W, Sood AK, Li C. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: A platform for near-infrared light-trigged drug release. J Control Release. 2012;158:319–28. doi: 10.1016/j.jconrel.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xiong C, Huang M, Zhang R, Song S, Lu W, Flores L, 2nd, Gelovani J, Li C. In vivo small-animal PET/CT of EphB4 receptors using 64Cu-labeled peptide. J Nucl Med. 2011;52:241–8. doi: 10.2967/jnumed.110.081943. [DOI] [PubMed] [Google Scholar]
- [15].You J, Zhang R, Xiong C, Zhong M, Melancon M, Gupta S, Nick AM, Sood AK, Li C. Effective photothermal chemotherapy using doxorubicin-loaded gold nanospheres that target EphB4 receptors in tumors. Cancer Res. 2012;72:4777–86. doi: 10.1158/0008-5472.CAN-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- [17].Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–9. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- [18].Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci U S A. 2004;101:5583–8. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, Zhu S, Hassanieh L, Smith DL, Buscarini M, Broek D, et al. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–80. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- [20].Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–93. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, Liu R, Manchanda PK, Ladner RD, Hawes D, Weaver FA, et al. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69:3736–45. doi: 10.1158/0008-5472.CAN-08-3232. [DOI] [PubMed] [Google Scholar]
- [22].Noble RP, Hatch FT, Mazrimas JA, Lindgren FT, Jensen LC, Adamson GL. Comparison of lipoprotein analysis by agarose gel and paper electrophoresis with analytical ultracentrifugation. Lipids. 1969;4:55–9. doi: 10.1007/BF02531795. [DOI] [PubMed] [Google Scholar]
- [23].Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- [24].Rijcken CJ, Soga O, Hennink WE, van Nostrum CF. Triggered destabilisation of polymeric micelles and vesicles by changing polymers polarity: an attractive tool for drug delivery. J Control Release. 2007;120:131–48. doi: 10.1016/j.jconrel.2007.03.023. [DOI] [PubMed] [Google Scholar]
- [25].Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release. 2008;132:164–70. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chung JE, Yokoyama M, Okano T. Inner core segment design for drug delivery control of thermo-responsive polymeric micelles. J Control Release. 2000;65:93–103. doi: 10.1016/s0168-3659(99)00242-4. 26. [DOI] [PubMed] [Google Scholar]
- [27].Chung JE, Yokoyama M, Yamato M, Aoyagi T, Sakurai Y, Okano T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate) J Control Release. 1999;62:115–27. doi: 10.1016/s0168-3659(99)00029-2. [DOI] [PubMed] [Google Scholar]
- [28].Prabaharan M, Grailer JJ, Steeber DA, Gong S. Thermosensitive micelles based on folate-conjugated poly(N-vinylcaprolactam)-block-poly(ethylene glycol) for tumor-targeted drug delivery. Macromol Biosci. 2009;9:744–53. doi: 10.1002/mabi.200800366. [DOI] [PubMed] [Google Scholar]
- [29].Soga O, van Nostrum CF, Hennink WE. Poly(N-(2-hydroxypropyl) methacrylamide mono/di lactate): a new class of biodegradable polymers with tuneable thermosensitivity. Biomacromolecules. 2004;5:818–21. doi: 10.1021/bm049955q. [DOI] [PubMed] [Google Scholar]
- [30].Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood) 2009;234:123–31. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhao Y. Rational design of light-controllable polymer micelles. Chem Rec. 2007;7:286–94. doi: 10.1002/tcr.20127. [DOI] [PubMed] [Google Scholar]
- [32].Hu FQ, Wu XL, Du YZ, You J, Yuan H. Cellular uptake and cytotoxicity of shell crosslinked stearic acid-grafted chitosan oligosaccharide micelles encapsulating doxorubicin. Eur J Pharm Biopharm. 2008;69:117–25. doi: 10.1016/j.ejpb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- [33].Zhao MD, Sun YM, Fu GF, Du YZ, Chen FY, Yuan H, Zheng CH, Zhang XM, Hu FQ. Gene therapy of endometriosis introduced by polymeric micelles with glycolipid-like structure. Biomaterials. 2012;33:634–43. doi: 10.1016/j.biomaterials.2011.09.077. [DOI] [PubMed] [Google Scholar]
- [34].Hu FQ, Liu LN, Du YZ, Yuan H. Synthesis and antitumor activity of doxorubicin conjugated stearic acid-g-chitosan oligosaccharide polymeric micelles. Biomaterials. 2009;30:6955–63. doi: 10.1016/j.biomaterials.2009.09.008. [DOI] [PubMed] [Google Scholar]
- [35].You J, Li X, de Cui F, Du YZ, Yuan H, Hu FQ. Folate-conjugated polymer micelles for active targeting to cancer cells: preparation, in vitro evaluation of targeting ability and cytotoxicity. Nanotechnology. 2008;19:045102. doi: 10.1088/0957-4484/19/04/045102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.